Article contents
Purification and properties of an extracellular proteolytic enzyme from Bacillus cereus
Published online by Cambridge University Press: 01 June 2009
Summary
A milk-clotting proteolytic enzyme was isolated and purified from the culture filtrate of Bacillus cereus strain x29 by fractionation with acetone or ammonium sulphate and subsequent column chromatography employing DEAE cellulose and DEAE Sephadex. The purified enzyme was found to be homogeneous by acrylamide gel electrophoresis from pH 3·5 to 8·6, with, a molecular weight of about 50000. The single absorption maximum of the native enzyme was at 277 nm and the value of 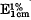 at 280 nm was 7·79. Purification resulted in a 9-fold enhancement of activity with 24 % yield. The optimum activity of the enzyme was at pH 8·0 at 40 °C with casein as the substrate. The enzyme was found to be most stable at pH 6·0 and was stable to freezing and freeze-drying. Heavy metal ions were found to inactivate the enzyme, but no metal ion activation was found. Enzyme activity was inhibited irreversibly by EDTA and reversibly by 1,10-phenanthroline. The enzyme has been identified as a Zn-containing neutral protease.
at 280 nm was 7·79. Purification resulted in a 9-fold enhancement of activity with 24 % yield. The optimum activity of the enzyme was at pH 8·0 at 40 °C with casein as the substrate. The enzyme was found to be most stable at pH 6·0 and was stable to freezing and freeze-drying. Heavy metal ions were found to inactivate the enzyme, but no metal ion activation was found. Enzyme activity was inhibited irreversibly by EDTA and reversibly by 1,10-phenanthroline. The enzyme has been identified as a Zn-containing neutral protease.
- Type
- Research Article
- Information
- Copyright
- Copyright © Proprietors of Journal of Dairy Research 1973
References
REFERENCES
- 8
- Cited by


