Introduction
The relationship between circumcision and HIV infection in Africa has been the source of numerous controversies (Connolly et al. Reference Connolly, Simbayi, Shanmugam and Nqeketo2008; Garenne Reference Garenne2006, Reference Garenne2008, Reference Garenne, Denniston, Hodges and Milos2010; Garenne et al. Reference Garenne, Giami, Perrey, Giles-Vernick and Webb2013; Rosenberg et al. Reference Rosenberg, Gómez-Olivé, Rohr, Kahn and Bärnighausen2018; van Howe Reference Van Howe1999, Reference Van Howe2015). In particular, the effect of circumcision on HIV incidence in randomized clinical trials (RCT’s) is different from the association between circumcision and HIV prevalence in real life populations. For instance, whereas RCT’s show a reduction of incidence by about half among newly circumcised men in the short run, the Demographic and Health Surveys (DHS) show no effect on prevalence in the long run (Garenne Reference Garenne2008; Garenne & Matthews Reference Garenne and Matthews2020). Among the 42 African DHS’s tabulating HIV infection by circumcision status, 21 find more HIV among circumcised men, and 21 find more HIV among intact men (DHS/StatCompiler 2021). Even though most of these differences are not statistically significant, the standardized relative risk of HIV by circumcision status is close to 1, and this was found already in earlier studies (Garenne Reference Garenne2008). There are many reasons why findings in populations could differ from those in RCT’s. National populations are complex and numerous interactions could exist between HIV, circumcision, socio-economic status, ethnicity, and a variety of exposure variables. For instance, in a population with two groups, one with high exposure to sexually transmitted infections (STI’s) and universal circumcision and the other one with the opposite features (low exposure to STI’s and no circumcision), one would find a relationship opposite to that expected from RCT’s. In addition, a 50% reduction in incidence from high level (say from 4% to 2% a year) still guarantees that men exposed continuously to HIV will eventually contract the infection because of repeated exposure over the years. This seems to be the case in South Africa, where fully circumcised groups have very high infection rates, among the highest in the world, as the Xhosa or the Shangaan (Rosenberg et al. Reference Rosenberg, Gómez-Olivé, Rohr, Kahn and Bärnighausen2018; South Africa/DHS 2019). The demographic impact of circumcision on HIV prevalence therefore depends on the local situation and on the level of infection.
Following the 2007 recommendation of the World Health Organisation (WHO) to promote circumcision as an additional tool for preventing HIV, 14 countries in Eastern and Southern Africa undertook mass campaigns of Voluntary Medical Male Circumcision (VMMC) (WHO 2012). These campaigns include not only the surgical act of circumcision but also health education and condom promotion, since regular condom use is necessary to really protect from HIV transmission in case of exposure. In addition, being based, in principle, on a voluntary decision and on informed consent, these campaigns are likely to select men who are better informed and are more motivated to prevent HIV. So, evaluating the impact of VMMC in a population includes not only the surgical procedure but also selection biases and changing behaviour.
Lesotho is an interesting case because at the onset of the HIV epidemic about half of the male adult population was already circumcised as a traditional custom, because levels of HIV prevalence were very high, and because the country undertook a major program of VMMC in recent years. The issue of the relationships between circumcision and HIV in Lesotho was addressed by several authors (Bulled Reference Bulled2015; Carrasco et al., Reference Carrasco, Rosen, Maile, Manda, Amzel and Kiggundu2020; Coburn et al. Reference Coburn, Okano and Blower2013; Low et al. Reference Low, Thin, Davia, Mantell, Koto, McCracken, Ramphalla, Maile, Ahmed, Patel, Parekh, Fida, Schwitters and Frederix2019; Maffioli Reference Maffioli2017; Makatjane et al. Reference Makatjane, Hlabana and Letete2016; Thin et al. Reference Thin, Frederix, McCracken, Letsie, Low, Patel, Parekh, Motsoane, Ahmed, Justman, Callaghan, Tembo and Schwitters2019; Warren Reference Warren2010).
Lesotho undertook several demographic surveys with information on circumcision and HIV prevalence, providing the information needed to investigate these relationships at population level. Lesotho was also one of the countries where HIV prevalence was significantly higher among circumcised men than among intact men in 2004 (RR=1.49, P<0.0003). The aim of this study was therefore to investigate what happened in Lesotho since 2004, to search for socio-economic and behavioural correlates of HIV and of circumcision, and to examine the association between the VMMC campaign and HIV prevalence. In a first part the paper provides background information necessary to understand the local situation, and the second part presents the statistical analysis of the complex relationships between HIV and circumcision, in the population and among couples.
Background
History
Lesotho is a territory embedded in South Africa. The territory was peopled by Southern Sotho tribes, who settled there in the 17th century. The country is therefore homogeneous in terms of ethnicity and language. The territory went through a troubled political period in the 18th century and early 19th century, with fights against nearby ethnic groups. The situation changed with the arrival of Europeans coming from the Cape colony. The country was first unified under the leadership of King Moshoeshoe (1823-1870). The current territory was delineated in 1868 when it became a British protectorate, named Basutoland. The territory refused to join the South Africa Union in 1910, and became independent in 1966, as a constitutional kingdom, following a British model. The first Christian missionaries (French Calvinists of the “Société des Missions Évangéliques de Paris”) arrived in 1833, soon followed by Roman Catholic missionaries (1862), by other European churches (Lutherans, Anglicans), and by African churches such as Christian Zionists (1912). By the early 20th century most people had converted to one of the churches. In the 2014 DHS, 98% of men age 15-59 acknowledge the Christian faith, the largest group being the Roman Catholic Church (38%), followed by the Pentecostal Church (25%), the Evangelical Church (17%), and the Anglican Church (7%). Religious affiliation could be important as it has an influence on marriage patterns and tolerance for sexual behaviour, and also as it can plausibly influence circumcision practices.
Geography
Lesotho is a small country (30,355 km2), located in high altitude, and mainly mountainous. Several rivers created large valleys permitting agriculture, in particular the Caledon valley, which borders South Africa, and the Senqu (Orange) river. The administration distinguishes four ecological zones, also used in censuses and surveys: “Lowlands” in the North-West, which includes the capital city, is a more developed area, and is close to South Africa; “Foothills”; “Mountains”, and “Senqu River Valley”. The lowlands is a peculiar area because it hosts a population more urbanized, wealthier and with a higher level of education. It was therefore considered separately in the statistical analysis.
Economics and Migration
Lesotho is a poor country, mainly rural, with low level of industrialization, and with a low income per capita compared with South Africa. The average Gross Domestic Product (GDP) per capita for the 2004-2014 period was 2300 $, that is 5.1 times less than in nearby South Africa (11700 $) (World Development Indicators 2017). The great imbalance in employment opportunities and wages induced huge migration flows from Lesotho to South Africa. Migration of Lesotho workers started with the development of the mining industry in the later part of the 19th century. By 1990, at the peak of the mines in South Africa, Lesotho men were accounting for 25% of the whole labour force in the gold mines, and 57% of foreign migrant workers (Kok et al. Reference Kok, Gelderbloom, Oucho and Van Zyl2006). The number of registered Lesotho workers in South Africa was about 100,000, which accounts for about 25% of the male population age 20-59. The pattern of migration changed after 1990, with fewer men and more women migrating. In recent years, it was estimated that 80% of migrant men worked in the mines and about 50% of migrant women were employed as domestic workers (Crush et al. Reference Crush, Dodson, Gay, Green and Leduka2010). Migrations played a major role in the spread of HIV in Lesotho. Male migrants were mostly married men, who kept a wife at home, and were visiting her on a regular basis, often monthly or several times a year. In contrast, female migrants tended to be single, widowed or divorced, who were coming back home less often. In both cases, men and women migrants were at high risk of contracting sexually transmitted diseases such as HIV in South Africa (Brummer Reference Brummer2002; Lurie et al. Reference Lurie, Williams, Zuma, Mkaya-Mwamburi, Garnett, Sturm, Sweat, Gittelsohn and Abdool Karim2003; Palk & Blower Reference Palk and Blower2015).
Demography
Due to the complex geography and arid climate, population size is moderate, about 2 million inhabitants. After a period of population growth since the 19th century, the population stagnated in recent years because of lower fertility, higher mortality (due to HIV/AIDS), and large out-migration flows. As a result, population size hardly changed between the last two censuses: 2.02 million in 2006 and 2.01 million in 2016. The country was essentially rural at the beginning of the 20th century, and urbanized rapidly after independence: 12% in 1976, 17% in 1996, and 34% in 2016. Migrations have an impact on the population counted as “resident and present”, the population interviewed in demographic surveys. For instance, at the 2016 census, some 16% of the adult population age 20-49 was considered as “absent”, mostly migrants to South Africa, 20% for men and 13% for women. Recent migration flows, with large numbers of women out of the country, created a surprising sex-ratio in the adult resident (de jure) population: 108 in the 30-39 age group. These patterns are unique in Africa, and signal an imbalance between sexes, which probably played a role in the spread of HIV/AIDS (Modo Reference Modo2001). Marriage is nearly universal in Lesotho, with some 6% of men never married by age 50 and 7% of women never married by age 40, values higher than the African average, but much lower than those in South Africa (Garenne Reference Garenne2016).
HIV in Lesotho
The dynamics of the HIV epidemic followed closely these in South Africa. First cases of HIV/AIDS were detected in 1986, followed by a rapid increase in seroprevalence between 1990 and 2004, and a stagnation afterwards due to maturation of the epidemic, changing behaviour, and arrival of anti-retroviral therapy (ART). By 2004, the level of HIV infection in Lesotho was comparable to that of the Black/African population in South Africa. HIV spread all over the country, with minor differences in seroprevalence by geographical location (urban and rural areas, ecological zone, district) (Lesotho/DHS 2005, 2010, 2016). The HIV epidemic in Lesotho had a mortality impact similar to that found in South Africa, with a major drop in life expectancy between 1990 and 2004, followed by a recovery (Gona et al. Reference Gona, Gona, Ballout, Rao, Kimokoti, Mapoma and Mokdad2020).
Circumcision in Lesotho
The Sotho tribes were probably used to practice male circumcision in the past, as many groups all over Africa. Circumcision was part of the initiation ceremony of young men (Lebollo la banna), a rite of passage from childhood to adulthood found in most African traditional societies (Mohlaloka et al. Reference Mohlaloka, Jacobs and de Wet2016). Traditional circumcision occurred at the end of adolescence, around age 16-20 (average = 18.8 years), sometimes earlier or later, and somewhat later on average than among other African groups. The practice of circumcision seems to have changed in the early 20th century, because Christian missionaries, as well as British authorities during the Protectorate, discouraged the practice which was causing numerous casualties. For instance, the Roman Catholic Church threatened its followers of excommunication in case of circumcision, and Chief Lentshwe, who converted to Christianity in 1892, prohibited circumcision. As a result, at the onset of HIV epidemic (1990) about half of adult men were circumcised. At the 2004 DHS, 51.4% of men age 15-59 were circumcised, with little variations by district (38.9% to 66.5%), and little variations by religious affiliation (47.5% among Roman Catholics; 48.3% among Evangelicals; 51.5% among Anglicans; 57.5% among Other Christians). This situation is rare in Africa, and constitutes a quasi- experimental situation, with similar numbers of circumcised and intact men, and no interaction with ethnicity or religion: a single ethnic group, almost all Christians, little differences by Church, whereas in other African countries circumcision is usually an ethnic or a religious custom. Traditional circumcision seems to have remained stable in the years 1970-2007, before campaigns of Voluntary Medical Male Circumcision (VMMC) were launched after 2008, following a recommendation by the World Health Organization, and financed largely by “The United States President’s Emergency Plan for AIDS Relief” (US-PEPFAR) and other international agencies (WHO 2012).
Data and Methods
DHS surveys
Lesotho conducted three Demographic and Health Surveys (DHS) with information on HIV and circumcision, every five years, in 2004, 2009, and 2014, the first survey being conducted at the peak of the HIV epidemic (Lesotho/DHS 2005, 2010, 2016). Circumcision was defined as declared by the respondent, and medical circumcision (VMMC) was asked only in 2009 and 2014, as there were very few medical circumcisions in 2004. HIV infection was defined by positive serology in a blood test. Details of the laboratory procedures are provided in the final reports (Lesotho/DHS 2005, 2010, 2016). In brief, blood samples are tested with two ELISA tests, and in case of discrepancy a Western Blot test is processed.
The DHS’s provide numerous indicators of socio-economic status, used in this study: urban residence, ecological zone, household wealth (quintile), and level of education defined by years of schooling. The DHS’s also provide several indicators of exposure to risk of STI’s and sexual behaviour: marital status, number of spouses (polygamy), age at first marriage, age at first sexual intercourse, life-time number of sexual partners, paid for sex in past 12 months, and condom use in past 12 months. Unfortunately, there was no information on previous migration to South Africa. For the statistical analysis, most variables were used as provided by the surveys, with some basic recoding, and traditional circumcision was distinguished from VMMC. For the multivariate analysis, age was replaced by the duration since first sexual intercourse, to better estimate the exposure to sexual transmission of HIV. Wealth was represented by the wealth quintiles defined by the DHS’s. Level of education was grouped into four categories of years of schooling: ‘0-1’; ‘2-4’; ‘5-7; ‘8+’. Other variables were taken as defined by the DHS.
Methods
The statistical investigation of the relationship between circumcision and HIV infection was conducted using classic cross-tabulations, and by linear-logistic regression on the probability of being infected, coding 1 for HIV positive and 0 for HIV negative. Several regression models were tested, independent variables being circumcision status and type of circumcision, socio-economic variables, exposure variables, and time trends. Calculations were done with SPSS-17. Details of the variables used in each model are provided in the results section.
Results
DHS samples
The three Lesotho DHS’s were based on representative samples of about 9,000 households and 11,000 men age 15-59 each, of which about a third was sampled for in-depth interview and HIV testing. The pooled sample used for this analysis included 9045 men, of which 8075 had complete information on HIV and circumcision (Table 1). In 2004, 48.1% of men were circumcised, 52.0% in 2009, and 72.2% in 2014, showing a major push of the VMMC campaigns: some 20.2% of the male adult population were circumcised within five years. HIV prevalence hardly changed over the period, with 18.8% (2004), 18.4% (2009) and 19.5% (2014) men infected. If anything, HIV prevalence did not decline over the period, and this was confirmed by a more recent survey conducted in 2016/2017 (Lesotho/PHIA 2019). These values were consistent with HIV prevalence among Black/African men age 15-59 in nearby South Africa: 18.1% in 2002, and 17.5% in 2017 (South Africa/HSRC, 2002, Reference Shisana, Rehle, Simbayi, Zuma, Jooste, Zungu, Labadarios and Onoya2014).
Table 1. Sample size of demographic surveys and basic characteristics, Lesotho, men age 15-59

Sources: Population: United Nations, Population Division, World Population Prospects, 2019. Other indicators: DHS survey reports and Author’s calculation from DHS surveys. TFR: Total Fertility Rate (children per woman); Under-five mortality: deaths of children age 0-4 per 1000 live births; Adult mortality: deaths of adults age 15-49 per 1000 survivors at age 15. (ns) not statistically significant.
The dynamics of the HIV epidemic varied by circumcision status: HIV prevalence was lower among the intact groups in 2004, but increased steadily from 15.1% to 21.6% over the period, whereas prevalence among the circumcised groups declined somewhat from 22.6% to 18.7%. As a result, the relative risk of HIV infection by circumcision status changed over the period, and declined from 1.49 [95% CI: 1.20-1.86] to 0.86 [95% CI: 0.70-1.06], the difference being statistically significant (P = 0.0004) (Table 1).This could be due to a variety of confounding factors, such as age (older men are more often intact and more infected), socio-economic status, changing behaviour, or the effect of VMMC.
The samples of adult men were more and more urbanized (from 21.5% to 33.8%), as in the general population. The proportion of men absent from the household, most of them migrant to South Africa, stayed at very high levels, and fluctuated from 33.1% to 39.7 %, without any obvious trend. In terms of exposure to sexual transmission of HIV, little changes could be observed over the study period: the median age at first intercourse declined somewhat (from 20.1 to 18.9 years), the mean age at first marriage increased somewhat (from 24.7 years to 26.0 years), and the proportion currently married decreased somewhat (42.6% to 40.0%). Polygyny is not frequent in Lesotho, and changes over the 2004-2014 period were not statistically significant (Table 1).
VMMC campaigns
By 2014 PEPFAR estimated that 84,735 men had been circumcised by the new VMMC campaign, that is 27.2% of the target population age 15-29 (UNAIDS 2019; WHO 2017). This corresponds roughly to the 2014 DHS estimate of 31.2% circumcised by a medical officer in the age group 15-29, whereas the proportion of men age 15-29 circumcised traditionally hardly changed over the years (39.9% in 2004, 43.3% in 2009, 39.4% in 2014). This reveals the magnitude of the VMMC campaign, which reached about half of the intact population within a few years. This effort was continued, and by 2018 196,456 men had been circumcised, close to the whole target population. On average VMMC was done at age 20.4 years, just above the median age at first sexual intercourse. However, it should be noted that medical circumcision was not restricted to the 15-29 target population. According to the 2014 DHS, only 72% of medical circumcisions were done at age 15-29, while 15% were done at age 0-14 and 13% above age 30. If this age distribution is taken into account, either the number of VMMC was under-estimated (possibly conducted by programs other than PEPFAR), or the proportion medically circumcised was over-estimated by the DHS. The change in the proportions circumcised appears clearly when the same cohorts are compared in 2004 and 2014: a major increase in proportions circumcised for men born after 1955 (age < 59 in 2014), and especially for the younger generations, so that 82.7% of men born in 1990-1994 (age 20-24 in 2014) were circumcised, a large increase from a baseline value of 58.6%, essentially due to the VMMC campaigns (Figure 1).
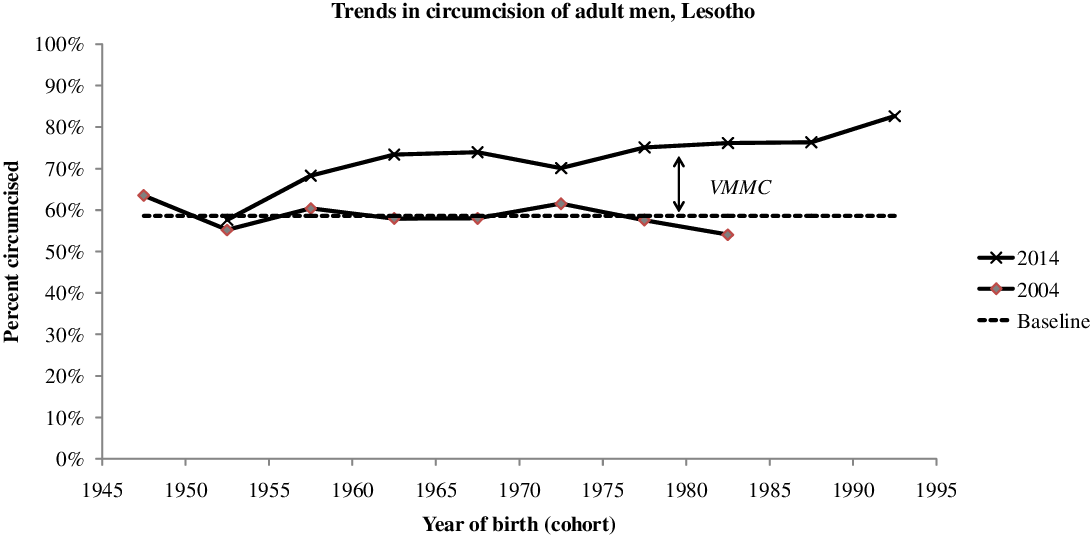
Figure 1. Trends in proportion of adult men circumcised, by year of birth, Lesotho, adult men.
Correlations of circumcision with socio-economic status
Male circumcision varied by demographic and socio-economic status (SES). Since traditional circumcision tended to be late in Lesotho, the prevalence of traditional circumcision in 2004 was about 60% in older adults (age 30-59), but only 40% at age 15-29. This difference changed with the VMMC campaigns and by 2014 prevalence of circumcision was about the same at all ages, ranging from 71% to 74% (Table 2). Differences between urban and rural areas were large in 2004 (from 32% to 52%), whereas they had almost disappeared by 2014 (68 to 74%). Differences were large by ecological zone (from 40% to 63%) in 2004, and were strongly reduced by 2014 (70% to 79%). Largest differences were found by wealth and education; they were also reduced over time, but had not disappeared by 2014. Traditional circumcision was concentrated in the poorer (70%) and least educated strata (79%), and even though prevalence increased over the years, it was only marginal (+4% and +5% respectively) in this group. In contrast, prevalence of circumcision was low among the wealthiest groups (30%) and the more educated (24%) at baseline, but increased considerably in these groups over the years (+40% and +39% respectively). In summary, there was a major push with the VMMC campaigns, and this push was concentrated in the least circumcised groups who were wealthier, more educated, more urbanized, and tended to live in the lowlands. There was no significant difference in the prevalence of traditional circumcision by religious affiliation.
Table 2. Demographic and socio-economic correlates of male circumcision, Lesotho, Men age 15-59
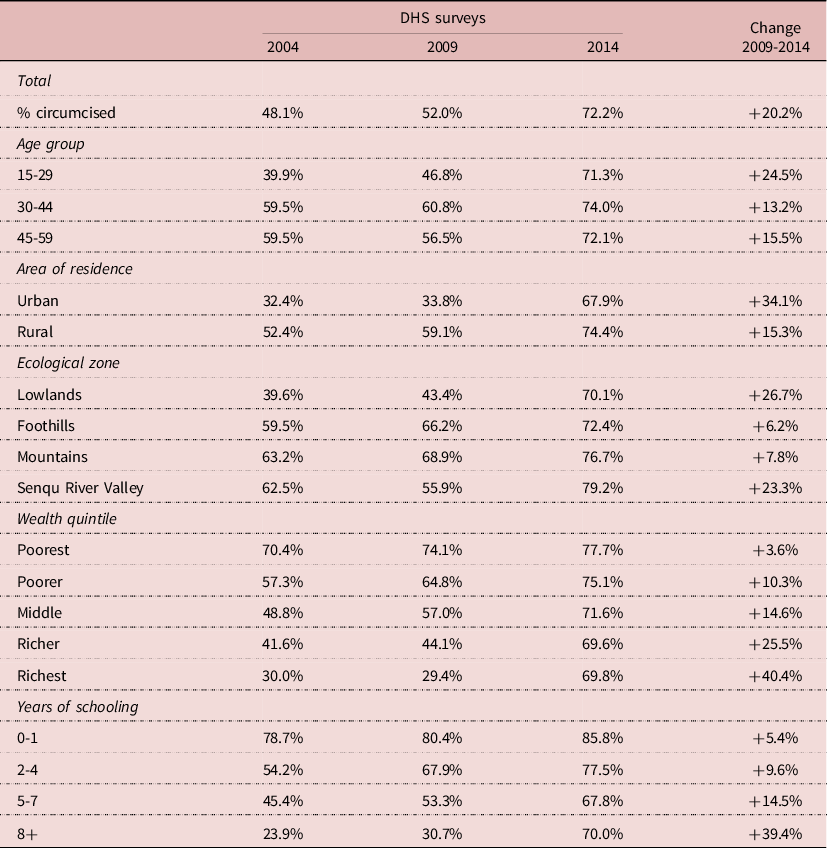
Source: Author’s calculation from DHS surveys
Correlations of HIV prevalence with socio-economic status
The pattern of HIV infection by socio-economic status was different, and often inversed compared with that of circumcision. Urban areas, lowlands, wealthier groups and less educated groups had higher HIV prevalence in 2004. HIV prevalence increased somewhat in the urban population whereas it tended to slightly decline in the rural population. HIV prevalence varied little by ecological zone, HIV being well spread all over the country: on average HIV prevalence was somewhat higher (+18%) in the lowlands, and the same in the other areas. The pattern of differentials by wealth was not linear, the group with the highest prevalence being the “richer” group; HIV prevalence declined somewhat only in the “poorer” group, but not in the others. When stratified by level of education, it tended to increase at all levels, and more so for the least educated. However, these differences remained small, and most of the time trends were not statistically significant (Table 3). Correlations between circumcision and HIV prevalence were inversed for some groups: more circumcision and less HIV for the more educated, for the poorer and middle wealth groups, for rural areas, for mountains, and for the 15-29 and 30-44 age groups, whereas the opposite pattern was found in the other groups (more circumcision associated with more HIV). These correlations show the complexity of the relationships between circumcision and HIV in populations.
Table 3. Demographic and socio-economic correlates of HIV infection, Lesotho, men age 15-59
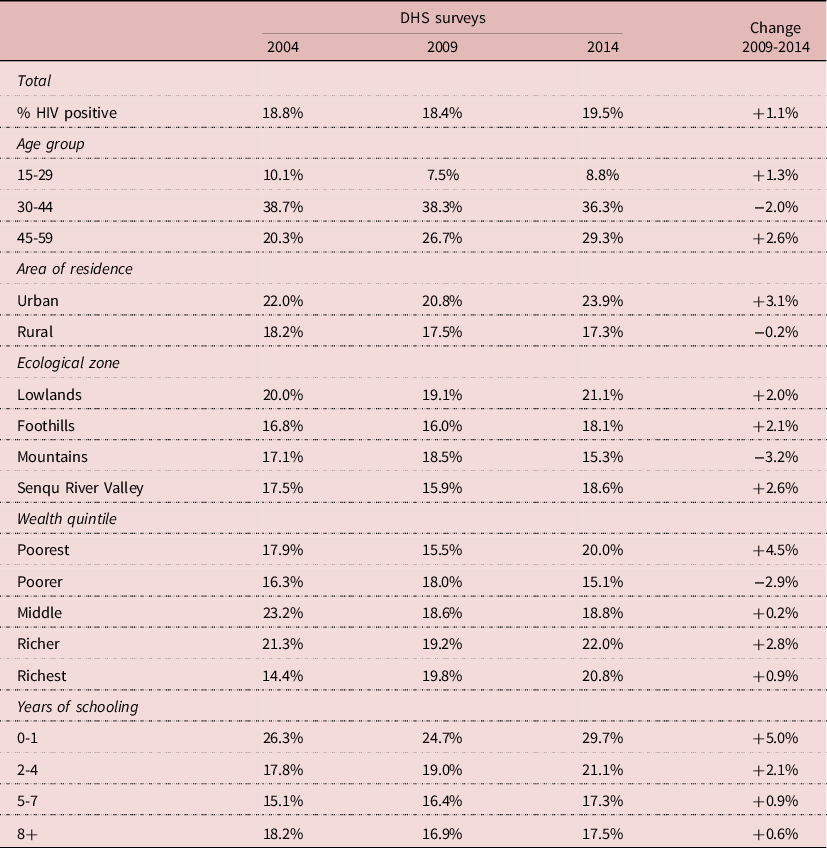
Source: Author’s calculation from DHS surveys
Interactions between HIV and circumcision and level of education
The relative risk (RR) of HIV infection by circumcision status changed with level of education. The relative risk tended to be higher at low level of education, and lower at high level of education. Furthermore, the pattern changed over time, especially at higher level of education, leading to a low 0.68 at high level of education in 2014 compared with a high 1.27 at low level of education in 2004, the difference being statistically significant (P= 0.013). This indicates a changing behaviour or a changing situation, possibly due to VMMC (Table 4).
Table 4. Relative risk of HIV infection by circumcision status and by level of education, Lesotho, men age 15-59
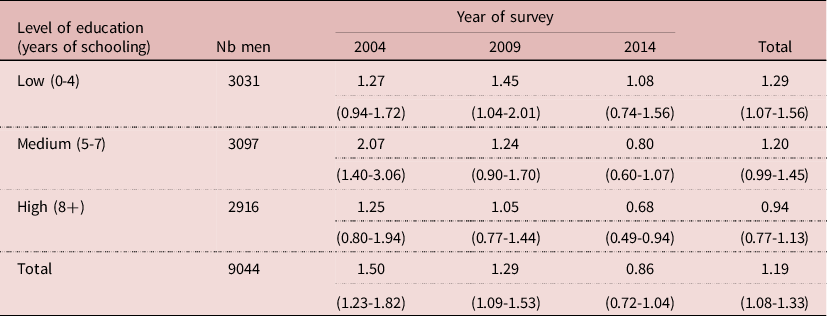
NB: RR= % HIV+ if circumcised/intact; (95% confidence interval)
Multivariate analysis of HIV prevalence
Various linear-logistic regression models were tried, linking HIV prevalence with circumcision, socio-economic status, exposure variables and other available variables. A parsimonious model included circumcision, duration since first sexual intercourse, marital status, number of unions, polygamy, number of sexual partners, wealth quintile and level of education. In this model, the effect of circumcision was nil (RR= 0.955, P= 0.495), whereas the effects of all other variables were highly significant, with P-values < 0.01 (The RR was 1.19 in univariate analysis). For exposure variables, duration since first sex induced an increase in prevalence of 15.3% for each additional 10 years; being ever-married multiplied the risk by 3.38; each additional union increased the risk by 1.61; polygyny reduced the risk by 33%; and 5-more partners increased the risk by 4.8%. These findings indicate that many cases of HIV transmission occur within unions. In addition, more wealth increased prevalence (RR= 1.69 for maximum quintile), whereas more education decreased prevalence (RR= 0.70 for 8 or more years of education). Due to the large sample size (8075 men), results were stable and significant, which gives a strong value to the lack of effect of circumcision (Table 5).
Table 5. Results of multivariate analysis of HIV infection, Lesotho, men age 15-59 (N= 8075 men)
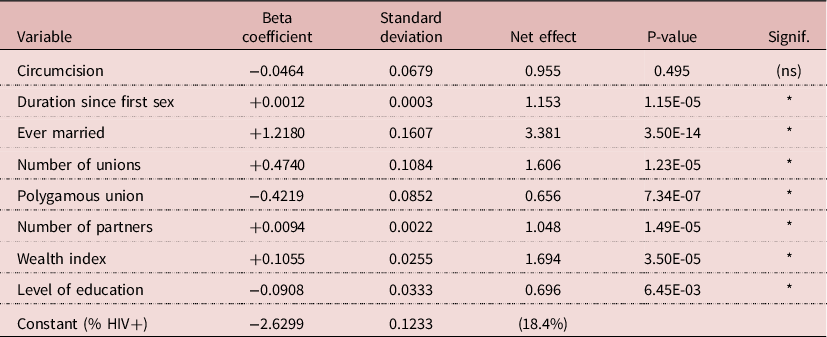
NB: Net effect for variables other that dummies: Duration since first sex = 10 years; Number of unions = each additional union; Polygamous union = each additional wife; Number of partners = five additional partners; Wealth = maximum of 5 categories; Education = maximum of 4 categories.
Adding other variables in the model
Adding other variables to the parsimonious model did not add any important information, and created perturbations due to multi-collinearity. Adding urban residence (RR= 1.19, P= 0.034) increased the effect of wealth, but decreased the effect of education. Adding ecological zone also had little effect: the lowlands had higher risk (RR= 1.24, P=0.002), with the same interactions with wealth and education as urban residence. Adding both urban residence and ecological zone wiped out the effects of wealth and urban residence. When added, the effect of condom use was positive, most likely because of reverse causality (men with HIV being more likely to use condoms). When added, the effect of ‘paid for sex’ was not significant.
Adding VMMC in the model
If circumcision never showed any effect on HIV prevalence in any model, this was not the case for VMMC. The 2014 sample, most appropriate for this testing, was too small to show the effect of VMMC (RR= 0.793; P= 0.129), and adding VMMC reduced the effect of education. When VMMC was included in the model with the three surveys, a significant effect was found (RR= 0.684; P= 0.002). But adding VMMC increased the net effect of circumcision, which became above 1 (still not significant), it decreased the effect of wealth, and that of education became not significant, indicating again interactions between education and VMMC. The net effect of VMMV could be shown by reconstructing the predicted value of HIV prevalence with and without VMMC for men age 50, who had first sexual intercourse at age 18, ever married, married only once in monogamic union, with 5 other partners. Calculations indicate that for poor and uneducated persons, HIV prevalence would be 37.8% without VMMC, and 30.0% with VMMC; for wealthy and educated persons, HIV prevalence would be 44.4% without VMMC, and 36.3% with VMMC. In both cases HIV prevalence would be reduced by about 20%. This could be compared with empirical values. For the 30-39 age group in 2014, the age group most likely to show an effect of VMMC, HIV prevalence was 36.6% in the intact group, 32.0% in the traditional circumcision group, and 33.1% in the VMMC group, that is an absolute difference of −3% compared with other groups. Similar differences were found at age 20-29, 40-49, and 50-59. Even though these differences were not statistically significant, they suggest a small correlation with VMMC. Whether this effect is due to the surgical procedure, to a selection bias, or to behaviour change is another issue (Table 6).
Table 6. Comparison of HIV prevalence by circumcision status, Lesotho, 2014 DHS, men age 20-59 (N= 2080)
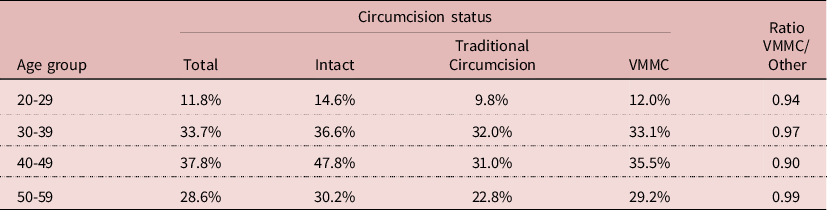
NB. Differences not statistically significant (P>0.05)
Correlates of VMMC in 2014
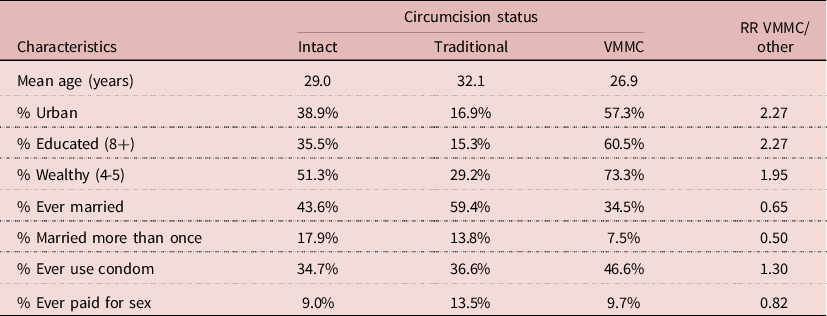
Many correlates of VMMC could be found in the 2014 DHS. Men who underwent VMMC were on average younger (mean age 26.9 years), more urbanized (57.3%), more educated (60.5%), and wealthier (73.3%) than either intact men or men who were traditionally circumcised (Table 7). In addition, they were less likely to be ever-married or married more than once. More important, they were more likely to use condoms (46.6%) than the two other groups. In multivariate analysis, after controlling for age, wealth, education and HIV status, condom use was still higher among VMMC men (RR= 1.39; P= 0.015). This indicates that even when controlling for exposure and socio-economic variables, the net effect of VMMC could be due to behaviour, in particular condom use, and not to the surgical procedure. Furthermore, VMMC could have selected a group of persons who were more aware of STD’s and less likely to take risks than others.
Table 7. Characteristics of men age 15-59 by circumcision status, Lesotho, 2014 DHS (N= 2926 men)
HIV and circumcision among couples
The DHS’s allow one to investigate HIV transmission within couples, by matching the HIV serostatus of husband and wife. The sample of available data was smaller, with 2068 couples with complete information. Overall, 84% of couples were concordant, whatever husband’s circumcision status, that is either both spouses not infected or both infected, which underlines the importance of HIV transmission within stable unions in Lesotho. HIV prevalence was always somewhat higher among husbands than wives, most likely because of age differences between spouses (+5.4 years). Differences in HIV prevalence in couples by husband’s circumcision status were not statistically significant, neither by VMMC status. The proportion of HIV infected women whose husband was infected was 65.6%, and the same in the three groups: 71.3% for intact husband, 63.6% for husband circumcised traditionally, and 64.5% for VMMC husband, none of the differences being statistically significant. Likewise, the proportion of HIV infected men whose wife was infected was 72.0%, and the same in the three groups: 77.3% for intact husband, 67.7% for husband circumcised traditionally, and 71.4% for VMMC husband, none of these differences being statistically significant. This indicates that when exposure is intense, as in a stable couple, circumcision (traditional or medical) has no effect on HIV transmission either way, from female to male as from male to female (Table 8).
Table 8. Relationship between HIV and circumcision in couples, Lesotho, DHS surveys
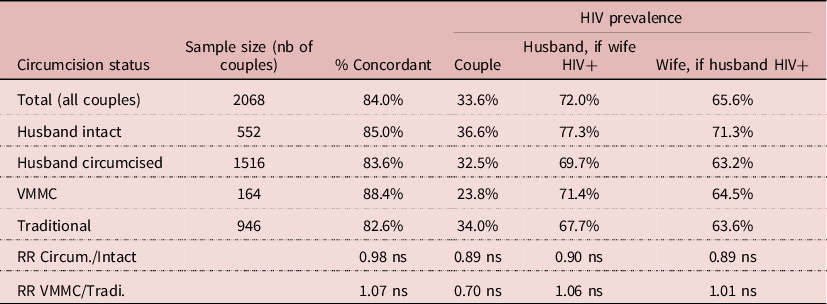
NB: (ns) difference not significant, P > 0.05
Discussion
The relationship between circumcision and HIV infection in real life population is a complex matter, likely to be country specific, or even specific to social groups, and likely to evolve over time, because of numerous interactions with selection biases and with behavioural change. This is why results differ from observations in RCT’s. Results from RCT’s could be transferred to populations only if exposure were uniform and behaviour constant, which obviously is not the case.
The Lesotho study did not find any effect of circumcision at individual level in multivariate analysis, after controlling for other factors. In addition, increasing circumcision by VMMC campaigns did not have any visible effect on trends in HIV prevalence. Even when including the 2016/2017 PHIA survey, changes in time trend of HIV prevalence among men age 15-34 between the 2004-2009 period (slope= −0.059 before VMMC) and 2009-2016 (slope= −0.046 after VMMC) were not significant (P= 0.582). Circumcising 20% of the male population, and about half of the intact men, did not have any effect on HIV trends. The study was unable to demonstrate any effect of circumcision, whether medical or traditional, on HIV transmission within couples, either way from male to female or from female to male.
In further multivariate analysis, there was a small correlation between VMMC and HIV prevalence: men who underwent VMMC had a 20% lower level of infection, after controlling for exposure. This could be due to selection bias or to a behavioural effect such as increased condom use, and unlikely to be due only to the surgical procedure. Whatever the mechanism, men age 30-39 had similar and high levels of HIV infection, irrespective of the circumcision status and the type of circumcision.
Even if one admits the net effect of VMMC, a massive campaign in a country such as Lesotho is unlikely to have any major impact on HIV prevalence in the population. With a 20% reduction in HIV infection and 20% of men undergoing VMMC, the reduction in HIV prevalence would be small (say from 20.0% to 19.2%), a small value hardly detectable in demographic surveys. These values are probably close to reality, and the small effect seems most likely due selection bias and to behavioural change, and not to the surgical procedure.
The VMMC campaigns hit not only young men and children but also older men. Circumcising men above age 30 makes no sense in this situation, since about a third of men (33.9%) were already infected by HIV. Unfortunately, the HIV status was not verified prior to circumcising. For children and minors, circumcision raises serious questions about medical ethics, informed consent, and children’s right to body integrity (Garenne Reference Garenne2007; Svoboda Reference Svoboda2013). Circumcision is also associated with numerous complications, especially when performed among minors (pain, loss of sensitivity, bleeding, infection, wounds, laceration, injury, meatal stenosis, etc.), some of which severe and definite, including death (Muula et al. Reference Muula, Prozesky, Mataya and Ikechebelu2007; Wilcken et al. Reference Wilcken, Keil and Dick2010).
This study has several limitations. Firstly, the analysis relied on self-reported circumcision status, which could be questioned, as there are sometimes confusion between the initiation ceremony and the circumcision act. In addition, the surgical procedure could be incomplete, and circumcision be partial, in particular in case of traditional circumcision. However, it should be noted that the information on traditional circumcision was consistent across surveys, and that the numbers of VMMC declared in surveys was consistent with VMMC program reports. Another limitation is that other confounding factors could have been omitted. This is the case in particular for migration to South Africa, which is a critical exposure factor in Lesotho. However, since a large fraction of the adult population worked or travelled to South Africa, this factor could have an effect on the level of infection, but is unlikely to affect the relative risk of infection associated with circumcision.
Many African countries were able to curb the course of the HIV epidemic with behavioural change and condom use, the best documented case being Uganda in the 1990’s, without any help of VMMC (Low-Beer & Stoneburner Reference Low-Beer and Stoneburner2003; Okware et al. Reference Okware, Kinsman, Onyango, Opio and Kaggwa2005; Stoneburner & Low-Beer Reference Stoneburner and Low-Beer2004). In contrast, despite VMMC, HIV prevalence did not decline in Lesotho. A large amount of money was spent on VMMC without much result. Was this effort worthwhile and money spent appropriately? Could it be better spent on more efficient health programs and health education?
Acknowledgements
This study did not receive any external grant. The author thanks the DHS programme and the Lesotho Bureau of Statistics for providing free access to survey data.
Conflict of interest
None declared.











