Article contents
Study of the precursors of ovine lactoproteins: primary structures of the ‘signals’ and enzymic processing of prelactoproteins by mammary microsomal membranes
Published online by Cambridge University Press: 01 June 2009
Summary
The radiolabelled primary translation products of ovine mammary mRNAs synthesized in a wheat germ cell-free system were isolated by immuno-precipitation and analysed by automated Edman degradation. The 3 ‘Ca-sensitive’ caseins (α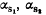 , α andβ ), k-casein, β-lactoglobulin and α-lactalbumin were found to be synthesized as precursors with amino terminal extensions of 15, 21, 18 and 19 amino acid residues respectively. The extra pieces of these various lactoproteins are similar to ‘signal’ peptides of other secretory proteins in their length and hydro-phobicity. The occurrence of an alanyl residue at the C-termini of the extra pieces of the 6 ovine prelactoproteins suggests that the mammary proteinase responsible for the cleavage of the signal peptides may have an elastase-like specificity.
, α andβ ), k-casein, β-lactoglobulin and α-lactalbumin were found to be synthesized as precursors with amino terminal extensions of 15, 21, 18 and 19 amino acid residues respectively. The extra pieces of these various lactoproteins are similar to ‘signal’ peptides of other secretory proteins in their length and hydro-phobicity. The occurrence of an alanyl residue at the C-termini of the extra pieces of the 6 ovine prelactoproteins suggests that the mammary proteinase responsible for the cleavage of the signal peptides may have an elastase-like specificity.
When mammary mRNAs were translated in a wheat germ cell-free system in the presence of mammary microsomal membranes, pre-β-casein was converted into authentic β-casein as demonstrated by amino terminal sequence analyses. Additionally, pre-β-casein was post-translationally converted into authentic β-casein by a specific proteinase(s) extracted from rough microsomes with Na deoxycholate.
- Type
- Section A. Biological Aspects of Milk Proteins
- Information
- Journal of Dairy Research , Volume 46 , Issue 2: Symposium on the Physics and Chemistry of Milk Proteins , April 1979 , pp. 175 - 180
- Copyright
- Copyright © Proprietors of Journal of Dairy Research 1979
References
REFERENCES
- 3
- Cited by


