Lactose intolerance is a metabolic disorder in which individuals are unable to digest significant amounts of lactose due to insufficient levels of the lactase enzyme (β-galactosidase (β-gal)) (Rusynyk and Still, Reference Rusynyk and Still2001; Suchy et al., Reference Suchy, Brannon, Carpenter, Fernandez, Gilsanz, Gould and Mennella2010). Fermented dairy products are good sources of lactase and can thus be alternatives for those with lactose intolerance.
Lactose (β-galactose-1, 4-glucose), also known as milk sugar, is a disaccharide sugar composed of glucose and galactose monosaccharide (Semenza et al., Reference Semenza, Auricchio and Mantei2001; Harrington and Mayberry, Reference Harrington and Mayberry2008). The lactase enzyme splits and hydrolyzes dietary lactose into glucose and galactose for transport across the cell membrane (Suchy et al., Reference Suchy, Brannon, Carpenter, Fernandez, Gilsanz, Gould and Mennella2010; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). In the human body, glucose acts as a source of energy, and galactose becomes a part of glycolipids and glycoproteins. Lactose is less sweet than other sugars which may make lactose a suitable carbohydrate for infant formulas and may help to prevent the development of a taste preference for sweet foods later in life. The Glycemic Index (GI) of lactose is relatively low and this can be beneficial for people who are prone to hyperglycemia (Schaafsma, Reference Schaafsma2008; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Lactose also has an effect on mineral absorption. For example, Abrams et al. (Reference Abrams, Griffin and Davila2002) reported that a lactose-containing infant formula absorbed 10.3% more calcium compared with a lactose-free formula. Overall, lactose and its derivatives have several nutritional benefits, primarily with regard to the promotion of gut health. For example, if taken in moderate dosages and distributed over meals, lactose may act as a prebiotic in lactose deficient populations (Schaafsma, Reference Schaafsma2008; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). The purpose of this article is thus to provide an overview of lactose intolerance along with a special emphasis on the roles of fermented foods and probiotics as a potential dietary regimen to alleviate lactose intolerance.
Lactose and lactase
Lactose is a disaccharide sugar composed of glucose and galactose (Semenza et al., Reference Semenza, Auricchio and Mantei2001; Harrington and Mayberry, Reference Harrington and Mayberry2008). The intestine can only absorb monosaccharides, therefore, it is the malabsorption of lactose that leads to the condition called lactose intolerance. Lactase, an enzyme (β-gal) found in the lining of the small intestine, acts as a catalyst in breaking down lactose into easily digestible glucose and galactose. In the small intestine, lactase activity is high close to the ileum and very low in the first portion of the duodenum and in the terminal part of the ileum (Swallow et al., Reference Swallow, Poulter and Hollox2001; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). People with lactose intolerance are unable to digest considerable amounts of lactose due to insufficiency of the enzyme produced by the expression of the lactase-phlorizin hydrolase gene in the cells lining the small intestine (Suarez et al., Reference Suarez, Savaiano and Levitt1995; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). The lactase gene (LCT) encodes the enzyme lactase or lactase-phlorizin hydrolase (LPH) and possesses lactase and phlorizin hydrolase activity (Torun et al., Reference Torun, Solomons and Viteri1979; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). LPH, the primary intestinal lactase, is an essential glycoprotein of the brush border of the upper small intestine (Swagerty et al., Reference Swagerty, Walling and Klein2002; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Lactase is synthesized as a pro- polypeptide which is glycosylated and proteolytically cleaved inside the cell in order to form the mature enzyme. Later, the active LPH enzyme is transferred to the outer surface of the brush border showing high activity of the LPH in the jejunum (Swagerty et al., Reference Swagerty, Walling and Klein2002).
In addition to hydrolyzing lactose into glucose and galactose, lactase also cleaves cellobiose, cellotriose, cellotetrose and, to a certain extent, cellulose. The phlorizin hydrolase activity breaks down β-glycosides into a large hydrophobic alkyl chain (galactosyl- and glycosyl-h-ceramides, phlorizin) (Torun et al., Reference Torun, Solomons and Viteri1979). Various roles of phlorizin site are useful in humans following the usual decline in enzyme expression after weaning from breast milk, and thus enzyme activity is retained even after the weaning period (Lomer et al., Reference Lomer, Parkes and Sanderson2008; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Although lactose is not a key nutritional component for adults, it is the primary source of energy during the first year of life of infants, providing almost half of an infant's total energy requirements (Vesa et al., Reference Vesa, Marteau and Korpela2000). After the weaning period, lactase activity decreases in most mammals, however, in some human ethnic groups such as western European Caucasians, lactase activity continues into adult life enabling the digestion of large quantities of dietary lactose (Troelsen, Reference Troelsen2005; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013).
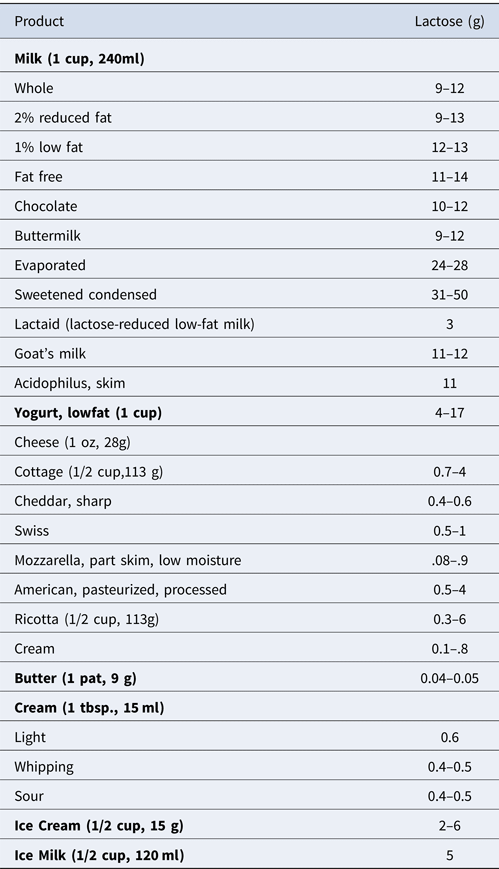
All vertebrates exhibit phlorizin hydrolase activity, whereas lactase activity has only been found in mammals (Leese and Semenza, Reference Leese and Semenza1973; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Lactose is present only in mammalian milk with approximately 7.2 g⁄100 ml in mature human milk and 4.7 g⁄100 ml in cow's milk (Miller et al., Reference Miller, Jarvis and McBean2006; Lomer et al., Reference Lomer, Parkes and Sanderson2008). A fuller description of the lactose content of various dairy products is given in Table 1. Lactose in a more or less pure form can be obtained from milk and whey and can be used as an ingredient in feed, food and pharmaceutical preparations (Schaafsma, Reference Schaafsma2008; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Examples of non-dairy products which may contain addded lactose include instant breakfast mixes, shakes, coffee whiteners, baby cereals, cake mixes, sausage, mayonnaise, frankfurters, ready-to-eat foods and certain processed foods (Montes and Perman, Reference Montes and Perman1991; Hertzler and Savaiano, Reference Hertzler and Savaiano1996; Cox, Reference Cox, Warrell, Cox, Firth and Benz2003; Harrington and Mayberry, Reference Harrington and Mayberry2008). Lactose has several applications in the food industry including the making of candy, confections, pancakes, waffles and pastries, mainly because of its limited sweetness, solubility, crystallization, and browning properties. It also provides better texture and binds water and color (van Griethuysen Dilber et al., Reference van Griethuysen-Dilber, Flaschel and Renken1988; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013).
Table 1. Lactose content of dairy products.
Types of lactose intolerance
Lactose intolerance can be classified into three basic types. These are:
Primary lactose intolerance: This type of lactose intolerance is genetically determined, and the low level of lactase is often developed after weaning (Greenberger and Isselbacher, Reference Greenberger, Isselbacher, Fauci, Braunwald and Isselbacher1998). Primary lactose intolerance is also referred to as adult-type hypolactasia, lactase nonpersistence, or hereditary lactase deficiency (Heyman, Reference Heyman2006; Ibrahim et al., 2013). This type may not become clinically evident until puberty or late adolescence (Escher et al., Reference Escher, De Koning, Van Engen, Arora, Büller, Montgomery and Grand1992; Lloyd et al., Reference Lloyd, Mevissen, Fischer, Olsen, Goodspeed, Genini and Mantei1992). Lactase levels start to decline during early childhood and continue to decline throughout life (Harrington and Mayberry, Reference Harrington and Mayberry2008). It is believed that the reduced syntheses of the precursor protein in the epithelial cells is associated with the reduction of lactase activity (Cox, Reference Cox, Warrell, Cox, Firth and Benz2003).
Secondary lactose intolerance: This usually occurs when the intestinal mucosa surface is damaged due to disease, surgery, radiation, or medications (National Dairy Council, 1978; Rusynyk and Still, Reference Rusynyk and Still2001; National Medical Association, 2009). This type of lactose intolerance can present at any age but most commonly occurs in infancy and may last for only a short time following infective gastroenteritis. Secondary lactose intolerance can cause mucosal diseases such as in celiac disease and Crohn's disease (Newcomer and McGill, Reference Newcomer and McGill1984; Rusynyk and Still, Reference Rusynyk and Still2001; Cox, Reference Cox, Warrell, Cox, Firth and Benz2003; Heyman, Reference Heyman2006).
Congenital lactose intolerance: This type of lactose intolerance is extremely rare and occurs only when the lactase enzyme is completely absent. This condition is typically characterized by infantile diarrhea and failure to thrive following the first exposure to breast milk (Lomer et al., Reference Lomer, Parkes and Sanderson2008). As congenital lactose intolerance remains lifelong, abnormal absorption of lactose leads to lactosuria and thereafter renal tubular acidosis, aminoaciduria, vomiting, and failure to thrive (Cox, Reference Cox, Warrell, Cox, Firth and Benz2003). This type of lactose intolerance is considered to be serious and life-threatening due to potential dehydration (Newcomer and McGill, Reference Newcomer and McGill1984; Saavedra and Perman, Reference Saavedra and Perman1989).
Non-probiotics dietary approach to alleviate lactose intolerance
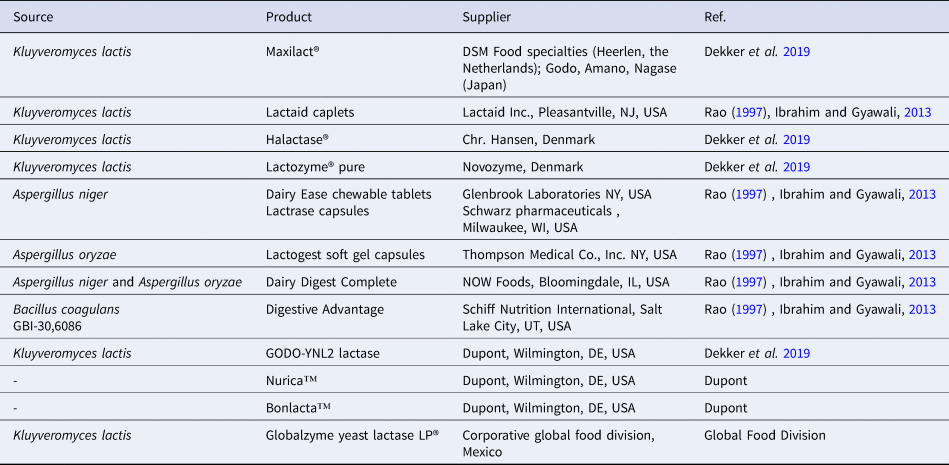
The total elimination of milk and dairy products from the human diet can have a negative impact on health. Therefore, several approaches have been investigated to reduce lactose intolerance symptoms and to manipulate the presence of lactose in dairy foods and ingredients. Lactase supplements from yeast and fungi have also been shown to reduce symptoms associated with lactose intolerance by replacing the body's missing lactase enzymes. The two major sources of lactase (β-gal) are the yeasts Kluyveromyces fragilis and Kluyveromyces lactis or the fungi Aspergillus niger and Aspergillus oryzae) (Solomons et al., Reference Solomons, Guerrero and Torun1985b; Rao, Reference Rao1997; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013), both of which have been used in industrial applications for many years. For example, β-gal from various strains of Aspergillus niger and A. oryzae are used commercially for the hydrolysis of lactose in whey for the alleviation of lactose intolerance and for the production of galacto-oligosaccharides (Nakayama and Amachi, Reference Nakayama, Amachi, Flinckinger and Drew1999; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Table 2 lists some available commercial lactase supplements.
Table 2. Commercially available lactose supplements.
A very few number of studies on lactase-based supplements have reported limited success in alleviating the symptoms of lactose malabsorption (O'Connel and Walsh, Reference O'Connell and Walsh2006a, Reference O'Connell and Walsh2006b). These authors suggested that none of the currently available commercial products meet the typical characteristics of an ideal supplemental lactase and that a higher dosage of these supplements is required in order to completely hydrolyze the lactose found in a dairy-based product. They (O'Connel and Walsh, Reference O'Connell and Walsh2006b) and ourselves (Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013) also reported that purified neutral lactase from Kluyveromyces marxianus is significantly more active in the small intestine than current commercial products. Purified enzymes obtained from Aspergillus fungus have been widely used for industrial applications; however, the purification method required is challenging and costly. Consequently, a better alternative would be the use of probiotic bacteria as a source of these enzymes (Alazzeh et al., Reference Alazzeh, Ibrahim, Alzzeh, Song, Shahbazi, Awaisheh and AbuGhazaleh2011; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013).
Use of fermented foods and probiotics to alleviate lactose intolerance
Fermented foods and dietary supplementation such as probiotics, prebiotics and synbiotics can be used to alter the composition and metabolism of colonic microbiota for the purpose of improving lactose digestion (Awaisheh et al., Reference Awaisheh, Hadaddin and Robinson2005; Awaisheh, Reference Awaisheh and Rigobelo2012; Ibrahim et al., 2013). Previous studies have shown that lactose digestion and the symptoms of lactose intolerance can be improved using probiotics that modify gut pH, express β-gal, and exert positive effects on intestinal activity and overall colonic microbiota (de Vrese et al., Reference de Vrese, Stegelmann, Richter, Fenselau, Laue and Schrezenmeir2001; Kopp-Hoolihan, Reference Kopp-Hoolihan2001; Roberfroid, Reference Roberfroid2000a; Rolfe, Reference Rolfe2000; Awaisheh, Reference Awaisheh2011). It has been demonstrated that lactic acid bacteria ferment lactose to produce lactate, hydrogen, methane, carbon dioxide and short-chain fatty acids (Hove et al., Reference Hove, Nørgaard and Mortensen1999; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013; Awaisheh et al., Reference Awaisheh, Rababah, Rahahleh, Haddad, Al-Groom and Ibrahim2016). During fermentation, lactase present in lactic acid bacteria cleaves unabsorbed lactose to glucose and galactose and is then absorbed into the body. In the small intestine at pH 6–8, lactase activity is optimal, but in the colon pH decreases to 4 and lactose is left unfermented due to the decrease in bacterial lactase activity (Heyman, Reference Heyman2000; Ibrahim et al., 2013). Undigested lactose can thus be considered to be a prebiotic that stimulates the growth of beneficial microflora by fermenting milk containing less lactose. The increase in the number of lactic acid bacteria is due to microbial digestion during fermentation (Lin et al., Reference Lin, Dipalma, Martini, Gross, Harlander and Savaiano1993; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). The degrees of lactose intolerance in different individuals are due to the variability of colonic microflora in fermenting lactose (Arola and Tamm, Reference Arola and Tamm1994). As a result, dairy foods that contain active cultures can be used to reduce the symptoms of lactose intolerance (Awaisheh, Reference Awaisheh and Rigobelo2012; Hadaddin et al., Reference Hadaddin, Awaisheh and Robinson2005). The consumption of active and live cultures in addition to the use of enzyme β-galactosidase (lactase) that break down lactose and the consumption of probiotics might be beneficial to individuals with lactose-intolerance. Table 3 presents some of the studies on the effects of probiotics on lactose intolerance. Corazza et al. (Reference Corazza, Benati, Sorge, Strocchi, Calza and Gasbarrini1992) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013) studied the impact of lactase from yeasts and molds ingested in the form of capsules and found a resultant significant increase in lactose digestion. Streptococcus thermophilus produces β-gal during its transit in the digestive tract, and this enzyme reduces the lactose content. This study was conducted on mice and showed that the produced enzyme is active and able to hydrolyze lactose resulting in an overall reduction of the lactose in feces (Drouault et al., Reference Drouault, Anba and Corthier2002; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Probiotics exhibit direct effects in the gut in the treatment of lactose digestion. For example, the administration of the probiotic Lactobacillus strain Lactobacillus planatarum has been shown to improve lactose digestion, decrease diarrhea and reduce bloating, flatulence and pain during irritable bowel syndrome (Nobaek et al., Reference Nobaek, Johansson, Molin, Ahrné and Jeppsson2000; Marteau et al., Reference Marteau, Vrese, Cellier and Schrezenmeir2001). In a clinical intervention study of lactose intolerance by Shaukat et al. (Reference Shaukat, Levitt, Taylor, MacDonald, Shamliyan, Kane and Wilt2010) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013), milk-containing L. acidophilus was compared to regular milk but was not shown to reduce gastrointestinal symptoms. There is also strong evidence to suggest that some probiotics can improve lactose digestion but do not alleviate the symptoms of lactose intolerance. Intake of yogurt causes fewer symptoms than intake of milk mainly due to the high β-gal activity in yogurt, partial hydrolysis of lactose and slower intestinal transit time that is a result of the digestion of lactose in yogurt (Cox, Reference Cox, Warrell, Cox, Firth and Benz2003; Rabot et al., Reference Rabot, Rafter, Rijkers, Watzl and Antoine2010). This could be related to the particular bacterial strains used in these yogurt products and the viability of the bacterial strains in the final products (Ibrahim and Carr, Reference Ibrahim and Carr2006). A systematic review of one controlled trial study showed the beneficial effects of probiotic supplementation on hydrogen breath test results and on lactose intolerant symptoms. Patients showed reduced exhaled hydrogen and improved abdominal cramping, diarrhea, vomiting, bloating and/or flatulence (Leis et al., Reference Leis, de Castro, de Lamas, Picáns and Couce2020). Similarly, Gingold-Belfer et al. (Reference Gingold-Belfer, Levy, Layfer, Pakanaev, Niv, Dickman and Perets2019) assessed the efficacy of probiotics with a β-gal activity on symptoms of lactose malabsorption and on the lactose hydrogen breath test. Authors found that probiotic formulation with a β-gal activity may serve as an optional treatment in the case of lactose malabsorption.
Table 3. Effectiveness of probiotics for the treatment of lactose intolerance.

He et al. (Reference He, Priebe, Zhong, Huang, Harmsen, Raangs and Vonk2008) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013) reported a decrease in lactose content by approximately 20–40% during fermentation by lactic acid bacteria. Their study showed that supplementation of Bifidobacterium longum and yogurt with B. animalis altered the number of bacteria and increased the β-gal activity in feces from lactose intolerant individuals. The lactic acid bacteria in fermented milk increases lactase activity in the small intestine and thus provides beneficial effects. However, it is still not clear whether it is the yogurt that is supplying lactase or if it is bacteria that produce lactase when they enter the gut (Fuller, Reference Fuller1991; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Lin et al. (Reference Lin, Yen and Chen1998) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013), studied the influence of nonfermented milk containing L. bulgaricus on lactose utilization. In this study, nonfermented reduced fat (2%) milk containing L. bulgaricus with a final bacterial population of 8 Log CFU/ml was prepared. Lactose maldigestion was then monitored by measuring breath hydrogen levels at hourly intervals for 8 h following consumption of 400 ml of milk samples. This study demonstrated that the lactose maldigestion symptoms significantly improved. The results indicate that L. bulgaricus is an effective choice for manufacturing nonfermented milk for lactose maldigesters. This study, among others, also shows clear evidence that consumption of fermented dairy products containing specific probiotic strains in appropriate amounts should be incorporated into the diets of lactose-intolerant subjects. The effectiveness of probiotics also depends on the tolerance of a strain to bile and acid in addition to the strain's lactase level or lactose transport (Mustapha et al., Reference Mustapha, Jiang and Savaiano1997; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013).
Several studies have been conducted for the purpose of establishing alternative approaches to enhancing microbial lactase (β-gal) activity and the selection of probiotic strains capable of producing large amounts of β-gal (Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). This food-grade classical approach can thus moderately increase β-gal concentrations in probiotic cultures to improve the potential of probiotic bacteria for treating the symptoms of lactose malabsorption in humans. Ibrahim and O'Sullivan (Reference Ibrahim and O'Sullivan2000) have developed a classical chemical mutagenesis protocol for improving the potential to treat symptoms of lactose malabsorption in humans, and this holds great potential for assessment of probiotics functionality. In this study, several strains of Bifidobacterium species (B. breve and B. longum) and one strain each of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus were tested by a single exposure to two chemical mutagens, ethyl methanesulfonate (EMS) and N-methyl-N′-nitro-N-nitrosoguanidine (MNNG). Mutants showed increased β-gal activity compared with the wild-type strains. Alazzeh et al. (Reference Alazzeh, Ibrahim, Alzzeh, Song, Shahbazi, Awaisheh and AbuGhazaleh2011) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013) reported similar results and showed that the chemical mutagenesis of L. reuteri led to enhancement of β–galactosidase which demonstrated that chemical mutagens could be applied as a practical approach for enhancing enzymatic activity (Ibrahim and O'Sullivan, Reference Ibrahim and O'Sullivan2000; Hsu et al., Reference Hsu, Yu and Chou2005; Donkor et al., Reference Donkor, Henriksson, Vasiljevic and Shah2007).
Alazzeh et al. (Reference Alazzeh, Ibrahim, Song, Shahbazi and AbuGhazaleh2009) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013), showed the influence of different carbohydrate and protein sources on the enhancement of alpha- and β-gal production in six strains of Lactobacillus reuteri. Based on this study, raffinose and lactose were the best carbohydrate sources to produce alpha- and β-gal, respectively. Yeast extract was the best protein source to produce both enzymes, and L. reuteri strain CF2-7F was the best producing strain under all experiment conditions. Therefore, these strains could be a food grade additive to provide benefits to lactose intolerant individuals. Ibrahim et al. (Reference Ibrahim, Alazzeh, Awaisheh, Song, Shahbazi and AbuGhazaleh2010) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013), also demonstrated the enhancement of α- and β-gal activity when Mn + 2 ions were added to growth medium of L. reuteri strain CF2-7F. Bhowmik et al. (Reference Bhowmik, Johnson and Ray1987) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013), evaluated the influence of different growth conditions on the activity of β-gal in L. acidophilus and found that the enzyme activity was stimulated by magnesium. Chowdhury et al. (Reference Chowdhury, Chakraborty and Raychaudhuri2007) and Ibrahim and Gyawali (Reference Ibrahim, Gyawali, Park and Haenlein2013), reported a higher production of β-gal from yogurt fermented with Lactobacillus acidophilus and Lactobacillus plantarum fortified with different types of herbs. The increase in β-gal activity could be attributed to the antioxidants present in the herbs. This metabolic pathway could enhance the production of specific metabolites of probiotics that ultimately lead to the production of functional compounds. Some of these compounds could also impact lactose metabolism (Gyawali and Ibrahim, Reference Gyawali and Ibrahim2012). Similarly, several mineral nutrients such as Mg2+, Mn2+, Fe2+, and Ca2+ have been shown to exert an impact on the growth, lactic acid production and functionality of probiotics (Boyaval, Reference Boyaval1989; Gyawali and Ibrahim, Reference Gyawali and Ibrahim2012).
Consequently, the presence of these minerals has been shown to create an environment that induces a new metabolic pathway. One limiting factor in the study of the impact of fermented dairy products on lactose intolerance has been the lack of a comprehensive assay for measuring the enzyme activity (lactase). There is a need to search for an effective technique that recovers the enzyme from bacterial cells (Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013), and the efficacy of such an extraction method needs to be evaluated. One recent study was conducted to evaluate chemical (solvents) and mechanical (sonication, bead-beater) methods for the recovery of the enzyme (Gyawali et al., Reference Gyawali, Oyeniran, Zimmerman, Aljaloud, Krastanov and Ibrahim2020). The study demonstrated that mechanical extraction using sonication provided the best tool for obtaining the highest enzyme recovery in dairy products.
The issue of lactose intolerance has led the food industry to remove dairy foods or ingredients from different food products. However, this could lead to nutritional inadequacy, particularly for calcium intake (Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Many individuals with lactose intolerance could consume dairy products without the resulting symptoms by simply consuming fermented milk products instead of unfermented ones. Other individuals do benefit significantly from lactose restriction, but care needs to be taken to ensure that calcium intake is provided. A greater understanding of the complexity of lactose intolerance, lactase deficiency and symptom generation would help clinicians treat patients more effectively (Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013).
Lactose-free dairy products
Milk is an important food that is nutritionally dense and essential in every diet in order to support proper human growth and development. However, the rising cost of healthcare and its associated implications for people diagnosed as lactose intolerant has put a thin line between health and nutrition when people are made to choose or purchase milk-based food products containing lactose (Roberfroid, Reference Roberfroid2000a, Reference Roberfroid2000b). Milk containing probiotics is therefore considered an important health attribute for both consumers of dairy and non-dairy products (McCarthy et al., Reference McCarthy, Parker, Ameerally, Drake and Drake2017). The condition of lactose intolerance is prevalent in many global populations thereby, many people are impaired with the ability to digest lactose in food products (Dekker et al., Reference Dekker, Koenders and Bruins2019). The antidote to this prevailing situation, however, is the development and introduction of lactose-free dairy products or their alternatives such as extracts from plant-based foods, often labelled as ‘milk’. There is an ongoing debate about the nutritional value of these products, as studies have confirmed that patients that routinely adhered to lactose-free diets were often disadvantaged due to nutritional deficiencies that led to ailments such as colon health and immune dysfunction (Wahlqvist, Reference Wahlqvist2015).
Total avoidance of lactose in food products leads to the omission of an important carbohydrate (lactose) that is naturally abundant in human and animal milk and provides a rich source of calcium for both children and adults (Heyman, Reference Heyman2006). A lactose-free alternative should not just be devoid of lactose but rather be benchmarked to the nutritional profile of lactose-containing products (Suri et al., Reference Suri, Kumar, Prasad, Tanwar, Goyal, Kaur and Singh2019). Due to the high prevalence of lactose intolerance globally and the media-dominated trend of vegetarianism, there is a paradigm shift and a high demand for lactose-free dairy products or their alternatives (Siro et al., Reference Siro, Kápolna, Kápolna and Lugasi2008). Moreover, it is reported in the United States that consumers seem to have a significant preference for lactose-free cow's milk-based on consumer studies for product acceptability and preference for alternative dairy products (Palacios et al., Reference Palacios, Badran, Drake, Reisner and Moskowitz2009). Lactose-free dairy products continue to gain attention and have a growing health appeal among many consumers even in countries where the majority of the populace are lactose tolerant (Dekker et al., Reference Dekker, Koenders and Bruins2019). The lactose-free dairy segment is projected to achieve a turnover of about €9 billion (approximately $11 billion) by 2022 and is presumed to be the fastest-growing category in the dairy industry (Dekker et al., Reference Dekker, Koenders and Bruins2019).
The increasingly varied product range for lactose-free products is making many consumers gravitate towards making lactose-free products a choice rather than an option. Interestingly, the term ‘lactose-free’ has been heralded as an important health claim for many new milk products rather than a marketing strategy for only niche products and has thus resulted in very high sales growth due to the increased demand from many health-conscious consumers (Dekker et al., Reference Dekker, Koenders and Bruins2019; Suri et al., Reference Suri, Kumar, Prasad, Tanwar, Goyal, Kaur and Singh2019). Consequently, the global dairy industry in its quest to meet the dietary calcium as well as the protein requirement for lactose-intolerant populations has introduced a neutral exogenous enzyme known as lactase (β-galactosidase) that can pre-digest the milk sugar (lactose) (Churakova et al., Reference Churakova, Peri, Vis, Smith, Beam, Vijverberga, Stora and Wintera2019). The enzyme, β-galactosidase has been commercially produced from the yeast Kluyveromyces lactis, Kluyveromyces fragilis, Saccharomyces lactis, or Kluyveromyces marxianus. It is noteworthy that this enzyme is only produced by four companies worldwide. In Europe, DSM Food Specialties (Heerlen, the Netherlands) exclusively markets the product under the trade name ‘Maxilact’ and in Japan, the companies Godo, Nagase, and Amano are solely responsible for its manufacture, however, their products are distributed through many channel operators, for example, the market name Halactase is linked to Chr. Hansen (Øresund, Denmark), Lactozyme® Pure is affiliated with Novozymes (Bagsværd, Denmark) and Dupont (Wilmington, DE, USA) under the brand name Godo YNL2® (Dekker et al., Reference Dekker, Koenders and Bruins2019).
It is significant to note that all industrially produced enzymes from Kluyveromyces lactis have the same performance index in the hydrolysis of lactose. The only difference exists in their grades of purity and their overall product strength (Dekker et al., Reference Dekker, Koenders and Bruins2019). Due to the heightened demand for lactose-free products there is, therefore, a strong and urgent need for product development valorizing the enzyme lactase (β-galactosidase) to ultimately fulfill the objective of addressing the issue of lactose intolerance among many people (Suri et al., Reference Suri, Kumar, Prasad, Tanwar, Goyal, Kaur and Singh2019). Moreover, there is a clarion call for researchers and the dairy industry to adequately develop nutritionally balanced, safe, and cost-effective lactose-free products to help alleviate the deficiency of lactose intolerance in global populations (Suri et al., Reference Suri, Kumar, Prasad, Tanwar, Goyal, Kaur and Singh2019).
Population diet culture and its link to lactose intolerance
The concept of lactase persistence has been prominent and has shown variance among different human populations (Yeo, Reference Yeo2017). Northern Europeans are believed to possess a higher lactase persistence activity, and this could be geographically attributed to the surge and dominance of dairy farming in their environment dating 10 000 years ago. This observation is termed as ‘the culture-historical hypothesis’ that asserted that lactase persistence was rarely observed prior to the advent of dairy farming (Lomer et al., Reference Lomer, Parkes and Sanderson2008). However, the onset of poor harvest led to the inclusion of milk as an integral component of the human diet (Lomer et al., Reference Lomer, Parkes and Sanderson2008). The highly enriched nutritional milk food became an excellent choice had a strong selection advantage for many who could digest it (Misselwitz et al., Reference Misselwitz, Pohl, Frühauf, Fried, Vavricka and Fox2013). Therefore, human evolution experienced the development of lactase persistence independently as different world regions began intense dairy farming (Misselwitz et al., Reference Misselwitz, Pohl, Frühauf, Fried, Vavricka and Fox2013). It is noteworthy that in global regions where long traditions of dairy farming were prevalent, it was not surprising to see lactase persistence as very common (Vesa et al., Reference Vesa, Marteau and Korpela2000). The geographical relevance of lactase persistence correlates significantly with the dairying culture in many different human populations (Gerbault et al., Reference Gerbault, Roffet-Salque, Evershed and Thomas2013). Another theory, known as the ‘reverse cause’ attempted to debunk the cultural−historical hypothesis by asserting that people with pre-existing lactase persistence were linked to dairy farming and consumption. There is little evidence to support this theory. There are examples of relatively localized Ethnic groups that support the culture-historical hypothesis. The frequent consumption of milk and milk products was intrinsic to the daily diets of Tibetans. A study conducted on lactose tolerance confirmed that Tibetans had a significantly higher lactose prevalence than that observed in Han Chinese (Dong et al., Reference Dong, Long and Kang2003). It was not surprising that the results confirmed Tibetans to have a higher lactose persistence as this concurred with historical facts of the high volumes of milk consumption by Tibetans in contrast to the lack of enough milk in the diet of the traditional Chinese (Jiang, Reference Jiang1995). Interestingly, how the milk consumption culture impacted the genetic diversity of the Tibetan population remains a mystery (Peng et al., Reference Peng, He, Zhu, Wu, Jin and Zhang2012). Globally, the situation is now more complex than it was. As societies evolve and become more mobile, it is interesting to note the observed trend of increasing numbers of lactase deficient adults residing in societies previously known for consuming dairy products, due to migration and cultural shifts (Ferguson, Reference Ferguson1981).
Concluding remarks
In conclusion, we affirm that lactase deficiency or low lactase activity is not synonymous with lactose intolerance. The symptoms of lactose intolerance can vary from individual to individual, depending on the amount of lactose consumed and the degree of lactase deficiency (Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Lactose intolerance can also vary based on several nutritional factors that are present when lactose is ingested (Lomer et al., Reference Lomer, Parkes and Sanderson2008). People with milder lactase deficiencies may be tolerant (no symptoms after ingestion of milk) to one glass of milk and yet intolerant (develop symptoms) to two glasses (Newcomer and McGill, Reference Newcomer and McGill1984; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). The prevalence of lactose intolerance is widespread globally, and it is one of the most common gastrointestinal disorders. People who are lactose intolerant usually avoid milk and dairy products in order to support gastrointestinal comfort and health. Very often, lactose intolerance is misdiagnosed as being a gastrointestinal disorder such as diarrhea and abdominal bloating. However, lactose intolerance can be effectively treated with dietary modification and education once a proper diagnosis has been made. For individuals who are lactose intolerant, milk should be consumed in amounts that can be tolerated rather than being completely eliminated as a lack of milk and dairy products, particularly calcium, can lead to nutritional deficiency (Lomer et al., Reference Lomer, Parkes and Sanderson2008; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Lactose is an excellent source of energy, however, for effective utilization, lactose must be hydrolyzed in the intestine by the enzyme β-gal, generally called lactase (Lomer et al., Reference Lomer, Parkes and Sanderson2008). By modifying their dietary pattern, lactose maldigesters can include milk and other dairy products in their diet without experiencing symptoms. For example, it is well known that lactose intolerant individuals can replace milk with yogurt or other fermented dairy products in order to meet the recommended daily nutritional requirements (Adolfsson et al., Reference Adolfsson, Meydani and Russell2004). Probiotics that include yogurt bacteria with high levels of lactase have been shown to alleviate lactose intolerance (Marteau et al., Reference Marteau, Vesa, Rambaud and Fuller1997; Ibrahim and Gyawali, Reference Ibrahim, Gyawali, Park and Haenlein2013). Future studies should focus on the selection of probiotic strains that can enhance the production of β-gal as the addition of such probiotics in various food products seems to be the most effective means of alleviating lactose intolerance. In order to elucidate the potential therapeutic relationship between probiotics and lactose intolerance, new studies on probiotic strains, their preparation, and establishing their respective concentrations should be conducted.
Acknowledgments
This publication was made possible by Grant or project Number NC.X308-5-18-170-1 from the National Institute of Food and Agriculture (NIFA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIFA. Authors would also like to acknowledge the support of NIZO Food Research BV, The Netherlands, Jarrow Formulas, USA, and the Department of Family and Consumer Sciences and the Agricultural Research Station at North Carolina Agricultural and Technical State University (Greensboro, NC, 27411 USA). The authors extend appreciation to the International Scientific Partnership Program ISPP at King Saud University for funding this research work through ISPP-153. This work was also partially supported by the Bulgarian Ministry of Education and Science under the National Research Programme ‘Healthy Foods for a Strong Bio-Economy and Quality of Life’ approved by DCM # 577 / 17.08.2018





