Introduction
Multicenter clinical trials are expensive, often have complex infrastructure and management system requirements, and are frequently unsuccessful or uninformative. Clinical trial networks have been established by several NIH Institutes and Centers to support clinical researchers working in a defined disease or discipline. These networks provide structure to support collaboration across multiple sites, deliver efficiencies in operationalizing trials, and increase opportunities for impact [Reference Bentley, Cressman, Van Der Hoek, Arts, Dancey and Peacock1,Reference Nemeh, Buchbinder, Hawley, Nelson, Waterkeyn and Reid2]. However, they are limited in the conditions they support, costly to maintain, and may not have the flexibility to respond to changes in the need for information. In 2006, the NIH launched the Clinical and Translational Science Award (CTSA) Program to support a national network of medical research institutions to improve the translational research process and begin to address clinical trial inefficiencies [Reference Zerhouni3]. Ten years later, recognizing that many clinical trials still experienced significant delays and roadblocks, the National Center for Advancing Translational Science (NCATS) leveraged the developed and functioning CTSA Program and its hubs to establish a disease-agnostic national platform for multicenter clinical trials: the Trial Innovation Network (TIN) [Reference Shah, Culp and Gersing4].
The TIN is the first collaborative initiative in the CTSA program to focus on providing consortium-wide resources to address barriers that limit the success of clinical trials. It was launched not only to support efficient and effective multicenter clinical trials but also to act as a national laboratory to study, understand, and innovate the process of designing and conducting trials. The TIN includes three Trial Innovation Centers (TICs), a Recruitment Innovation Center (RIC), and Hubs based in the 60-plus organizations with a CTSA. CTSA Hubs and their affiliate partners touch at least 93 million patients whose demographic characteristics closely reflect the diversity of the US population, including those from urban and rural areas, ranging from neonates to older adults. When the TIN was introduced, Liaison Teams were established at each CTSA Hub to support collaboration across the national network (see Fig. 1). More than 60 CTSA Hubs across the United States created Liaison Teams between 2016 and 2018.
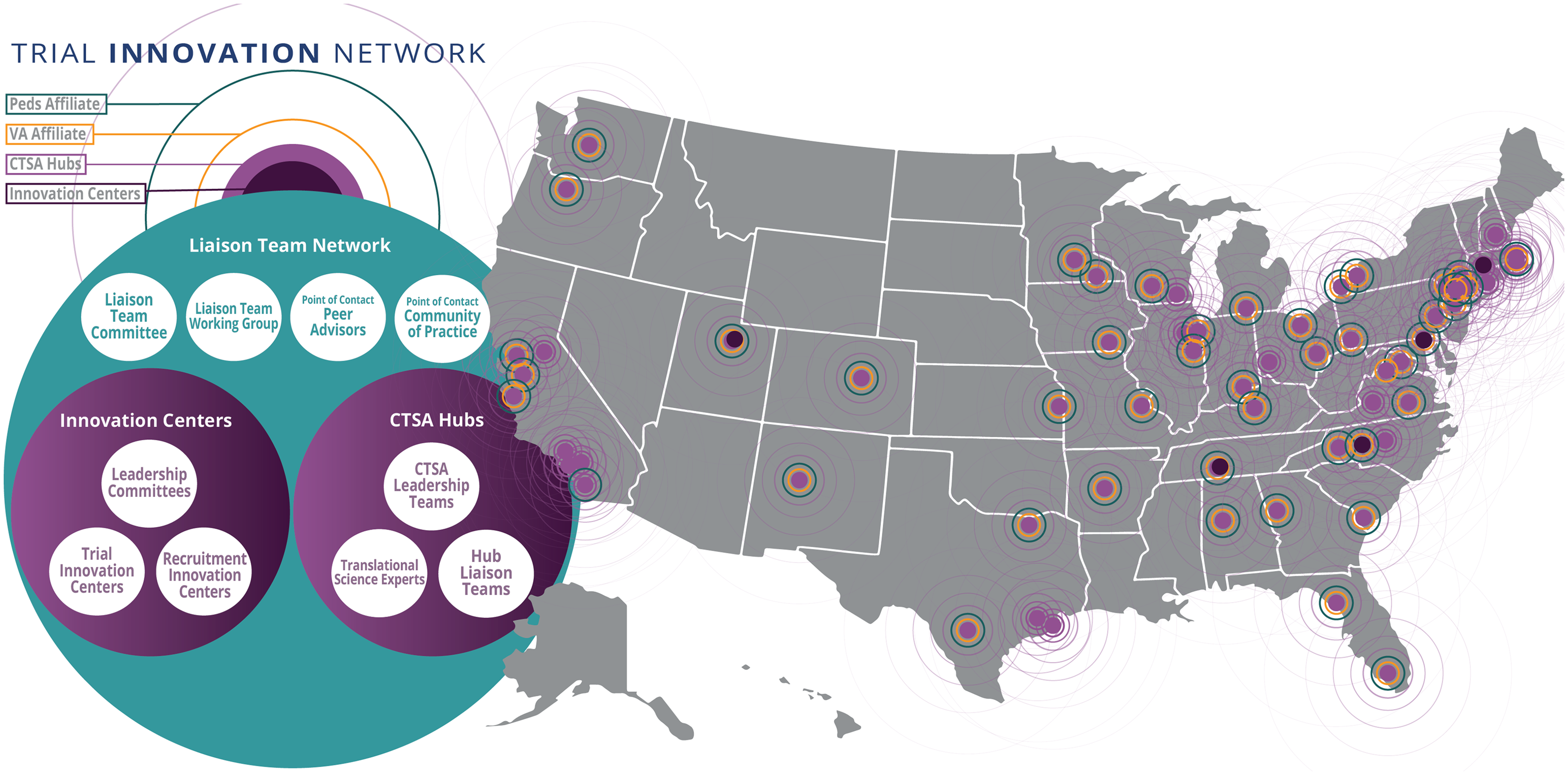
Figure 1. The Liaison Team network, including CTSA Hubs (with veterans affairs and pediatric affiliates), Innovation Centers, and network connections spanning the United States; CTSA = Clinical and Translational Science Award; Peds = pediatric; VA = veterans affairs.
In this paper, we review the structure, function, communications, and development of the Liaison Team network, discuss lessons learned during the first TIN funding cycle, and outline a path toward further network maturity, involving role expansion and amplified engagement of affiliates. Lessons from the wider development of the TIN, including governance, trial consultations, and response to public emergencies, have been published in a separate manuscript [Reference Harris, Dunsmore and Atkinson5].
CTSA Hub Liaison Team Description and Structure
The idea of a national platform for efficient clinical trials required connecting multiple CTSA Hubs; therefore, starting in 2015, a new function called “Liaisons to Trial Innovation Centers” and “Liaisons to Recruitment Innovation Centers” was described as a required element for CTSA programs (RFA-PAR-15-304). Liaisons were envisioned to be a team of experienced research professionals who would provide oversight, consultations, and education, help with quality control and assurance, and connect people and organizations. Liaisons would be positioned to support the TICs and the RIC (hereafter collectively referred to as the Innovation Centers) within the newly launched TIN. The announcement indicated that CTSA support should focus on providing a foundation that promotes quality, efficiency, and collaboration and that this support should be used to identify roadblocks to clinical trial conduct and to foster innovation to overcome them.
Those in a Liaison role were expected to facilitate clinical research within their CTSA Hub, and each hub was required to develop a core that streamlined and supported study startup, implementation, and the recruitment of trial participants. There were specific instructions to focus on accelerating Institutional Review Board (IRB) review, budgeting, contracting, and other startup timelines through process re-engineering and parallel rather than sequential work steps. Liaisons would connect to the Innovation Centers and prepare their organizations, and affiliate partners, to act as sites for multicenter clinical trials. They would have a vital role in identifying investigators at their site who could participate in multicenter clinical trials, and they would collaborate with other institutions to implement IRB reliance and pre-negotiated master subcontracts.
CTSA Hubs structured their Hub Liaison Teams (HLTs) with individuals who had the authority and experience to meet the requirements described above. While the CTSA announcement provided some guidance regarding the Liaison role, there was also significant latitude in how the HLT at each CTSA would be structured. Most CTSA Hubs identified a Hub Medical Director, who was in some cases also the CTSA Principal Investigator (PI), to provide Hub leadership. They also often appointed a primary HLT Point of Contact (POC) responsible for managing communication and expectations, an IRB contact to facilitate single IRB efforts, a contracting expert to streamline multicenter contracts, and an informatics contact to manage electronic health records (EHR) cohort requests.
In 2020, we explored existing TIN HLT membership models via a CTSA-wide survey that asked about local HLT composition, meeting frequency, and activities. The survey had a response rate of 89% of CTSA Hubs and was answered mainly by HLT POCs (91%). We found many different models and no single “right” way to structure and maintain a HLT. Responses indicated that organizational context and CTSA structure make a difference in the development and maintenance of a functional HLT. Survey respondents reported differences in HLT composition (Fig. 2), meeting frequency, regularity of expert engagement, approaches to tracking TIN EOIs, and strategies for identifying potential site PIs. A variety of structural and operational approaches are being tested across the network and HLT members are being presented with different models and ways of working in HLT meetings and having facilitated discussions that allow learning from one another.
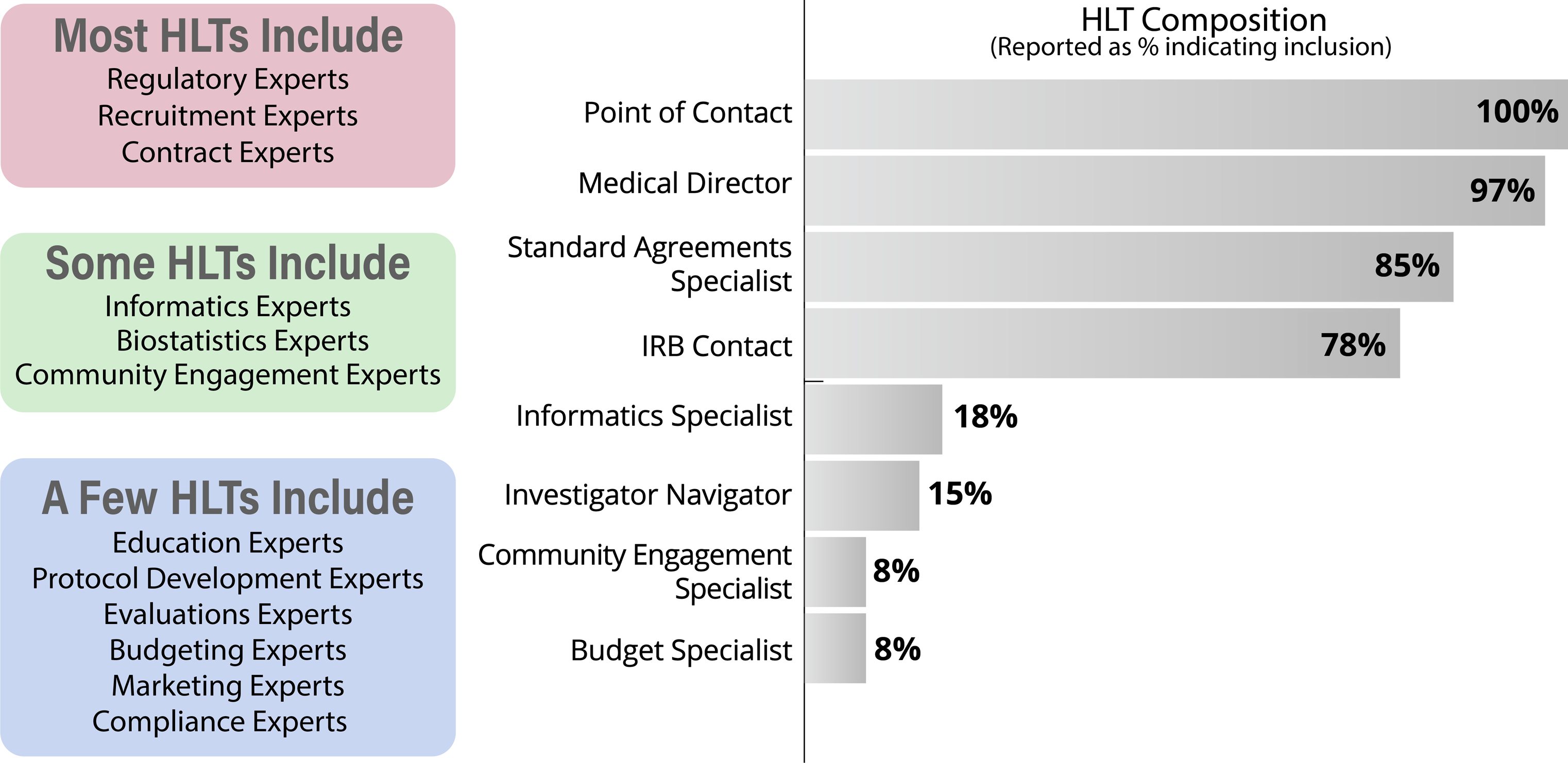
Figure 2. Hub Liaison Team (HLT) structure as self-reported (right) and HLT expertise gathered via 2020 survey (left); IRB = Institutional Review Board.
CTSA Hub Liaison Team Function
The CTSA Program has other cross-CTSA Hub groups that support national collaboration, including special interest groups, Enterprise Committees, and project-specific collaborations. However, HLTs are supported at each CTSA Hub and are uniquely positioned to be an outward-facing connector while also being agnostic to disease area, design methodology, and investigator career stage. This allows the possibility of a broader and more flexible role with a focus on collaboration and connection to the wider national network, coordinating processes, and communicating in bidirectional ways. The role of HLTs requires openness to collaboration and transparency to support team science.
HLT POCs play an essential role in supporting the connection between the CTSA Hubs and the Innovation Centers. As set out in the 2015 CTSA announcement, they serve as expert, informed intermediaries who work bi-directionally, connecting investigators with TIN support, and connecting the TIN with potential site PIs. HLT POCs are internal subject matter experts who can help PIs access local services and stakeholders to facilitate multicenter research. They can also connect relevant PIs with TIN resources. During a TIN consultation, the investigator has a series of meetings with relevant experts to discuss study planning, recruitment and retention, and clinical trial innovations [Reference Harris, Dunsmore and Atkinson5,Reference Palm, Edwards and Wieber6]. HLT POCs are invited to TIN consultations and can act as an ongoing link between the Hub, investigators, and the Innovation Centers, to ensure that support and resources provided locally and nationally are complementary.
HLTs facilitate their institutions entering into sharing and conduct agreements (e.g., SmartIRB Authorization, IRB Reliance Exchange Portal, Federal Demonstration Partnership-CTSA Standard Agreement, and Confidential Disclosure Agreement covering TIN Trial Solicitation Information Exchange) to support and streamline collaboration within the TIN. HLT POCs also field TIN requests for clinical trial sites. They receive requests for EHR-based patient cohort discovery numbers and expressions of interest (EOIs) that may also include questions about protocol design, budget feasibility, and named site PIs who have expressed an interest in being part of the proposed trial. The central call for EOIs and systematic collection of responses has been important to TIN-supported multicenter study site selection and expansion [Reference Nelson, Drury and Hood7]. These requests are often circulated beyond the hub site, to affiliates, and affiliate responses are frequently collected centrally by POCs. This is an important role, and it requires the organizational knowledge to navigate a variety of disease areas and departments to identify and liaise with relevant individuals and investigators. HLT POCs do not just initiate these activities; they also monitor and track investigator progress and site engagement requests. Below we describe how HLT POCs were engaged in the co-development of resources that support this monitoring and oversight.
CTSA Liaison Team Communication Pathways
An active communication strategy is essential to create and maintain network support [Reference Best, Berland, Greenhalgh, Bourgeault, Saul and Barker8]. Different communication pathways were developed by the TIN to support collaboration, provide network updates, share good practices, discuss HLT management strategies, share operational developments, and provide trial proposal details. HLTs began meeting together in late 2016 at Collaboration Webinars. At the time of this publication, there have been almost 100 Collaboration Webinars. Communications pathways evolved over time, and meetings targeting different audiences and with different goals were launched (Table 1).
Table 1. Overview of Trial Innovation Network webinars, meetings, forums, and work groups, showing movement from early Innovation Center leadership (rows 1-4) to later Hub Liaison Team and investigator leadership (rows 5-7)
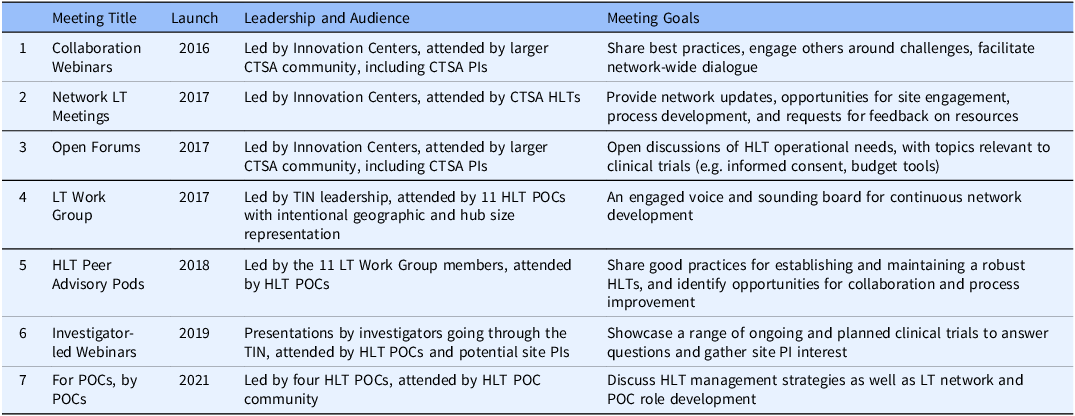
CTSA =Clinical and Translational Science Award; HLT = Hub Liaison Team; LT = Liaison Team; PI = Principal Investigator; POC = Point of Contact; TIN = Trial Innovation Center.
In addition to interactive communication pathways, a TIN newsletter, launched in early 2017, has a current distribution list of over 750 individuals. At the time of writing, 58 newsletters have been circulated. To support non-centralized communications, a Listserv of CTSA Hubs is included on the TIN website with the contact details of all the PIs, Medical Directors, administrators, and HLT POCs. This list, maintained by the TIN, supports communication and collaboration among CTSA Hubs across the network.
This overview of HLT communications shows the evolution of the Liaison Team Network, which started with the intention to collaborate in bidirectional ways and grew from the top-down creation of platforms and forums for sharing and feedback to bottom-up leadership. The Open Forum began with Innovation Center presenters interested in engaging the HLTs, and later became more diverse, showcasing good practices from across the CTSA Hubs. In 2021 the Open Forum became bi-monthly to share the time slot with the “For POCs, by POCs” meetings, as the network matured and the HLT POCs took on a leadership role, actively moving the network in directions that they felt were important. The development of TIN communication pathways taught us the importance of identifying communication needs and building platforms and pathways that could evolve with network growth. Flexibility and transparency were essential to bringing in a range of voices and priorities from across the TIN and supporting open dialogue.
CTSA Hub Liaison Team Resource Development
Development of the Liaison Team Network has been iterative and collaborative, with the Innovation Centers engaging with CTSA Hub PIs and HLT POCs about training and information priorities, support needs, and process development. Regular evaluative activity, such as the collection of feedback on Collaboration Webinars and Network LT meetings, is used to shape network activities and resources. The Innovation Centers also conduct surveys to understand HLT needs and gather ideas.
A survey conducted in 2019 asked about the challenges that HLTs were facing. Respondents reported challenges related to communications, lack of information, unclear processes, and motivating PIs to engage with the TIN. They requested tools for PI engagement, clearer timelines, formal HLT POC expectations, and process explanations. As a result of the requests and suggestions, the Innovation Centers and LT Work Group partnered to standardize communications. An informational TIN slide deck was created, a two-page TIN consultation summary to support PI engagement was developed, and guidance to orient HLT POCs to the TIN was launched [9].
In 2020, a training needs assessment was sent, with a response rate of just over 50% (33/65) of CTSA Hubs. The survey asked about training and information sessions ahead of a half-day TIN HLT workshop. Responses indicated that some CTSA faculty and staff who had not experienced the TIN consultation process did not understand its value and were interested in knowing more. As a result, the workshop included a mock TIN consultation to bring the concept to life and allow attendees to ask questions. The audience of over 100 attendees from more than 40 organizations included CTSA PIs, CTSA medical directors, CTSA investigators, and HLT POCs.
The development of network resources mirrors the evolution of the communications activity, with top-down requests for feedback on Innovation Center resource development complemented by resources that are requested and/or led by the LT Working Group. Essential network processes, including TIN proposal submission, EHR-based cohort discovery, EOI requests (see Fig. 3), and TIN investigator testimonial collection, were developed by the Innovation Centers in partnership with HLTs. These processes were presented for feedback in Open Forums and discussed and launched during Network LT meetings.

Figure 3. Vignette of Innovation Center and Hub Liaison Team collaboration to develop the Expression of Interest process.
The network dashboard, an internal part of the TIN website available only to HLTs, was iteratively developed and continuously improved based on HLT needs and activities. The dashboard includes a listing of trial proposals that have been submitted to the TIN, the NIH Institutes and Centers that have been engaged, and the therapeutic areas represented. As this space was built, HLTs advised on the information that they wanted to access and how it should be displayed. HLTs are now able to sort by their organization and track the progress of studies that have been submitted by investigators from their hub, or studies for which they are acting as a recruiting site. This allows HLTs to identify study slowdowns or barriers and positions them to support Hub investigators to move forward efficiently.
There were many lessons learned in the development of TIN resources for use by HLTs. Innovation Center staff who led development of tools, platforms, and processes learned to engage HLTs early, show responsiveness, iterate towards a solution, and return to HLTs following the use of resources with a view to continuously improving them. Because the TIN is comprised of HLTs that operate under different models, resource development required an understanding of diverse needs and the creation of resources with inbuilt flexibility.
CTSA Hub Liaison Team Timeline
The timeline below (see Fig. 4) starts with the launch of HLTs in 2016 and shows the development of network structures, communications, and resources. The bidirectional and responsive nature of the network is highlighted by arrows indicating connections between requests and delivery. The timeline captures CTSA feedback; however, it is important to note that this manuscript is focused on the development of the Liaison Team network. While investigators who underwent TIN consultations were asked to provide feedback in the form of anonymous survey, and CTSA PIs were invited to sit on an advisory board to comment on the direction of the network, these results are provided in a separate manuscript [Reference Harris, Dunsmore and Atkinson5].
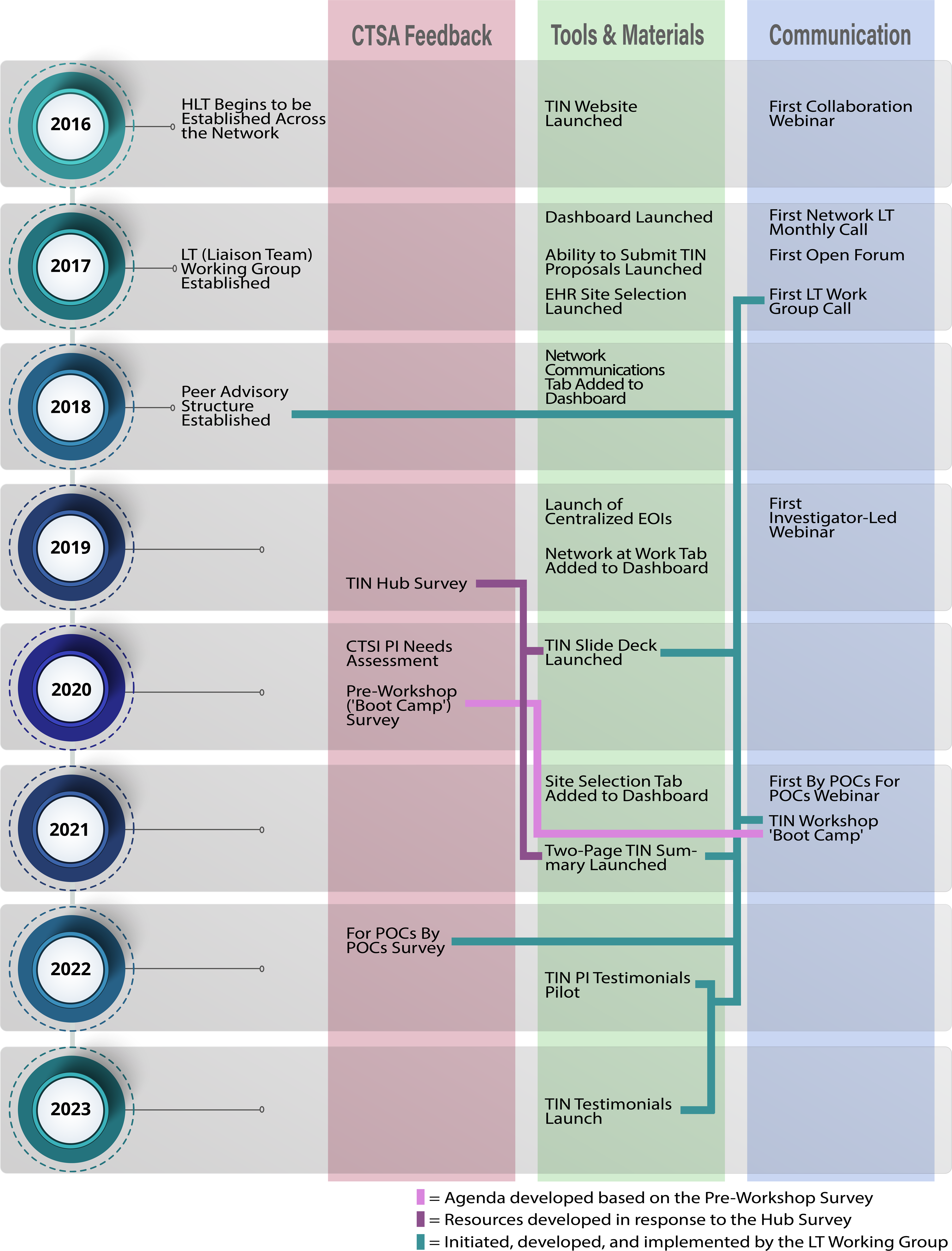
Figure 4. Timeline of Liaison Team network development, including CTSA Hub feedback, development of tools and materials, and communication pathways.
CTSA Liaison Team Network Future Development
The Liaison Team network is now a mature and functional national network at the cutting edge of team science in clinical and translational research. HLTs show active engagement with the TIN, with 94% of HLTs supporting investigators to submit proposals to the TIN portal. Of the 48 EOIs circulated in the first TIN funding cycle, 100% of HLTs have responded to one or more requests for patient counts and/or interested site PIs, with an average response rate of 64%. This indicates that HLTs are effectively responding to requests for trial sites and actively engaged in supporting trial investigators.
The internal HLT structures and the external network structure have been developed in collaborative and iterative ways, with methods for shared learning and continuous process improvement. A strong framework has been established that can be modified as the network continues to grow and evolve. Lessons learned about the importance of varied and targeted communications pathways, including HLTs in early discussions about resource development, and having HLTs lead important efforts, will serve the network well in the future.
The CTSA Program continues to evolve, and the most recent CTSA funding announcement (PAR-21-293) indicates that each CTSA Hub must appoint an HLT to function as an interface between the Hub and the national collaborative activities of the CTSA Program. Current national collaborative activities include the development and application of innovative approaches to collection, analysis, use, and sharing of various types of data; collaborative infrastructure to support scientific, training, governance, workgroup, and other types of CTSA consortium activities; consideration of participation in clinical trials; identification and dissemination of innovations in clinical trials; and a collaborative informatics community. The HLT is expected to be a connector to their local research community as well as Innovation Centers and to NCATS Consortium-Wide Resources. Communication pathways that allow HLTs to share strategies for effective hub development and growth will be important as their role within the CTSA Program continues to evolve.
The strong foundations of a mature network paired with the expansion of the HLT role are an opportunity for continued collaboration and the growth of network-wide team science. The HLT role will be supported by existing structures, with communication pathways and resource development avenues that are bidirectional and provide both top-down and bottom-up opportunities. Methods for the implementation of new processes and strategies have been developed, and dissemination can flow through the communication channels that have been refined and continue to improve. CTSA Hub leadership is committed to supporting their HLTs, and they are working with TIN leadership to explore how to expand the HLT role given their effectiveness in supporting multicenter trials. In the future, HLTs may play a role in connecting informatics expertise across sites, developing and disseminating clinical trial resources, and supporting local investigators to use TIN-developed resources. As the TIN transitions to its second funding cycle and the HLT role expands within the CTSA Program, ongoing partnership efforts include facilitating CTSA affiliate outreach to enhance diversity in clinical research and supporting investigators who are moving from single to multicenter trials.
Conclusion
The TIN leveraged the strengths of the CTSA Program to create a connected network of disease-agnostic HLT clinical science professionals who are supporting and driving translational research in the United States. The future of the network will bring more partnership and shared learning opportunities. HLT members have demonstrated diverse structural and operational approaches and shown how this diversity can be used to help the network continue to learn, develop, and iterate. While performing an essential role within the TIN, the Liaison Team Network has also functioned as a national laboratory for team science and for the development of translational science.
Acknowledgments
Authors acknowledge the partnership and engagement of the Hub Liaison Teams from across the CTSA Program Hubs, particularly the Liaison Team Medical Directors, and the Points of Contacts (POCs). We also acknowledge contributions from specific contributing CTSA Institutions and past and present representative members of the Liaison Team Working Group: Albert Einstein College of Medicine, Ilija Atanasovski, Zoe Tsagaris; Children’s National Hospital, Rachel Walega; Mayo Clinic, Laura Meiners; Medical College of Wisconsin, Amit Gode; Medical University of South Carolina, Amanda Cameron; New York University, Patricia Corby; The Scripps Research Institute, Shelly Meese, Emily Spencer; Stanford University, Maya Berdichesky; University of Arkansas Medical Sciences, Amy Jo Jenkins, David Avery, Monica Smith; University of California-San Francisco, Carmela Lomonaco, Nimit Ruparel; University of Colorado-Denver, Aaron Mobley, Maya Koch; University of Michigan-Ann Arbor, Santinio Jones; University of New Mexico Health Sciences Center, George Garcia, Susan Tigert; University of North Carolina-Chapel Hill, Laura Viera; University of Rochester, Yonjong Choi; University of Texas Health Sciences-San Antonio; Patricia Dahia; Yale University, Helen Seow. Authors would also like to recognize TIC and RIC members for their communications and coordination expertise 2017-2023: Duke University, Maria Cecilia Santiago-Turla, Kristi Romero, Princess Abbott-Grimes, Alyssa Frey, Eilene Pham, Sonya Sutton, Eve Marion; Johns Hopkins University, Krista Vermillion; University of Utah, Michelle Aponte, Monse Lopez, Eun Hea Unsicker; Vanderbilt University, Julia Dunagan, Wendy Lloyd, Kaysi Phillips. In addition, Vanderbilt University, Michelle Jones has been an important partner, working closely with Hub Liaison Teams to develop the dashboard and create tools to support POCs. Finally, authors are very grateful to University of Utah, Caroline Foley for her design skills in creating the manuscript figures.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding statement
This work is supported by the NCATS and the National Institute on Aging, in support of the Trial Innovation Network under grant numbers U24TR001579 (Vanderbilt University), U24TR001597 (University of Utah), U24TR001608 (Duke/Vanderbilt Universities), and U24TR001609 (Johns Hopkins/Tufts Universities).
Competing interests
The authors have no conflicts of interest to declare.







