Introduction
The use of vaccines to prevent Coronavirus disease 2019 (COVID-19) is critical to the reduction of disproportionate pandemic-related morbidity and mortality in racial and ethnic minority communities that have seen declines in life expectancy due to COVID-19 [Reference Killerby, Link-Gelles and Haight1–Reference Andrasik, Broder and Wallace4]. Early in the pandemic, there were low racial and ethnic minority participation rates in phase I and II clinical trials [Reference Flores, Frontera and Andrasik5]. Historical underrepresentation of minorities in clinical trials [Reference Heiat, Gross and Krumholz6,Reference Hussain-Gambles, Atkin and Leese7], including vaccine trials [Reference Djomand, Katzman and di Tommaso8,Reference Sobieszczyk, Xu, Goodman, Lucy and Koblin9], presented a critical challenge to the successful development, testing, and use of a safe and efficacious vaccine by those who need it most. Underrepresentation of participants from diverse racial and ethnic backgrounds may have implications for the generalizability of clinical trial results, given the efficacy and safety of medical treatments may differ by race or ethnicity [Reference Ramamoorthy, Pacanowski, Bull and Zhang10]. Enhanced representation of diverse groups in vaccine trials may also enhance subsequent vaccine uptake [Reference Flores, Frontera and Andrasik5], increase equitable access to other timely or novel treatments, and contribute to our understanding of health disparities. Thus, early in the inception of COVID-19 vaccine trials, the US National Institutes of Health (NIH) identified increasing accessibility to underrepresented populations as a priority and vital to providing equitable protection from COVID-19.
To address enrollment gaps in COVID-19 clinical trials, we collaborated with NIH-sponsored Phase 3 COVID-19 vaccine trial teams in Los Angeles County to convene a Community Consultant Panel (CCP), an advisory group designed to provide community feedback and recommendations to improve the recruitment and retention of minority participants. The goals of the CCP were to help increase participant diversity and representation – by race/ethnicity, essential worker occupation, and geography by recruiting CCP members from underrepresented racial and ethnic groups from communities with high rates of infection, morbidity, and mortality – in local COVID-19 vaccine clinical trials and provide access to accurate information about clinical trials to communities disproportionately affected by COVID-19. Assuring diversity among trial participants was particularly relevant as California has the highest number of COVID-19 cases nationwide, with 32% of cases in Los Angeles County, one of the country’s most populated and racially diverse counties [11]. We describe our approach to forming the CCP, how recruitment strategies were modified, and enrollment outcomes across three local vaccine trials. We summarize actionable strategies recommended by the CCP to improve the engagement of minority populations in COVID-19 vaccine trials within our academic institutions.
Materials and Methods
The CCP was rapidly formed by the UCLA Clinical and Translational Research Institute’s (CSTI) Community Engagement & Research Program (CERP) to support three COVID-19 clinical trial research teams across Los Angeles County with clinical trial recruitment and retention (UCLA CARE Center, Harbor UCLA/Lundquist Institute, and UCLA Vine Street Clinic) enrolling into two NIH-funded COVID-19 clinical trials (AstraZeneca and Moderna). The CCP had several roles: to consult with academic clinical trial researchers, health professionals, and other Los Angeles-based community leaders, to identify barriers and facilitators to COVID-19 vaccine clinical trial participation across diverse communities in Los Angeles County, and to provide recommendations for enhancing clinical trial participation in diverse communities.
Deliberative Community Engagement
We used a Deliberative Community Engagement (DCE) approach to understand and enhance clinical trial recruitment and implementation and better understand and address the pervasive lack of diverse representation in clinical trials. DCE has been used to examine and obtain community input on a variety of complex health and social issues [Reference Dry, Garrett and Koenig12–Reference Abelson, Blacksher, Li, Boesveld and S.15]. The process allows participants to consider relevant information from multiple points of view and involves: recruiting a sample of relevant stakeholders (regarded as experts in how the topic at hand concerns or affects the population at risk) to serve as deliberates; engage in educational activities to ensure stakeholders have a working knowledge of the technical issues at hand, as well as clinical, social, and other trade-offs; facilitate discussion so participants can clarify their values and understand others’ perspectives; and develop and discuss specific recommendations [Reference Dry, Garrett and Koenig12].
CCP Member Recruitment
Representatives from communities with a high risk of COVID-19 due to race/ethnicity and/or age, occupation (e.g., essential service industries), or geographic region served were identified by members of the CERP academic-community collaborative based on occupations, regions, poverty level, and ages with higher than average COVID-19 cases reported by Los Angeles County. A list of recommended candidates was circulated to the CERP community partners and academic faculty, who then ranked individuals to ensure representation from all potential professional sectors and communities (race/ethnicity, occupation type or organization, and geographic area) as well as noting any previous experience working with the individual such as previous CBPR projects, CTSI community partners, and personal and professional networks. The clinical trial team tabulated, reviewed, and discussed ratings to select 13 candidates. Invitations were sent to panelists defining the role of the consultant, participation expectations, and compensation.
CCP Structure and Curriculum
Because of the immediate need to implement trial protocols and design equitable engagement plans, CCP met weekly over zoom with UCLA researchers for eight weeks (90 min per meeting) in Summer 2020 to identify barriers to trial participation. Members were compensated for their time commensurate with the expected time commitment, both during and outside the scheduled meetings, and were offered a tablet with free internet access if needed for CCP participation. Before the first meeting, each participant received a clinical trial briefing booklet with information on COVID-19, clinical trial stages and processes, the importance of diverse participation in research, and the protection of human subjects. These and other materials were also made available to participants on a website, which was regularly updated with evolving information.
Weekly CCP meetings aimed to promote bidirectional exchange on trial processes, COVID-19, vaccines, and other topics determined by the CCP. The academic team (principal site investigators, clinical trial staff, and CERP staff) developed and shared brief educational presentations about COVID, vaccine development, regulatory approval, emergency authorization processes, and vaccine risk, benefits, and safety. Discussions were framed around community concerns related to trial participation, strategies for clear and culturally appropriate recruitment messaging, and trial participation barriers of high-risk groups. Discussions were driven by new information about the virus, vaccines, trials, and other topics determined by the CCP. Supplemental Table 1 provides a brief outline of the educational objectives of each session and the discussion topics covered during the CCP meeting. For some of the sessions, representatives from a marketing and communications firm hired by two of the local clinical trials participated and had an opportunity to interact directly with community members on language and design of outreach materials, approach, and potential outlets for messaging. The meetings were recorded, and an academic team member took notes during each session.
Clinical trial investigators reviewed the CCP feedback, discussed the feasibility of suggestions with the CCP, and identified strategies to incorporate proposed recommendations when possible. For these analyses, we summarize the recommendations from the CCP and whether and how the recommendations were acted upon.
After the last session, CCP participants were invited to complete a survey that included demographic characteristics, previous research or consulting experience, perceived experience as a community consultant, the perceived value of the community-academic team [Reference Matthews, Anderson, Willis, Castillo and Choure16], and how they used and disseminated information gained through the CCP. A five-point Likert scale was used to gauge the success of each metric.
Cross-Collaboration across COVID-19 Vaccine Clinical Trial Leads in Los Angeles County
In addition to the CCP meetings, the three participating interdisciplinary vaccine trial teams met in weekly 60-min Zoom sessions to share experiences. Investigators used these meetings to share approaches to recruitment and engagement, help answer questions raised during the CCP meetings, and incorporate feedback from the CCP with the vaccine trial research teams.
Results
The final community panel included 13 participants (Table 1). Participants identified themselves as Black (31%), Latino (39%), Asian (15%), or White (15%). Participants self-identified as representing the following community sectors and special populations: community health workers (54%), essential workers (46%), health care professionals (31%), low-income (69%), individuals with chronic conditions (39%), and LGBTQ (23%). Most participants had experience serving on a community advisory board (69%).
Table 1. Community Consultant Panel (CCP) demographics and experiences (N = 13)

a Provided options were: Black/AA, LatinX/Latino, White/Caucasian, Asian/Pacific Islander, Native American, LGBTQ, Low income, Chronic Disease, Other (please specify).
b May include community advisory boards for academic or non-academic institutions.
c Response of “Very Satisfied” or “Extremely Satisfied” on a Likert scale.
All the participants reported gaining new information about clinical trials, COVID-19, and/or the COVID-19 vaccine due to CCP participation. All thirteen participants reported applying the new information they learned in the community, including sharing the information with family (85%), friends (77%), co-workers (92%), and others (8%). The vast majority, 92%, reported that they felt welcomed as a member of the CCP, and 92% felt that the academic team valued their comments (Table 1).
Actionable Strategies Incorporated into Local COVID-19 Vaccine Trials
Clinical trial investigators could incorporate several proposed recommendations (Table 2). The recommendations addressed three domains. First, the participants recommended increasing trust and transparency in the research process by clarifying vaccine science or trial processes, utilization of trusted messages and messengers, and highlighting trial participation benefits in addition to risks. The second group of suggestions related to the importance of inclusive community-engaged approaches to promote more effective outreach and recruitment, developing culturally tailored approaches for engagement, and recruitment communications that promote inclusion, including engagement strategies for responding to questions, concerns, misinformation, and disinformation prevalent in the community. Third, the CCP strongly endorsed enhancing trial accessibility and acceptability related to the social determinants of health, including improving local practices that promote a welcoming environment and address the needs of low-income individuals with competing financial and social demands and study protocols. They specifically recommended strategies to reduce barriers to participation, such as providing transportation services, language translation services for visits and all study documents, offering child care services, and addressing concerns about access to medical visits for vaccine-related side effects for those who lack health care coverage. CCP members specifically endorsed the importance of creating a welcoming study environment through intentional “customer service” efforts to improve trust, acceptability, and retention of underrepresented populations in research. The panel recommended that participants receive access to food and refreshments, accommodations for additional instruction, and a clear plan for visits and follow-up to address health literacy, language translation services, and recognition for their participation and time. Lastly, participants recommended clinical trial sites be located directly in the community to improve accessibility and reduce social burden.
Table 2. Community Consultant Panel (CCP) recommendations for improved participation in clinical trials and modifications made by investigators
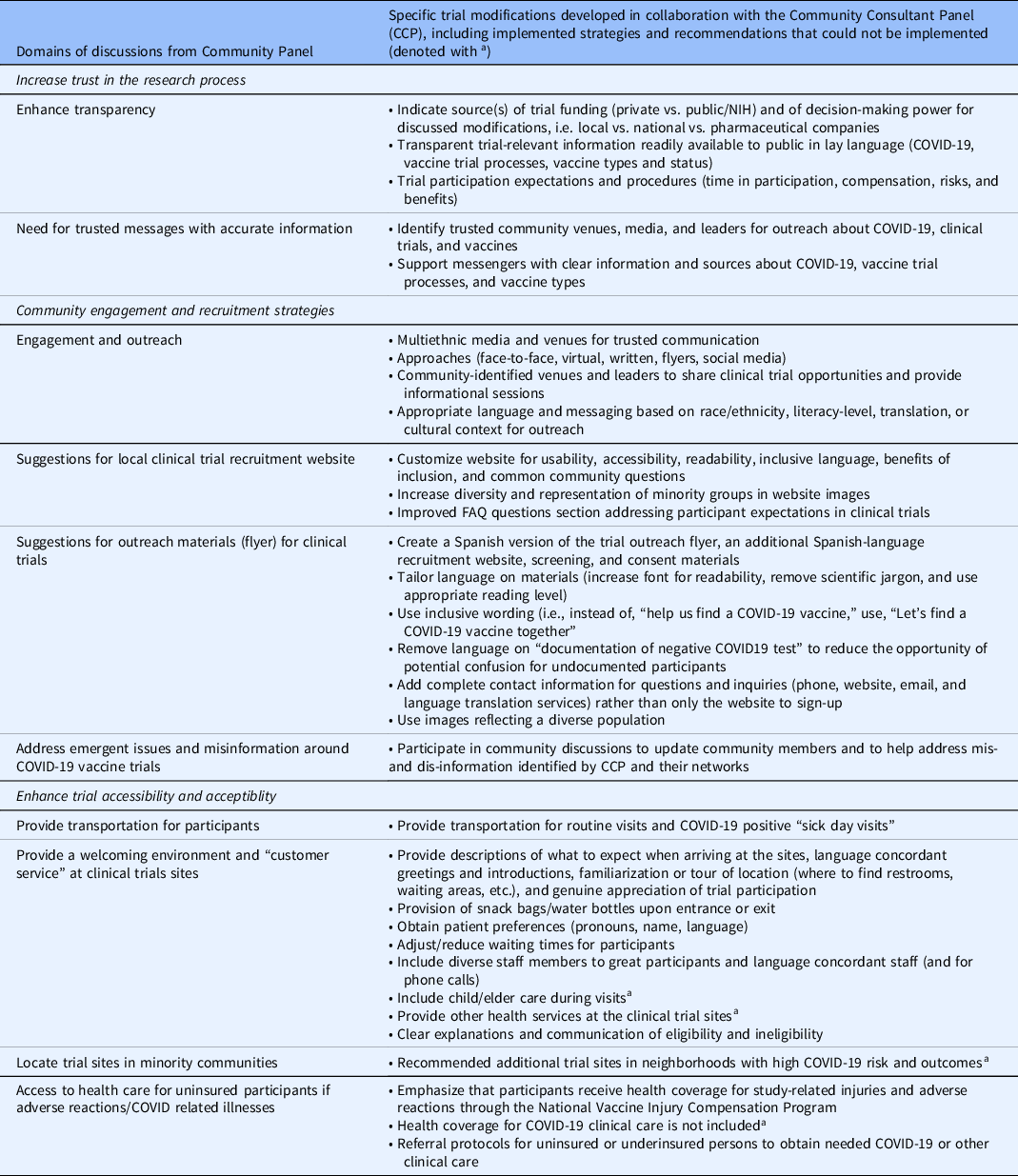
a Recommendations the Los Angeles COVID-19 trial teams were unable to implement (see narrative for additional detail).
Discussions allowed for the iterative development of locally tailored strategies to modify engagement practices related to outreach, recruitment, and retention. During these discussions, the panelists identified individuals, agencies, organizations, media outlets, and community members they viewed as trusted messengers, provided introductions to key stakeholders in the community, and helped craft messages for trial information and dissemination. To enhance transparency, the team shared information on NIH and industry funding for the trials in Los Angeles County and links to websites with that information. Structured discussions were held on trial protocols for each vaccine (“clinical trial basics”), participant expectations related to the timing of procedures, vaccine types, risks such as side effects and the potential for lack of efficacy, as well as the potential benefits of participation, including compensation, early access to promising vaccines, and medical screenings.
The panelists and the study teams developed, tested, and customized messaging for different communities in Los Angeles County to improve recruitment. Investigators and panelists collaborated on 112 community discussions for organizations represented by the CCP members, including churches, Filipino social clubs, LGBTQ podcasts, and parent organizations, reaching over 10,000 individuals. Due to the pandemic, many of these events occurred virtually.
Tailoring of the recruitment messaging incorporated readability, inclusive language, diversity in images of participants represented, and updating frequently asked questions in response to trial and vaccine development progress, changes in the pandemic, and CDC and local public health guidance. For example, initial drafts of clinical trial recruitment materials listed eligibility criteria indicating the need for “documentation” of a negative COVID-19 test. The CCP noted the word “documentation” was a potential trigger for deterring undocumented persons or families with mixed documentation status from participation due to the burden of proof and misconceptions participation in public benefits (access to free COVID-19 test) can interfere with future immigration eligibility, also known as fear of public charge [Reference Katz and Chokshi17,Reference Perreira, Yoshikawa and Oberlander18]. Ensuring undocumented populations felt safe in clinical trial enrollment was particularly important to the CCP, considering it was unknown at that time if COVID-19 vaccines would only be covered by health insurance.
To improve accessibility and acceptability for trial participation, participants suggested ways to reduce participation barriers related to social determinants of health and suggested improving trial retention through a genuine focus on the participant. Social barriers were mitigated by providing transportation for participants (both for routine visits and “sick day visits,” e.g., visits to the clinical trial site to address symptoms that might represent infection or side effects related to the vaccine), Spanish translation services, and referrals to resources for those who were uninsured or underinsured. Although most of the participating clinical trial sites had a strong record of collaboration with diverse communities, the extensive “customer service” recommendations from the CCP were important reminders of the need to build rapport with participants to enhance retention. Recommendations included a welcoming environment for participants, many of whom had never participated in a clinical trial yet were now doing so in the context of COVID-19 distancing requirements and other restrictions, increased personal and community stressors, and competing clinical and social demands brought about by the pandemic. Specific recommendations included thanking participants for their time, providing clear directions before their appointment, a tour and introductions, asking about gender pronouns, and supplying water, snacks, or a “goodie bag.”
The local clinical trials could not address some CCP recommendations. For example, participants strongly endorsed the need for more clinical trial locations in minority communities through mobile trial sites and partnerships with minority services institutions. However, most vaccine trial sites were identified based on prior NIH accreditation, and available mobile vans could not process the clinical trial samples. Other suggestions, such as a need for after-hours availability, including weekends and weeknights, and on-site child care, were not feasible due to union, staffing, or resource limitations. Finally, some panelists’ inadequate staff diversity at some sites and lack of fluency in languages other than English and Spanish were major concerns, particularly for representatives of Asian and Pacific Islander communities. Panelists and clinical research teams endorsed the need for lay health workers from these communities who could facilitate participation in the participant’s preferred language, study materials (including consent forms and informational materials), and staff and/or translators who could address these participants’ needs in real time.
Recruitment of Diverse Communities in the Local COVID-19 Vaccine Trial
Deploying several of the CCP’s recommendations for trial engagement, recruitment, messaging, accessibility, and acceptability, our three local trials reported more than 50% underrepresented minority participation, with the following ranges: 32%–47% Latino, 20%–31% White, 11%–21% Black/African American, 5%–21% Asian American, 2% Native Hawaiian or other Pacific Islander, 1%–4% American Indian/Alaska Native, and 0.5%–5% Other/Multiracial. The racial and ethnic breakdown of local COVID-19 vaccine clinical trial enrollment closely mirrored the racial and ethnic composition of Los Angeles County (Fig. 1) and showed larger proportions of racial and ethnic minorities than the aggregate Moderna and AstraZeneca trial enrollments [Reference Falsey, Sobieszczyk and Hirsch19,Reference Baden, El Sahly and Essink20].

Fig. 1. Racial and ethnic composition of participants enrolled in partnered clinical trials compared to Los Angeles County population. *Los Angeles County data from 2019 American Community Survey.
Discussion
The disparities in COVID-19 and mortality rates in communities of color represent longstanding systemic health inequities [Reference Thompson, Manning and Mitchell21,Reference Carrion, Colicino and Pedretti22]. Underrepresentation of minorities in COVID-19 clinical trials may result in limited generalizability of outcomes and decreased vaccine confidence and uptake among communities most impacted by the COVID-19 pandemic. We describe a community-engaged approach to developing community-centered recommendations to improve racial and ethnic diversity in COVID-19 vaccine clinical trials. We found that in addition to leveraging dedicated resources to help vulnerable communities overcome barriers related to the social determinants of health, engagement is critical for reaching diverse participant pools, building trust and transparency, and reducing obstacles to participation. Others have also advocated for the implementation of strategies leveraging community-partnered research [Reference Flores, Frontera and Andrasik5], such as partnering with Black church leaders and other trusted community leaders [Reference Jaklevic23], and acknowledging the role of racism and history of systematic abuse and mistreatment both in health care and medical research for racial and ethnic minorities in the USA [Reference George, Duran and Norris24].
The DCE approach to COVID-19 vaccine trial recruitment presented an opportunity to understand and reduce community participation barriers, address informational needs, and improve acceptability. Some suggestions put forth by the CCP were adopted through modifications to each clinical trial team’s recruitment approach. Although we could not incorporate all CCP recommendations, the diverse panelists, investigators, and staff allowed for robust discussions of the policies and practices needed to effect long-term, fundamental change in the planning for and implementation of clinical trials in nontraditional settings to engage more diverse participant populations.
Our project had some limitations. This process relied on longstanding community ties and may be difficult to replicate. The rapid implementation timeline also required significant funding to support the DCE approach, staff trained in community engagement, and adequate compensation for CCP participants, given the demands on their time and the need for a quick turnaround for feedback. This study took place in a racially and ethnically diverse urban setting with access to several recruitment sites, so it may not generalize to other locations. Finally, many CCP members had prior experience with research and were highly educated. The perspectives of the members of the CCP may not represent the community at large or those with less favorable views of research; however, we intentionally recruited community leaders with experience working with high-risk communities and racially and ethnically diverse community members to inform our approach. While recruiting diverse participants in clinical trials is essential, future research should focus on retention strategies for participants from underrepresented groups in underresourced communities.
Our results have important policy implications. High-risk communities should be involved in clinical trial planning to address the profound health disparities during the COVID-19 pandemic. An established community partner network, organizational infrastructure, and leadership that supports this process allowed us to leverage trusted relationships from a vast network of community stakeholders. The panelists were able to effectively collaborate with clinical trial leadership and staff to provide insight and practical advice on community concerns, share updated and valuable information for their communities, and enhance researchers’ awareness of unique barriers and facilitators to participation in COVID-19 vaccine clinical trials from the perspective of diverse local communities. To promote the generalizability of clinical trial outcomes and address the needs of populations at the highest risk for health inequities, policies are needed to enhance representation in the biomedical workforce, promote collaborations with trusted community members and organizations, and develop and monitor metrics for diversity in clinical trials beyond race/ethnicity, age, and gender (i.e., socioeconomic status, insurance status, sexual identity and orientation, languages spoken, language preferences). Such policies will build confidence, engage community stakeholders early in the clinical trial process, and overcome social disparities that contribute to health inequities. Ensuring ample funding for community investment and capacity building to create mutually beneficial and reciprocal relationships between researchers and communities is essential to improve the representation of diverse communities in clinical trials.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cts.2022.471
Acknowledgments
We would like to thank the devoted efforts of the Los Angeles COVID-19 Vaccine Trials Community Consultant Panel members: Adel Domingo, Alexys Watson, Arden Caffrey, Dontá Morrison, Helena Williams, Jacquelyn DuPont-Walker, Jim Mangia, Luis Pardo, Mimi Chang, Octavio Clarin, Veronica Arciga Barriga, Xochitl Cervantes-Luna, and Yey Coronel.
This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 (YC, EN, RB, ALW, SC, DM, SV, AC, AB) and the NIH CEAL/STOP COVID-19 CA Grant Number 21–312-0217571-66106L (YC, RL, SC, SV, AC, AB) and NIH/NIAD UM1 AI069424-16 (RL, RH, ED, PC, CB).
Disclosures
The authors have no relevant financial or non-financial interests to disclose that are relevant to the content of this article. RL serves on the Scientific Advisory Board for Merck and Gilead and has served as a consultant to Cepheid.





