Introduction to extracellular vesicles
According to the International Society for Extracellular Vesicles, the term extracellular vesicles (EV) indicates ‘particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate’ (MISEV 2018, 2018). Among EV, exosomes (EXO) are plasma membrane-derived biological nanoparticles of endocytic origin, ranging from 30 to 100 nm in size, and they are secreted by multiple cell types under normal and pathological conditions. The term exosome was first used in 1981 by Trams et al. (Reference Trams, Lauter, Salem and Heine1981) and the biological role of these EV was later reconsidered by Kassis et al. (Reference Kassis, Lauter, Stojanov and Salem1986) and Johnstone et al. (Reference Johnstone, Adam, Hammond, Orr and Turbide1987). However, only in the last few decades have EXO gained popularity and became the object of significant research (Witwer and Thery, Reference Witwer and Théry2019). The growing interest in EXO depends on the increasing knowledge of their biological meaning, corroborated by the new advances in genomic and proteomic platforms, which are now affordable for many researchers, especially when performed as an external service.
According to Edgar (Reference Edgar2016), there are at least three reasons for the explosion of interest in EXO. Firstly, EXO are largely involved in cell−cell communication and in the transfer of macromolecules among cells in relation to the onset and development of many diseases (Théry et al., Reference Théry, Zitvogel and Amigorena2002). Secondly, EXO contain proteins and nucleic acids such as mRNA, microRNA (miRNA), rRNA, long noncoding RNA, tRNA and variably DNA, which can be shuttled from one cell to another, affecting the recipient cell's protein production (Valadi et al., Reference Valadi, Ekstrom, Bossios, Sjostrand, Lee and Lotvall2007; Hata et al., Reference Hata, Murakami, Nakatani, Yamamoto, Matsuda and Aoki2010; Yamamoto et al., Reference Yamamoto, Kosaka and Ochiya2019). The cargo is specific to the donor cell and can be defined as its ‘fingerprint’ or ‘signature’, spreading nucleic acids, lipids and protein in recipient cells. Thirdly, the ability of EXO to deliver their cargo has raised the interest, over the last few years, in using these nanoparticles to deliver drugs (Ha et al., Reference Ha, Yang and Nadithe2016). Moreover, EXO can fuse with cell membranes and are better tolerated by the host, since they do not trigger an immune response (Edgar et al., Reference Edgar, Eden and Futter2014). It has been suggested that preparations of EXO may be used for clinical purposes as effective carriers of various drugs, including proteins, lipids, RNAs and other compounds to mammalian cells (Yamamoto et al., Reference Yamamoto, Kosaka and Ochiya2019). Furthermore, unlike typical nanoparticulate systems such as liposomes or polymeric nanoparticles, EXO can deliver their cargo directly into the cytosol, avoiding the lysosomal/endosomal pathway, thus the transfection efficiency is increased (Ha et al., Reference Ha, Yang and Nadithe2016).
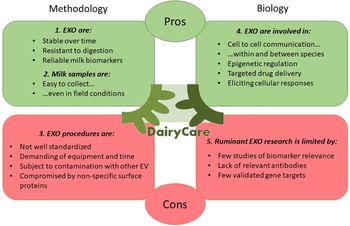
EXO have been detected in several biological fluids, such as blood, urine, saliva, colostrum and milk (Lasser et al., Reference Lässer, Alikhani, Ekström, Eldh, Paredes, Bossios, Sjöstrand, Gabrielsson, Lötvall and Valadi2011; Yamamoto et al., Reference Yamamoto, Kosaka and Ochiya2019). Since EXO could provide diagnostic information that can be used to monitor metabolic conditions and immune response of the organism, this review discusses some of the opportunities and limitations of the use of milk EXO and their cargo as markers of metabolism and health in dairy ruminants (Fig. 1).

Fig. 1. Reasons and limits to include the study of exosomes in mammary gland biology of ruminants. (1) EXO are stable under many conditions, since the encapsulation protects cargoes against enzymatic and non-enzymatic protection. Therefore, EXO cargo resists digestion and heat treatment and is stable over time, including in commercial milk. (2) Samples of milk can be easily collected in commercial farm conditions two or three time a day and for several days, without interfering with the cows. For research purposes, milk samples can be collected several times in a day. (3) The milk of ruminants contains casein, which limits a straight application of methods in EXO isolation developed for human milk. In the literature there is not a consensus of protocols and commercial kits used in EXO isolation are not available for ruminant's milk. Ultracentrifugation and identification of EXO requires a dedicated laboratory and skilled personnel. There are no exclusive markers for EXO, it is not easy to differentiate them from the other extracellular vesicles and contamination is possible. (4) The mechanism of EXO delivery varies, from endocytosis to fusion or interaction with surface proteins of recipient cells. This latter route enables target drug delivery. EXO elicit a response from recipient cells that is cargo specific, thus influencing the expression and the activity of proteins in the recipient cell as well as epigenetic regulation. (5) In dairy ruminant research, a limited number of studies of milk EXO-derived miRNAs and proteins as markers of metabolism and health have been done. The identification of proteins is limited by the availability of antibodies and requires proteomic approaches. Known miRNA gene targets of milk EXO of ruminants are mainly based on nucleotide sequences and only a few are validated.
Milk exosomes: regulators of mammary gland biology
Milk contains numerous nutrients and other bioactive molecules, including growth factors (Colitti, Reference Colitti2015), metabolic hormones and cytokines (van Hooijdonk et al., Reference van Hooijdonk, Kussendrager and Steijns2000; Sgorlon et al., Reference Sgorlon, Fanzago, Guiatti, Gabai, Stradaioli and Stefanon2015), and it is widely considered a good source of nutrients for humans and for the newborn. Milk also contains other signaling molecules which can modulate cellular functions of the mammary gland. For instance, in mammary glands of dairy ruminants there is a dynamic balance between proliferation and apoptosis, the former prevailing in early lactation, the latter from the peak of lactation onwards (Stefanon et al., Reference Stefanon, Colitti, Gabai, Knight and Wilde2002). Survival, proliferation, differentiation and apoptosis are controlled by specific signals that are responsible for the cellular fate (Colitti and Farinacci, Reference Colitti and Farinacci2009). Other animal and environmental factors such as parity, milking frequency, diet and farm management may alter the lactation cycle. Therefore, the association of these factors with modifications of lactation has been studied in order to develop strategies to improve milk yield or to reduce the effect of diseases, which commonly decrease milk yield and quality.
Among the signaling molecules, miRNAs are a class of small non-coding RNAs of approximatively 22 nucleotides that act as post-transcriptional regulators of gene expression primarily through RNA silencing. The exosomal miRNAs can be delivered to recipient cells by endocytosis or by fusion of the EXO with the plasma membrane. Exosomes may also bind to a receptor and activate specific signaling pathways (Guay and Regazzi, Reference Guay and Regazzi2017).
Results obtained with next-generation sequencing (NGS) techniques indicated a high similarity between miRNAs expressed in human, bovine and goat milk. About 95% of the miRNAs expressed in bovine milk are also expressed in goat milk and 91% of the miRNAs expressed in goat and bovine milk are also expressed in human milk (Golan-Gerstl et al., Reference Golan-Gerstl, Elbaum Shiff, Moshayoff, Schecter, Leshkowitz and Reif2017). Since miRNAs are regulatory factors that can affect the activity of economically important tissues for farm animals, such as skeletal muscle, adipose tissue (Wang et al., Reference Wang, Gu and Jiang2013) and mammary gland (Benmoussa and Provost, Reference Benmoussa and Provost2019), the study of their role can find applications to improve livestock genetics through the identification of genomic variation controlling an economically relevant phenotype.
Several published studies of milk miRNAs, recently reviewed by Benmoussa and Provost (Reference Benmoussa and Provost2019), did not define if extracted miRNAs were either derived from EV or EXO or, alternatively, were not encapsulated and directly released by the mammary cells. This aspect is important, since free miRNAs are not stable and their collection, storage and other preparative procedures can degrade them (Howard et al., Reference Howard, Kusuma, Baier, Friemel, Markham, Vanamala and Zempleni2015). Conversely, miRNAs contained in milk EXO are stable following heat treatment and during storage. This feature allows for easier sample handling and produces results that are more reliable over time in terms of milk EXO activity (Shandilya et al., Reference Shandilya, Rani, Onteru and Singh2017). Furthermore, miRNAs in EXO are resistant to RNA degradation and gut digestion in vitro and, once adsorbed by the intestinal cell, regulate recipient cell functions (Benmoussa and Provost, Reference Benmoussa and Provost2019) or modulate macrophage activity of the host (Izumi et al., Reference Izumi, Tsuda, Sato, Kosaka, Ochiya, Iwamoto, Namba and Takeda2015). It is acknowledged that bovine milk EXO are bioavailable after intake in other species (Lasser et al., Reference Lässer, Alikhani, Ekström, Eldh, Paredes, Bossios, Sjöstrand, Gabrielsson, Lötvall and Valadi2011) and some delivered miRNAs may regulate gene expression and, therefore, protein expression (Zempleni et al., Reference Zempleni, Aguilar-Lozano, Sadri, Sukreet, Manca, Wu, Zhou and Mutai2017) in human. In experiments conducted on mice, Manca et al. (Reference Manca, Upadhyaya, Mutai, Desaulniers, Cederberg, White and Zempleni2018) found that bovine milk EXO were accumulated primarily in the liver and, to a lesser extent, in the spleen. Liao et al. (Reference Liao, Du, Li and Lönnerdal2017) reported that miRNA 148a in human milk EXO is absorbed by intestinal cells and down-regulates the expression of DNA methyltransferase 1, a gene involved in epigenetic regulation. Therefore, milk provides not only nutrients, but also elicits epigenetic regulation of recipient cells, due to the transfer through the intestine of miRNAs contained in EXO (Melnik and Schmitz, Reference Melnik and Schmitz2017). The transfer of miRNAs through EXO from the milk to the host is a novel route of communication within and between species, and for this reason, the EXO content of milk could be included among milk quality factors.
The EXO cargo
Studies have reported that milk of cattle (Chen et al., Reference Chen, Gao, Li, Huang, Sun, Dong, Tian, Gao, Dong, Guan, Hu, Zhao, Li, Zhu, Yan, Zhang, Zen and Zhang2010), goats (Golan-Gerstl et al., Reference Golan-Gerstl, Elbaum Shiff, Moshayoff, Schecter, Leshkowitz and Reif2017), humans (Lässer et al., Reference Lässer, Alikhani, Ekström, Eldh, Paredes, Bossios, Sjöstrand, Gabrielsson, Lötvall and Valadi2011) and rodents (Izumi et al., Reference Izumi, Kosaka, Shimizu, Sekine, Ochiya and Takase2014) includes different classes of biologically active EXOs. Interestingly, the comparison of miRNAs in milk EXO of human, swine, cow and panda showed that the most abundant miRNAs are conserved among mammals (van Herwijnen et al., Reference van Herwijnen, Driedonks, Snoek, Kroon, Kleinjan, Jorritsma, Pieterse, Hoen and Wauben2018). These authors found that the let-7 family members, namely the let-7a, let-7b, let7f and miR-148a, which are involved in immune response, signal transduction and regulation of cell growth, were the most abundant and similar between these species, having high sequence homology and suggesting an evolutionary conservation of their functions.
The cargo of EXO is a controlled and non-random process and the miRNA repertoire of EXO varies as a function of the donor cell and its physiological and developmental state (Barile and Vassalli, Reference Barile and Vassalli2017). Considering the strong relation between the lactation curve and the plethora of pathways involved in the mammary gland, specific EXO of the donor cell can change their cargo during the lactation cycle. Moreover, at the end of lactation, other membrane coated vesicles, such as apoptotic bodies, are secreted and these can shuttle miRNAs to neighboring cells as well (Crescitelli et al., Reference Crescitelli, Lässer, Szabó, Kittel, Eldh, Dianzani, Buzás and Lötvall2013). It has been recently stated that the profile of miRNAs in goat milk EXO changes through different phases of lactation, affecting milk fatty acid (FA) content through transcriptome modifications in mammary epithelial cells. For instance, miR-27a (Lin et al., Reference Lin, Luo, Zhang, Wang, Shi and Zhu2013) and miR-183 (Chen et al., Reference Chen, Shi, Sun, Luo, Zhang, Hou and Loor2018) promoted the content of unsaturated FAs and medium chain FAs. The likely mechanism underlying the variation of FA profile in goat milk is the silencing of key genes involved in lipid metabolism.
Two studies quantified differentially expressed miRNAs in milk EXO in experimentally induced infection of the mammary gland with Staphylococcus aureus (Sun et al., Reference Sun, Aswath, Schroeder, Lippolis, Reinhardt and Sonstegard2015; Cai et al., Reference Cai, He, Jia, Chen, Wang, Shi, Liu, Xiao and Lai2018). Although the number of differentially expressed miRNAs in milk EXO between the healthy and infected cows was equal to 13 in both studies, only two miRNAs, bta-miR-142-3p and bta-miR-223, overlapped (Table 1). Another study identified miRNAs in milk EXO during relocation stress in dairy cows in early lactation and reported 15 differentially expressed miRNAs (Colitti et al., Reference Colitti, Sgorlon, Licastro and Stefanon2018). Interestingly, 4 of these miRNAs (bta-miR-142-5p, bta-miR-146a, bta-miR-146b and bta-miR-221) overlapped with the study by Cai et al. (Reference Cai, He, Jia, Chen, Wang, Shi, Liu, Xiao and Lai2018) and 2 of them (bta-miR-183 and bta-miR-378-2) with the results by Sun et al. (Reference Sun, Aswath, Schroeder, Lippolis, Reinhardt and Sonstegard2015). During heat stress, the miRNAs bta-miR-146a and bta-miR-146b in cow serum were associated with stress and immune response (Zheng et al., Reference Zheng, Chen, Zheng, Li and Wang2014), but no information is available for milk-derived exosomal miRNAs.
Table 1. Milk-derived exosomal miRNAs significantly affected by challenge with Staphylococcus aureus (Infection) or by stress of relocation (Stress) during early lactation of dairy cows.

Similarly to miRNA, the protein cargo of EXO is deeply involved in cell-to-cell communication either within and between organisms and varies during the lactation cycle. The pattern and abundance of exosomal proteins were reported to be very similar among cows at mid lactation (Reinhardt et al., Reference Reinhardt, Lippolis, Nonnecke and Sacco2012), but higher protein diversity in milk EXO is expected in animals at different stages of lactation and fed on different diets. By proteomic analysis, enzymatic and transport differences have been distinguished between milk EXO and milk fat globule membranes, which also have a plasma membrane origin (Reinhardt et al., Reference Reinhardt, Lippolis, Nonnecke and Sacco2012). Samuel et al. (Reference Samuel, Chisanga, Liem, Keerthikumar, Anand, Ang, Adda, Versteegen, Jois and Mathivanan2017) found that 1372 proteins contained in EXO were similar between colostrum and milk, but the abundance of proteins implicated in inflammatory reaction, acute phase proteins and innate immune response were more than 3-fold higher in the colostrum. Indeed, no experiments have yet associated specific challenges with a modification of EXO proteins in bovine milk. Crookenden et al. (Reference Crookenden, Walker, Peiris, Koh, Heiser, Loor, Moyes, Murray, Dukkipati, Kay, Meier, Roche and Mitchell2016) analyzed the cargo of EXO isolated from blood in high and low risk cows at calving and identified unique proteins for the former group, namely α-2 macroglobulin, fibrinogen and oncoprotein-induced transcript 3, suggesting that EXO cargo can be used as an earlier biomarker of metabolic status in dairy cows. However, changes in exosomal proteins in relation to modifications of metabolic conditions or immune response are not yet demonstrated.
In conclusion, there is an increasing interest in studying the cargo of milk EXO in dairy ruminants to investigate the biology of mammary gland and lactation. Some studies were dedicated to defining protocols for the isolation of EXO from milk, since casein content can still represent a methodological constraint (Hata et al., Reference Hata, Murakami, Nakatani, Yamamoto, Matsuda and Aoki2010; Vaswani et al., Reference Vaswani, Koh, Almughlliq, Peiris and Mitchell2017). Top date, few studies have associated the modifications of exosomal cargo in relation to specific challenges and more research is needed to validate them as early biomarkers of mastitis and metabolic conditions in dairy cows.
Acknowledgements
This article is based upon work from COST Action FA1308 DairyCare, supported by COST (European Cooperation in Science and Technology, www.cost.eu). COST is a funding agency for research and innovation networks. COST Actions help connect research initiatives across Europe and enable scientists to grow their ideas by sharing them with their peers. This boosts their research, career and innovation.