Introduction
Functional foods are one of the most valuable parts of the dairy industry and contribute to human health by decreasing the risk of ailments and modulation of colonic microbiota. Fermented milk products, such as cheese and yogurt, are widely consumed due to their healthy nutritional content, sensorial attributes, and compatibility with various diets (Delgado-Fernández et al., Reference Delgado-Fernández, Moreno, Corzo and Nöbel2020). Yogurt is a widely marketed and popular dairy product worldwide, prepared through the fermentation of milk by thermophilic lactic acid bacteria (LAB), especially Lactobacillus delbrueckii subsp. Bulgaricus and Streptococcus thermophilus. These strains are conventionally selected as starter cultures (Fazilah et al., Reference Fazilah, Ariff, Khayat, Rios-Solis and Halim2018; Meybodi et al., Reference Meybodi, Mortazavian, Arab and Nematollahi2020). In the process of milk fermenting with a pH dropping to 4.5, electrostatic repulsion between the casein micelles subsides, consequently accumulating in a homogeneous gel structure (Delgado-Fernández et al., Reference Delgado-Fernández, Moreno, Corzo and Nöbel2020). Due to the different forms, manufacturing processes, and components of yogurt as well as diverse consumer preferences, it is challenging to standardize the quality of yogurt. In dairy products, the majority of safety concerns are related to the presence of contamination in milk, including residuals of veterinary drugs, chemical pollutants, pathogens (such as Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes), spoilage agents (such as molds), anaerobic spore-forming bacteria (such as Clostridium tyrobutyricum) and aerobic spore-forming bacteria (such as Stearothermophilus and Bacillus cereus). Nowadays, milk and milk products are widely available, and these contaminants can jeopardize the health of many individuals. Therefore, the production of safe and high-quality dairy products can contribute to protecting public health and satisfy consumers (Ortuzar et al., Reference Ortuzar, Martinez, Bianchini, Stratton, Rupnow and Wang2018; Prabhurajeshwar and Chandrakanth, Reference Prabhurajeshwar and Chandrakanth2019; Zubairi et al., Reference Zubairi, Ishak, Sani, Kasim and Nurzahim2021). Food safety requires conformity with good manufacturing practices (GMP) which would typically require a food safety management system (FSMS) and a sanitation standard operating procedure (SSOP) as well as good hygiene practices (GHP), also called operation prerequisite programs (OPRPs), and adherence to the principles of hazard analysis of critical control points (HACCP) (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). The HACCP program is a global approach to risk management designed to identify and predict potential hazards during each stage of food manufacturing, from farm to fork (Hoolasi, Reference Hoolasi2005). The origins of HACCP date back to the 1960s during the collaboration of Pillsbury Company, the United States Army Laboratories at Natick and NASA to develop the tool for monitoring and managing food safety in manned space missions. Later, HACCP was standardized by Codex Alimentarius in 1996 and is currently widely employed for ensuring food safety because it maintains, identifies, evaluates, controls and monitors each manufacturing point (Murphy, Reference Murphy and Griffiths2010). HACCP is considered synonymous with food safety and was initially developed as a ‘zero defects’ program (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016). It is a scientific method systematically employed to identify food safety risks and prevent the consumption of unsafe food. HACCP emphasizes preventative measures instead of depending on end-product evaluation for control, and in addition to identifying and preventing hazards, recommends precautions for their control (Murphy, Reference Murphy and Griffiths2010). There is no doubt that HACCP is a well-known, efficient and preventive method of managing food safety. Following its implementation in the industry, the principal benefits and barriers associated with HACCP are identified and discussed. Currently, the HACCP system has been adopted by numerous countries and international organizations such as the WHO and FAO (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). Food safety systems (HACCP principles), prerequisite programs (PRPs), and OPRPs have been described for quality control in small-scale yogurt production factories (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). Psomas and Kafetzopoulos (Reference Psomas and Kafetzopoulos2015) investigated the effectiveness of HACCP between ISO 22000 certified and non-certified dairy companies, while Abd Rabo et al. (Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016) gave safety specifications for dairy processing. These studies were designed based on the HACCP program, as defined by its 7 principles in the Codex Alimentarius (Codex Alimentarius, 2020), which is abbreviated hereafter as classical HACCP. To the best of our knowledge, only a limited number of studies have addressed the implementation of HACCP in yogurt production. Since yogurt occupies a special place in the diet of many people, the production of safe and high-quality yogurt is crucial. Implementing HACCP in yogurt production is one of the most effective methods for ensuring yogurt safety. Hence, the purpose of this study was to review the literature about implementing and evaluating the hazard analysis and critical control point (HACCP) during different stages of the yogurt manufacturing process.
Materials and methods
Search strategy
We undertook a qualitative descriptive study in the form of a literature review, conducted in May 2022. The literature study method involved various written sources in the form of books, journals, scientific articles, etc. Articles were searched in databases in May 2022, and there was no limitation on searching for them. The chosen databases were Scholar, PubMed, Science Direct, Web of Science, and Scopus. The keywords used in the systematic search included: (‘yogurt’ or ‘dairy’) and (‘HACCP plan’ or ‘food management systems’ or ‘ISO: 22000’ or ‘CCP’ or ‘chemical hazard’ or ‘biological hazard’ or ‘physical hazard’ or ‘control measures’).
Methods
The implementation of international standards in companies is a crucial component of promoting their competitiveness in the marketplace. Food producers are obligated to fulfill their responsibility for hygienic and safe food, consumer care and compliance with environmental standards. Therefore, implementing management systems and verifying them with an emphasis on the product's safety and quality to ensure consumer health and enhance their confidence is critical for foodstuff firms. According to numerous studies examining the experience of companies in diverse sectors, FSMS may be implemented for widely varying reasons, for instance, interpretation and inferences of information and identification or description of FSMS problems. HACCP system is an essential part of the food safety management system, therefore, many studies focus on HACCP. However, a number of the factors that lead to the adoption of HACCP would also be attributed to the implementation of ISO 22000 (Chen et al., Reference Chen, Liu, Chen, Chen, Yang and Chen2020; Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020).
Pre-requisite programs of HACCP
Elaboration of PRPs
The HACCP team is accountable for organizing and implementing corrective measures to enhance conformity to the PRPs (GAP, GHPs, GMPs, and SSOPs). Prior to implementing HACCP, it is necessary to execute pre-requisite programs efficiently, otherwise the implementation of HACCP is intricate and severely challenging. PRP is an umbrella term to describe all activities from the farm to the final consumer. These refer to good hygiene practices that are the fundamental requirements and actions for providing a hygienic environment, including premises and structure (such as appropriate and adequate employee facilities, hygienic facilities, and proper structure of floors, walls, and doors); equipment calibration; technical maintenance; sanitation and cleaning; area delineation (such as avoiding cross-contamination); controlling and eliminating environmental contaminations; control of raw material suppliers (such as ingredients, additives, packaging material); storage, distribution, and transport; pest control; waste management; hygiene of employees; training and supervision; provision of working instructions (Ali, Reference Ali2015).
Elaboration of OPRPs
In food safety, O-PRPs refer to specific PRPs that are recognized by hazard analysis necessary for controlling the probability of introducing food safety hazards, therefore O-PRPs are directly related to PRPs. O-PRPs are control measures or incorporation of control measures used to eliminate or diminish a significant food safety hazard to an allowable level (Chen et al., Reference Chen, Liu, Chen, Chen, Yang and Chen2020).
Elaboration of the HACCP/implementation of the HACCP plan
Twelve steps for implementing a HACCP plan in different process of yogurt production according to Codex Alimentarius were considered (Table 1).
Table 1. Steps of HACCP implementation
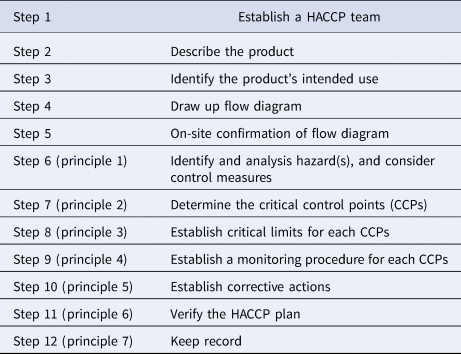
Step 1: Establish a food safety team
The HACCP team must comprise experts from a range of disciplines to ensure that decisions are based on a complete comprehension of the commodity system and can precisely detect potential hazards and, further, that their implementation and control is undertaken by individuals who possess the suitable combination of professional training, skill and experience to recognize the potential hazards to consumer health and prevent them from occurring. One of the strength points of HACCP is the food safety team which should include:
-
− Team leader: must be a trained person and experienced in HACCP teamwork in order to coordinate and guide the team's work.
-
− Specialists: a group of experts each of them knowledgeable about specific hazards associated with the product and process, such as, chemist, toxicologist, microbiologist, QC manager, and process engineer.
-
− Production specialist: with a well-developed knowledge of the commodity system.
-
− Depending on the situation and necessity, other people may also participate in the team; for example, hygiene specialists, packaging experts, purchasers of raw materials, distribution staff or production staff, farmers, etc.
Team members may require training before implementing HACCP. Therefore, to effectively contribute to the HACCP plan, they should be fully trained and updated with a comprehensive knowledge of the HACCP principles (Ali, Reference Ali2015).
Step 2: Prepare a description of the product
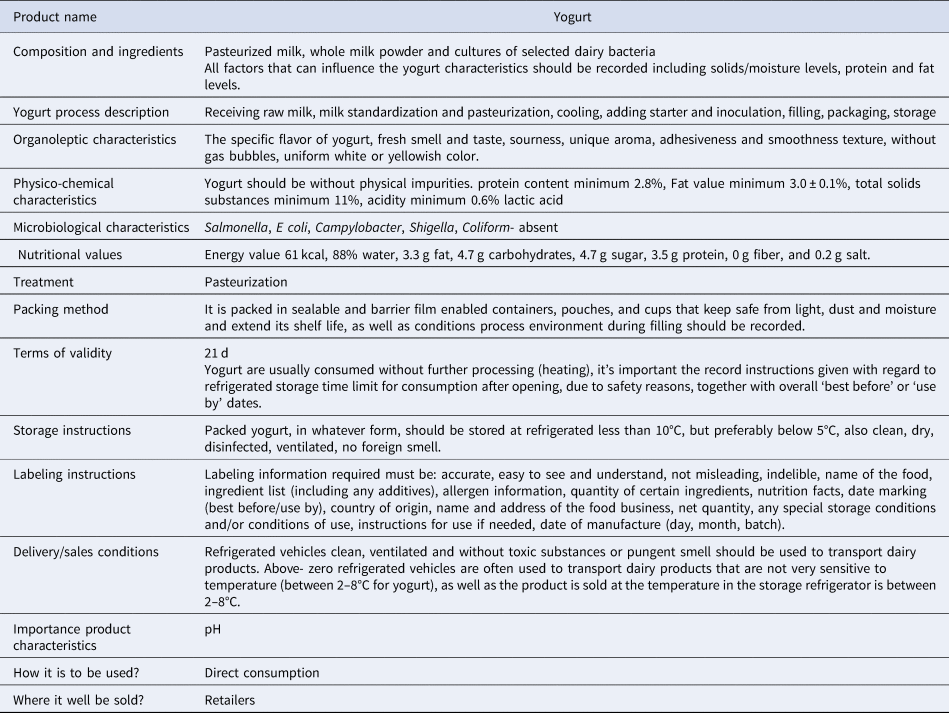
To initiate a hazard analysis, a detailed description of the yogurt should be prepared, including information regarding its ingredients and its processing, biological, chemical and physical properties as well treatments to be applied, durability of the product, storage conditions required and distribution systems. This information will guide the team to identify potential hazards at different stages of process which must be considered. Table 2 summarizes yogurt's typical characteristics.
Table 2. Description of yogurt's properties
Step 3: Identify the intended use
It is crucial to consider how the product will be used. Intended use and consumer group information are part of the product and process description. Information such as whether the product will be consumed directly, or be cooked or further processed as well as information on storage methods, all influence hazard analyses. Furthermore, the characteristics of the target group of the product may also be pertinent, especially in the case of infants, the elderly, malnourished individuals and those with compromised immune systems. Yogurt is a suitable product for consumption by all individuals of all ages except vulnerable people (those with a milk allergy or intolerance) and marketed to final consumers through food retailers (Ali, Reference Ali2015).
Step 4: Prepare a flow diagram
In a HACCP study, flow diagrams are designed to elicit a comprehensive evaluation of the process, then documented in a way that facilitates and guides the following stages (Hoolasi, Reference Hoolasi2005). The flow chart of yogurt production is illustrated in Fig. 1.
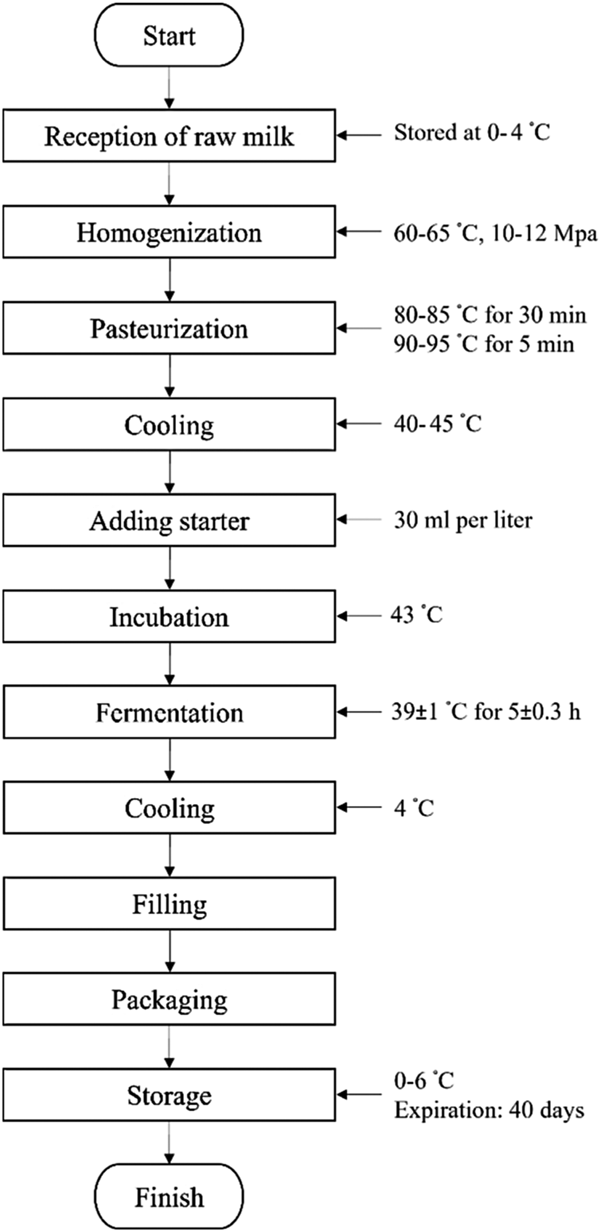
Figure 1. Flow diagram of yogurt processing (Fazilah et al., Reference Fazilah, Ariff, Khayat, Rios-Solis and Halim2018).
Step 5: Confirmation of flow diagrams on site
The HACCP team should validate the flow diagram on-site. A flow diagram should include any effect of shift patterns and weekend working, as well as any reclaim or rework activity. It should be noted that in case of any change in the production process, a new flow chart must be prepared (Hoolasi, Reference Hoolasi2005).
Step 6, principle 1: Identify and analyze hazards, and consider control measures
Food safety hazards are known as contaminants, which can make food products unsafe for manufacturers. Food safety hazards in the manufacturing process of dairy products are generally classified into major groups: chemical, biological, and physical (Kristiningrum and Permatasari, Reference Kristiningrum and Permatasari2020). Also, the Codex Alimentarius Commission identified the hazards as chemical, biological, and physical contamination in food products or the specific condition of a food product with a potentially harmful impact on human health (Codex Alimentarius, 2020). Dairy chain hazards can occur at every stage, including receiving milk (microbial such as coliforms, somatic cell count, and chemical, such as antibiotic residues), formulation and preparation (microbial e.g. Staphylococcus aureus, Coxiella burnetii, chemical such as acidity, physical such as foreign materials), packaging and labeling (physical such as foreign materials) transportation and storage (microbial such as total count increase, chemical such as acidity). From raw milk receipt during processing to the consumer, milk is threatened by numerous hazards, which affect the quality and safety of the final food product. Some of these types of hazards may be caused by animal husbandry techniques, during feeding, milking, and processing (Tiwari et al., Reference Tiwari, Babra, Tiwari, Williams, De Wet, Gibson, Paxman, Morgan, Costantino and Sunagar2013). Hazard analysis is developed from the receiving of raw material stage to the delivery of the final food product (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). The food supply chain (from farm to fork) contains a wide variety of observable risks (biological, chemical, and physical). The global food safety initiative (GFSI) identified hazards in different groups: allergens (including mislabeling) at 46.2%, cross-contamination (including biological hazards) at 40%, chemical hazards at 2.3%, physical hazards at 9.3% and others at 2.1% from 2008–2018 (Soon et al., Reference Soon, Brazier and Wallace2020). Hazards may possibly enter a food chain from the food product ingredients and cause contamination through food processing. HACCP is a preventive and management system that is considered to guarantee safety and security of food. It enables product protection and error correction, minimizes the expenses resulting from quality defects and improves the food control. From the primary manufacturer to the last customer, the HACCP system can be applied to the whole food supply chain. It helps to find the best approaches to control hazards by avoiding their entry into the process, eliminating them, or decreasing the contamination to an acceptable limit (Manning et al., Reference Manning, Baines and Chadd2006).
Physical hazards
Physical hazards generally include solid particles (for instance, glass pieces, metal and bone fragments, insects or their parts, jewelry, stones/soil/dust and hair/fur) and relative to other hazards are usually easily detectable (Codex Alimentarius, 2020). They can happen as accidental contamination and are more associated with contact with different objects, packaging and labeling (McSwane et al., Reference McSwane, Rue and Linton2003; Van Asselt et al., Reference Van Asselt, Noordam, Pikkemaat and Dorgelo2018). The safety and quality of raw milk are of paramount importance, as it potentially ensures the safety and quality of the dairy products derived from it. Ideally, in addition to having a pleasant taste without off-odor, the milk needs to be free of physical hazards such as forage pollutants (Merrzlov et al., Reference Merrzlov, Lomova, Narizhniy, Rudakova, Snizhko and Viktor Voroshchuk2018).
Biological hazards
Organisms that can result in serious harm through intoxication or infection such as pathogenic bacteria, toxigenic molds or fungi and parasites are known as biological hazards (Codex Alimentarius, 2020). Biological and chemical hazards are more important and more common than physical ones, due to the complexity of their effects on body interactions (Merrzlov et al., Reference Merrzlov, Lomova, Narizhniy, Rudakova, Snizhko and Viktor Voroshchuk2018). Recently, food safety legislative demands have increased due to extensive food scares such as microbiological hazards (including Salmonella, E. coli), contaminants (including dioxins), and also animal disease (Kendall et al., Reference Kendall, Kaptan, Stewart, Grainger, Kuznesof, Naughton, Clark, Hubbard, Raley and Marvin2018). Several investigations have identified pathogenic organisms in milk, containing Shiga-toxin-producing E. coli (STEC), Campylobacter jejuni, Salmonella spp., L. monocytogenes, and Yersinia enterocolitica. The skin and gastrointestinal tracts of livestock and the environment of farms are potential sources of many pathogens found in milk (Oliver et al., Reference Oliver, Jayarao and Almeida2005). These pathogenic microorganisms could arrive in meat products and milk through slaughter and milking processes (McEwen and Fedorka-Cray, Reference McEwen and Fedorka-Cray2002). Most of the dangerous microbiological hazards can be removed by thermal treatments (e.g. sterilization or pasteurization) (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016). Recently, food-borne disease outbreaks have been highly related to four genera of organisms, namely Salmonella spp., Campylobacter spp., L. monocytogenes and E. coli (Yilmaz et al., Reference Yilmaz, Moyer, MacDonell, Cordero-Coma and Gallagher2009). In the study of Makita et al. (Reference Makita, Desissa, Teklu, Zewde and Grace2012), the annual incidence rate of staphylococcal poisoning through consumption of informally-marketed milk and home-made yogurt was calculated at 20 per 1000 people. Additionally, this study found that milk fermentation reduced staphylococcal poisoning risk by 93.7%, so that when this step was eliminated, the annual incidence rate increased to 315.8 per 1000 people (Makita et al., Reference Makita, Desissa, Teklu, Zewde and Grace2012). However, cow's raw milk contains numerous types of pathogens including Escherichia coli O157:H7 (Tsegaye and Ashenafi, Reference Tsegaye and Ashenafi2005) and Brucella abortus (Tesfaye et al., Reference Tesfaye, Tsegaye, Chanie and Abinet2011). E. coli O157:H7 can survive for up to 72 h even when pH has fallen to 3.8 or 3.9 (Tsegaye and Ashenafi, Reference Tsegaye and Ashenafi2005), but Brucella will be inactivated quickly when the acidity decreases below pH 4, and very quickly below pH 3.5 (Codex Alimentarius, 2020).
Chemical hazards
Long-term or short-term exposure to toxic chemicals can lead to adverse effects on human health. Naturally occurring toxins, veterinary drug residues, pesticide residues, heavy metals, direct and indirect food additives, environmental contaminants and chemicals coming from packaging material are classified as chemical hazards. In the study, 66% of milk samples contained H2O2, which is usually added in the summertime to increase milk quality (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016).
Step 7, principle 2: Identify the critical control points
Critical control points (CCPs) refer to the stages where hazards are more likely to occur and must be meticulously controlled. The CCP decision tree of the Codex is a practical approach that is commonly applied by HACCP teams (Fig. 2). Critical limits need to be assessable and recognized for total CCPs (Codex, Reference Codex1997). Mureşan et al. (Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020) implemented ISO 22000:2018 in the production of yogurt containing 3.6% fat on a small-scale, and findings revealed that pasteurization was the most significant CCP as it could eliminate many pathogenic bacteria (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). Implementing proper management practices, especially monitoring the temperature and time of pasteurization (72°C for 15 s) will greatly improve pasteurization efficiency (Azar and Rofehgari-Nejad, Reference Azar and Rofehgari-Nejad2009). The cooling stage was reported as the second CCP, where the temperature declined from 85 to 2–8°C in 1 h. This stage can be effective in preventing the growth of thermos-tolerant bacteria through careful time and temperature monitoring. The last CCP was delivery and sales, however, the reception of milk, packaging, and reception of lactic acid bacteria starter cultures were also considered as CPs (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). Musaj et al. (Reference Musaj, Bijo, Hoxha and Gjinovci2012) reported eight CCP, namely receipt of fresh milk, filtration, separation, pasteurization, fermentation, filling of cups, packaging and storage, and in the manufacture of yogurt with honey products 6 CCP were determined: receiving of raw milk, receipt of raw non-dairy materials, cooling and reservation steps, pasteurization, fermentation and packaging (Merrzlov et al., Reference Merrzlov, Lomova, Narizhniy, Rudakova, Snizhko and Viktor Voroshchuk2018). Chountalas et al. (Reference Chountalas, Tsarouchas and Lagodimos2009) identified 8 CCPs for yogurt production which were receiving raw milk (CCP1), raw milk and cream storage (CCP2), heat treatment (CCP3), starter culture ingredients storage (CCP4), flavorings storage (CCP5), cream receipt (CCP6), packaging (CCP7) and finished product quarantine (CCP8), as did Shapton and Shapton (Reference Shapton and Shapton1991) for manufacture of yogurt with added fruit or nut puree, which in this case were receiving milk, fruit or nut removed from the container and stabilizer added, pasteurization, starter incubation, filling, chill storage of fruit or nut processed off-site and distribution (Shapton and Shapton, Reference Shapton and Shapton1991). Aly et al. (Reference Aly, Hathout and Sahab2011) reported that identified CCPs in probiotic Talbina processing (a dairy product) included raw milk receipt, pasteurization treatment, probiotic bacteria addition, container filling and storage. Due to the possibility of contaminating the final product through damage to filling containers and presence of toxic residuals in them, the filling containers stage was considered CCP. Furthermore, storage is one crucial stage to provide food safety because storage at higher temperatures may increase the growth of different fungal species and/or pathogenic microorganisms (Aly et al., Reference Aly, Hathout and Sahab2011). The HACCP study of yogurt produced by small and medium-sized enterprises in the Semarang region of Indonesia reported 8 CCPs in raw materials and manufacturing process, and it was emphasized that the suppliers require to be chosen carefully and the storage condition (time and temperature), equipment sanitization, instrument calibration, performance control and record keeping should all be well controlled (Kristiningrum and Permatasari, Reference Kristiningrum and Permatasari2020). In Table 3 HACCP plan for different process steps in production of yogurt defines hazards with the related control measures, critical limits and monitoring. Deviations detected by monitoring are treated by the proper corrective actions.

Figure 2. HACCP decision tree for the determination of critical points in HACCP plans (Jervis, Reference Jervis2002).
Table 3. HACCP plan for the management of the manufacture of yogurt (Ali, Reference Ali2015; Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020)

Step 8, principle 3: Establish critical limits for each CCP
The establishment of critical limit(s) is recognized as a criterion for distinguishing acceptability from unacceptability. A critical limit is known as a maximum and/or minimum assessment to which a biological, chemical, or physical hazard must be carefully controlled at a CCP in order to prevent, remove or reduce to an acceptable level the risk of incidence of a food safety hazard. It should be quantifiable in real-time (throughout the yogurt preparation process) and might contain determinations of time, temperature, acidity, pH, moisture, the phosphatase activity for pasteurized milk efficiency and ATP testing method to determine cleaning efficiency (Hoolasi, Reference Hoolasi2005). In the Galochkina study, changes in temperatures have been applied as the main criterion for the determination of CCPs for the probiotic dairy product (Galochkina and Glotova, Reference Galochkina and Glotova2019).
Step 9, principle 4: Establish a monitoring procedure for each CCP
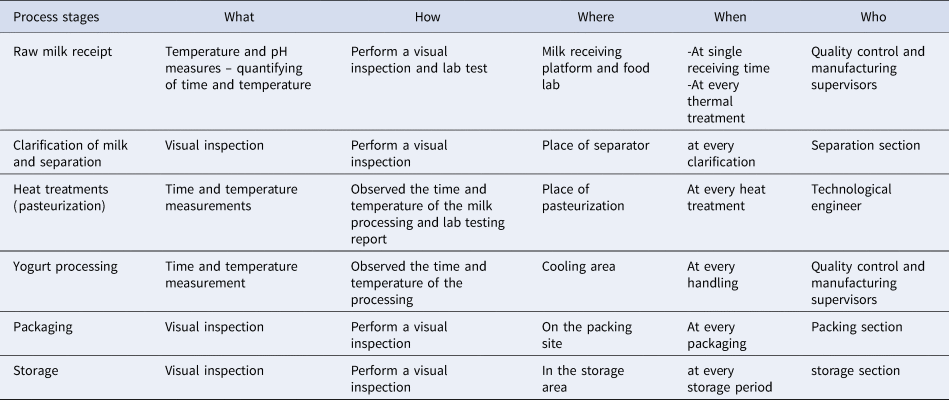
Monitoring is a good guarantee for ensuring whether the critical limit at every single CCP can be constantly performed. Additionally, monitoring contains an organized system of measurements or observations against critical limits to measure whether a CCP is entirely under control. Deviations in the CCPs can occur due to various reasons, such as failures in monitoring, absence of enough equipment and problems with providers (Cusato et al., Reference Cusato, Gameiro, Corassin, Sant'Ana, Cruz, Faria and de Oliveira2013). Steps involved in monitoring are shown in Table 4 and might include the following items: object (e.g. additive concentration); method (requirement for inspection report); frequency (each batch); personnel (operators) (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016). Strict monitoring of technological procedures and the corrective actions will reduce the risk in the manufacture of unsafe fermented dairy products like yogurt (Galochkina and Glotova, Reference Galochkina and Glotova2019). The implementation of HACCP plays an important role in improving monitoring tactics and making validation data which leads to the effectiveness of the processing control. Monitoring can take place offline when corrective action might involve rejecting each implicated product, or online with automated corrective action such as flow diversion devices on pasteurizers. Physical and chemical tests are considered over microbiological measurements because they can be performed quickly and often indicate the conditions controlling the microbiology of the product (phosphatase test for pasteurized milk is an example: Hoolasi, Reference Hoolasi2005).
Table 4. Monitoring procedure (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016; Chountalas et al., Reference Chountalas, Tsarouchas and Lagodimos2009)
Step 10, principle 5: Establish corrective actions
Corrective actions for dairy products would include the following procedures:
1) Receiving milk and other raw materials from approved providers and registered vendors.
2) Rejecting milk when the contamination is detected during raw milk receipt.
3) Calibration and adjusting of all equipment effecting quality and safety of yogurt.
4) Re-heat process (temperature and time) when the monitoring outcomes are modified (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020)
5) Training of operators and staff for operational controls and laboratory tests and also re-training for personnel hygiene.
6) Providing control devices for monitoring the temperature and relative humidity in stores, production facilities etc.
7) Washing and sanitizing plastic cups with hot water (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016).
The aim of monitoring is to control the critical control limits in the specified intervals, and in case of leaving this range, corrective measures will be taken. These actions assist in controlling the process before critical limits are exceeded or will remove the product that has violated the critical limits. Monitoring and corrective actions should be undertaken by all HACCP team members, and appropriate decisions should be taken. Also, the individuals who are responsible for executing corrective actions should be identified and all records must be documented (Hoolasi, Reference Hoolasi2005). Cusato et al. (Reference Cusato, Gameiro, Corassin, Sant'Ana, Cruz, Faria and de Oliveira2013) showed that incorrect monitoring and the lack of corrective actions in the yogurt production line resulted in increasing the temperature of the cold storage up to 24°C and led to the deterioration of several yogurts kept there. As a solution, the group of personnel was trained for the dispatching process, and subsequently, monitoring was carried out successfully.
Step 11, principle 6: Verification of the HACCP plan
Verification procedures affirm that the performance of HACCP program is effective. Validation and verification are recognized as confirmation tools. These are distinct and a variety of activities are well-defined by Codex (2020) (Codex Alimentarius, 2020).
Validation
Obtaining sufficient evidence to prove the parts of the HACCP plan are effective.
Verification
The employing of techniques, tests, procedures, and other measurements, also monitoring, to evaluate conformance to the HACCP plan. A complete HACCP system needs a verification procedure, like randomized sampling and different analyses, to ensure the HACCP system can successfully control and ensure food safety. Verification actions were established to verify whether HACCP controls work efficiently and correctly during yogurt processing (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016). The verification would be completed by validation system so that it can satisfy all Codex needs and be updated in the event that the materials or manufacturing process stages change. The verification procedure for yogurt includes checking thermal treatment records, analysis of the final product, checking the calibration of monitoring instruments and testing the transport of raw milk and other materials, frequently (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016). Furthermore, verification of conformity with equipment maintenance, the pest control process, and the sanitation of manufacturing spaces, work equipment and annexes have been reported (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). For instance, the alkaline phosphatase activity may be evaluated to verify the efficiency of thermal treatment (Harding, Reference Harding1991).
Step 12, principle 7: Record keeping and documentation
Documentation and record-keeping consists of a team member list and their tasks, a detailed description of the food product and intended use of it, a flow chart, hazards related to every CCP and prevention measures, identification of CCP and establishment of critical limits for every single one of them, monitoring, corrective action for deviation from critical limits, HACCP plan, maintenance of records and actions for verification of HACCP system (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016).
The HACCP approach will be an integral component of the documentation, drawing the CCPs and their controlling methods (critical limits, monitoring, and corrective actions), which shows the validation of the HACCP plan. The success of HACCP is heavily dependent upon the maintenance and keeping of HACCP records. Documents can be archived in both paper and computer forms (Kamboj et al., Reference Kamboj, Gupta, Bandral, Gandotra and Anjum2020). Documentation must contain the precise details of total operations such as times, temperatures and microbiological factors accurately. Moreover, the operator's responsibilities related to that particular section of the production line should be recorded. Complaints from consumers or authorities provide evidence that the adopted HACCP program failed to effectively control the manufacturing process. To determine whether the HACCP program is completely implemented and provides the indicated control, it is necessary to audit all monitoring and corrective action archives. In addition, validation records must be reviewed, and additional testing at determined CCPs may be necessary to confirm the effectiveness of the control measure (Hoolasi, Reference Hoolasi2005).
Effectiveness of the HACCP plan
HACCP plan's implementation is a reliable and cost-effective method to ensure food safety (Papademas and Bintsis, Reference Papademas and Bintsis2010). Implementation is advantageous in all stages of the yogurt production process and contributes to the production of a higher quality and safer product, increasing customer satisfaction, boosting factory profitability, restricting financial losses, and improving brand reputation and credibility (Musaj et al., Reference Musaj, Bijo, Hoxha and Gjinovci2012).
According to the study by Mureşan et al. (Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020) HACCP plan's implementation in a small-scale yogurt factory, reduced the microbiological load of the final product. The study identified pasteurization, cooling/storage and distribution stages as CCPs. The implementation of the HACCP plan resulted in decreased microbial load of raw milk, pasteurized milk and final product (yogurt). The results of the study indicated that after the HACCP implementation, the CFU in the raw milk decreased from 250 000 to 80 182 CFU/ml, and the CFU of Enterobacteriaceae in the final product (yogurt) decreased from 3 to 0 CFU/ml. These values were within the maximum allowed (Mureşan et al., Reference Mureşan, Marc, Jimborean, Rusu, Mureşan, Nistor, Cozma and Suharoschi2020). In addition, Abd Rabo et al. (Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016) reported similar results and detected a decrease in mold, yeast, Staphylococcus aureus, Coliform group, and Salmonella species following the implementation of HACCP in raw milk, fresh yogurt, and yogurt stored for 7 and 14 d (Abd Rabo et al., Reference Abd Rabo, El-Bialy, Ahmed and Sobhy2016).
Another research by Musaj et al. (Reference Musaj, Bijo, Hoxha and Gjinovci2012) indicated microbiological results before the implementation of HACCP were beyond the limits of the Kosovo national standard and after the implementation of HACCP, the results were within the standard. In this study, when HACCP plans were implemented, the recognized CCP diminished from 8 to 2, as well as the results indicated a significant diminution in the CFU number of Escherichia coli and Coliform (Musaj et al., Reference Musaj, Bijo, Hoxha and Gjinovci2012). In contrast to Musaj et al. (Reference Musaj, Bijo, Hoxha and Gjinovci2012) study, Cusato et al. (Reference Cusato, Gameiro, Corassin, Sant'Ana, Cruz, Faria and de Oliveira2013) found no substantial difference between the mean total coliform and fecal coliform counts before and after HACCP implantation. However, after HACCP implementation, the amount of yeast and mold decreased (Cusato et al., Reference Cusato, Gameiro, Corassin, Sant'Ana, Cruz, Faria and de Oliveira2013).
Ibrahim (Reference Ibrahim2019) analyzed the physicochemical, chemical, and microbiological properties of yogurt samples in two companies, only one of which was implementing FSMS, and realized that FSMS had a positive effect on the mentioned parameters. In the company without an FSMS, yogurt samples were contaminated with coliforms, yeast, and mold. Moreover, the chemical components and total solids content of yogurt samples in the company that implemented FSMS were higher (Ibrahim, Reference Ibrahim2019). Hoolasi (Reference Hoolasi2005) evaluated raw milk, the manufacturing process, and yogurt before and after the implementation of HACCP to assess its effectiveness. In the case of raw milk, 4004 samples were analyzed. The average percentage of butterfat in 434 raw milk samples following HACCP adoption was 3.761, while it was 3.982 for the same number of samples prior to HACCP adoption. Moreover, HACCP implementation improved the microbiological quality, shelf life, and overall quality of raw milk. In the production process, 1638 samples were tested. There was statistical significance in the pH value of the yogurt in the intermediate tank. In addition, 2898 yogurt samples were analyzed, and results demonstrated that HACCP implementation enhanced final product quality, such as improving the viscosity and PH, reducing the presence of foreign objects and decreasing yeast and mold count (Hoolasi, Reference Hoolasi2005).
According to the reviewed literature, the following operational recommendations for different stages of yogurt production can enhance the effectiveness of the HACCP program:
Receiving raw milk
Microbiological hazards can be controlled by cooling milk rapidly to 0–4°C following collection and keeping it at that temperature of 0–4°C throughout transportation and storage. Moreover, foreign substances can be removed by filtering milk (Chountalas et al., Reference Chountalas, Tsarouchas and Lagodimos2009; Ilozue et al., Reference Ilozue, Bello and Lawan2012).
Homogenization
The objective of the homogenization process is to diminish the diameter of milk fat globules to below 2 microns. As a result, the formation of the cream is prevented. The homogenization temperature must be above 50°C because the milk fat must remain in a liquid like state to prevent any accumulation (Ali, Reference Ali2015). It is recommended that homogenization be carried out at 60–65°C under 10–12 MPa pressure (Merrzlov et al., Reference Merrzlov, Lomova, Narizhniy, Rudakova, Snizhko and Viktor Voroshchuk2018).
Pasteurization
The objectives of the pasteurization of yogurt are: (1) to kill foodborne pathogens (2) to diminish food spoilage agents to permissible levels, including molds such as Aspergillus, Penicillium, Rhizopus, and Fusarium, and yeasts such as Candida spp., Debaryomyces, and Saccharomyces spp. (3) to minimize the total number of microorganisms in order to form a suitable environment for the growth of starter microorganisms (Hoolasi, Reference Hoolasi2005). Also, heat treatment through denaturing whey protein has a substantial impact on yogurt texture formation. Milk pasteurization is performed at a temperature of 80–85°C for 30 min to 90–95°C for 5 min (Soukoulis et al., Reference Soukoulis, Panagiotidis, Koureli and Tzia2007).
Cooling of milk
After pasteurization, milk must be immediately cooled to 40–45°C (Pal et al., Reference Pal, Tefera, Tasew, Jergefa and Deressa2015).
Adding starter and incubation
The starter must be free of bacteriophages and contaminating bacteria and must grow rapidly. The starter culture consists of Streptococcus thermophilus and Lactobacillus bulgaricus in proportion 1:1 should be added at 40–45°C. The temperature of the incubation period should be 43°C and during this period the temperature and acidity must be persistently monitored (Pal et al., Reference Pal, Tefera, Tasew, Jergefa and Deressa2015; Sandrou and Arvanitoyannis, Reference Sandrou and Arvanitoyannis2000).
Cooling
After reaching the desired acidity (pH = 4.5–4.6), the fermented yogurt immediately cools, and the fermentation process is stopped by decreasing the temperature to 4°C (Pal et al., Reference Pal, Tefera, Tasew, Jergefa and Deressa2015).
Filling and packaging
After cooling, the filling operation should be done promptly and fruit or nut puree is added to the yogurt at this stage (Sandrou and Arvanitoyannis, Reference Sandrou and Arvanitoyannis2000). Yogurt is packaged in plastic containers with metal or plastic foil (Sandrou and Arvanitoyannis, Reference Sandrou and Arvanitoyannis2000).
Storage
Yogurt should be stored at a temperature of 0–6°C and its expiration date is about 40 d after the production date (Chountalas et al., Reference Chountalas, Tsarouchas and Lagodimos2009).
Conclusions
Yogurt is one of the most widely consumed dairy products in many communities. The implementation of the HACCP plan with practical preventive procedures for manufacturing this product can guarantee food safety. Numerous potential hazards in terms of biological, chemical, and physical have been reported during yogurt processing (from the stage of raw materials until distribution). It is necessary to watch out for the cooling system and pasteurization step because both of them are more likely to have contamination potential. The records and documentation of the HACCP system can provide traceability of the origin of contamination, and prevent poor-quality products and reduce the material consumption, personnel and financial issues. Moreover, the implementation of the HACCP plan can lead to complaints reduction from the consumers through improving the safety and quality of the yogurt, efficient management of control points and CCP, and augments in sales and reputation of the company.
Acknowledgements
This work is a report on the food safety and hygiene division in the School of Public Health at Tehran University of Medical Sciences.








