Introduction
Androphilia refers to sexual attraction and arousal towards adult males whereas gynephilia refers to sexual attraction and arousal towards adult females. Numerous studies have shown that older brothers increase the odds of androphilia in later-born males. The observed increase in odds is typically between 15 and 50% per older brother (e.g. Blanchard & Bogaert, Reference Blanchard and Bogaert1996; Blanchard et al., Reference Blanchard, Zucker, Siegelman, Dickey and Klassen1998; Blanchard & Lippa, Reference Blanchard and Lippa2007). This phenomenon has been termed the fraternal birth order effect. This effect is most easily demonstrated when the mean number of older brothers is elevated among androphilic, compared with gynephilic, males and the mean sum of other siblings (i.e. older sisters+younger brothers+younger sisters) is similar across these groups. If the mean sums of other siblings are not similar, or if there are other large demographic differences between groups, statistical corrections are sometimes needed to see the effect (e.g. Blanchard, Reference Blanchard2014).
The best-developed explanation of the fraternal birth order effect is the maternal immune hypothesis (Blanchard & Bogaert, Reference Blanchard and Bogaert1996; Bogaert & Skorska, Reference Bogaert and Skorska2011). This hypothesis argues that antigens from male fetuses’ cells enter maternal circulation during pregnancy, promoting an immune response to these male-specific antigens. This immune response would, in turn, produce long-lasting effects on the brain of the male fetus, preventing its neurons from making a male-typical pattern of connections, resulting in attraction towards men rather than women. In its general form, the maternal immune hypothesis does not specify which male-specific proteins are most likely to be involved. Based on considerations like tissue distribution and prenatal expression, it has been conjectured (see Blanchard et al., Reference Blanchard, Zucker, Cavacas, Allin, Bradley and Schachter2002; Blanchard, Reference Blanchard2004) that two likely proteins are PCDH11Y (Blanco et al., Reference Blanco, Sargent, Boucher, Mitchell and Affara2000) and NLGN4Y (Jamain et al., Reference Jamain, Quach, Betancur, Rastam, Colineaux and Gillberg2003). As noted by Blanchard (Reference Blanchard2008), the maternal immune hypothesis does not challenge the long-standing theory that sexual orientation is primarily influenced via prenatal sex hormone exposure; rather, it proposes that sexual orientation in the human male brain is influenced by two systems: one driven by prenatal sex hormones and a supplementary system driven by male-specific proteins under direct genetic control.
Several lines of research support the plausibility of the maternal immune hypothesis. To begin with, fetal cells and fetal molecular material have been found in maternal circulation during early pregnancy and postpartum, a phenomenon called microchimerism (Lo et al., Reference Lo, Lo, Watson, Noakes, Sargent, Thilaganathan and Wainscoat1996; O’Donoghue et al., Reference O’Donoghue, Chan, de la Fuente, Kenna, Sandison and Anderson2004; Gammil et al., Reference Gammil, Guthrie, Ayedelotte, Adams Waldorf and Nelson2010). Further evidence points to a specific T-cell-mediated immune response towards antigens arisen from the Y-chromosome, called male antigens (HY) (Piper et al., Reference Piper, McLarnon, Arrazi, Horlock, Ainsworth and Killby2007; Khan & Baltimore, Reference Khan and Baltimore2010; Lissauer et al., Reference Lissauer, Piper, Goodyear, Kilby and Moss2012; Dierselhuis et al., Reference Dierselhuis, Jankowska-Gan, Blokland, Pool, Burlingham, van Halteren and Goulmy2014) as well as polymorphisms to minor histocompatibility complexes (Christiansen et al., Reference Christiansen, Kolte, Dahl, Larsen, Steffensen, Nielsen and Hviid2012), which might play a significant role in the maternal immune response to male fetuses. Additional evidence shows higher prevalence of male fetus miscarriages in women with a supposedly more HY-reactive HLA (Hiby et al., Reference Hiby, Regan, Lo, Farrell, Carrington and Moffett2008; Nielsen et al., Reference Nielsen, Steffensen, Varming, Van Hakteren, Spierings and Ryder2009), and the number of sons a woman has throughout life has been associated with age-linked inflammation (Marttila et al., Reference Marttila, Nevalainen, Kananen, Jylhävä, Jylhä, Hervonen, Ilonen and Hurme2015), thus providing further evidence of a male-specific maternal immune response.
In addition, the fraternal birth order effect does indeed appear to be prenatal in origin. First, relatively low birth weight provides a marker of prenatal exposure to a maternal immune response (for review, see VanderLaan et al., Reference VanderLaan, Blanchard, Wood, Garzon and Zucker2015) and androphilic males who have older brothers exhibit lower birth weights (Blanchard & Ellis, Reference Blanchard and Ellis2001; Blanchard et al., Reference Blanchard, Zucker, Cavacas, Allin, Bradley and Schachter2002; VanderLaan et al., Reference VanderLaan, Blanchard, Wood, Garzon and Zucker2015). Hence, even at the time of birth, there seems to be a physical marker of sexual orientation (i.e. birth weight) that is related to the number of older brothers. Second, Bogaert (Reference Bogaert2006) examined the association between male sexual orientation and biological siblings (i.e. born from the same mother) and non-biological siblings (i.e. adoptive, step or paternal half-siblings). Whether and how long probands were reared with these siblings was also considered. Biological older brothers significantly predicted male sexual orientation regardless of whether or how long probands were reared with these brothers. In contrast, the remaining sibling categories, including non-biological older brothers, did not.
By virtue of suggesting that the fraternal birth order effect is prenatal in origin, the maternal immune hypothesis posits mechanisms that have the potential to operate in any situation where a mother gestates a male fetus in more than one pregnancy. As such, one would predict the fraternal birth order effect to be nearly ubiquitous – with the exception of populations where people do not have older brothers (e.g. China: Xu & Zheng, Reference Xu and Zheng2015). One approach for establishing the ubiquity of this effect has been to examine a variety of sample types. To date, the fraternal birth order effect has been documented in university and community convenience samples, national probability samples, clinical samples of male-to-female transsexuals, clinical samples of men who are primarily attracted to prepubescent or pubescent children, clinical samples of natal male children and adolescents who are likely to be androphilic as adults, and archival samples of men interviewed decades ago (for reviews, see Blanchard, Reference Blanchard1997, Reference Blanchard2004; Bogaert & Skorska, Reference Bogaert and Skorska2011; VanderLaan et al., Reference VanderLaan, Blanchard, Wood and Zucker2014; Blanchard & VanderLaan, Reference Blanchard and VanderLaan2015). In addition, this effect has been documented in several countries (e.g. Canada: Blanchard & Bogaert, Reference Blanchard and Bogaert1996; Italy: Camperio Ciani et al., Reference Camperio Ciani, Corna and Capiluppi2004; The Netherlands: Schagen et al., Reference Schagen, Delemarre-van de Waal, Blanchard and Cohen-Kettenis2012; Samoa: VanderLaan & Vasey, Reference VanderLaan and Vasey2011; Spain: Gómez-Gil et al., Reference Gómez-Gil, Esteva, Carrasco, Cruz Almaraz, Pasaro, Salamero and Guillamon2011; Turkey: Bozkurt et al., Reference Bozkurt, Bozkurt and Sonmez2015; UK: King et al., Reference King, Green, Osborn, Arkell, Hetherton and Pereira2005; USA: Schwartz et al., Reference Schwartz, Kim, Kolundzija, Rieger and Sanders2010).
Despite the consistency with which the fraternal birth order effect has been observed, there has been debate regarding its ubiquity and, by extension, the role of maternal immune factors in the development of male sexual orientation. Some studies failed to replicate this effect, raising scepticism about its importance (e.g. Currin et al., Reference Currin, Gibson and Hubach2015; Frisch & Hviid, Reference Frisch and Hviid2006; Kashida & Rahman, Reference Kishida and Rahman2015). Other research reported that androphilic males show elevations in older brothers and older sisters, raising the question of whether the male sexual orientation difference in birth order is specific to older brothers (e.g. King et al., Reference King, Green, Osborn, Arkell, Hetherton and Pereira2005).
Blanchard and VanderLaan (Reference Blanchard and VanderLaan2015) addressed both of these challenges. First, their re-analyses of the data presented by Frisch and Hviid (Reference Frisch and Hviid2006) and Kashida and Rahman (Reference Kishida and Rahman2015), respectively, indicated that the fraternal birth effect was, in fact, evident in these samples. Further, they noted that failures to replicate (Type II error) are to be expected in some proportion of studies, as is the case with any true effect. Second, they explained that number of older brothers tends to be correlated positively with number of older sisters. Thus, although one would expect to observe older sister effects in some proportion of samples, older sister effects should not be observed as consistently as older brother effects. Indeed, Blanchard and VanderLaan (Reference Blanchard and VanderLaan2015) presented a meta-analysis showing that only the older brother effect was reliably associated with male sexual orientation across previously published studies.
An additional means of continuing to evaluate the reliability and ubiquity of the fraternal birth order effect is to examine birth order in relation to male sexual orientation in previously unexamined populations. The present study did so by comparing numbers of older brothers and numbers of other siblings in a sample of Brazilian male-to-female transsexuals who are attracted to men vs a comparison group of non-transsexual gynephilic men.
Methods
Participants
Participants (N=261) were recruited at the Hospital de Clínicas de Porto Alegre (HCPA) from 2008 to 2013. All male-to-female transsexual participants (n=118) were at least 18 years of age and were patients of the Gender Identity Program (PROTIG) who met the DSM-IV-TR criteria for Gender Identity Disorder (GID; American Psychiatric Association, 2000). Prior to assessment by PROTIG, all had previously used hormonal medications without medical guidance, but none had undergone sex-reassignment surgery. Three individuals assessed by PROTIG were excluded from the present study because they evidenced psychotic symptoms that limited the ability to make an accurate diagnosis concerning GID. None had a disorder of sex development.
The comparison group of gynephilic men (n=143) consisted of medication-free volunteers who had no current, past history, or first-degree family history of a major psychiatric disorder, dementia or mental retardation. The sample was collected from non-psychiatric medical patients and companions at the outpatient clinics at HCPA, aged 18 years old or greater.
Measures
Male-to-female transsexuals and men completed a questionnaire about their age, year of birth and numbers of biological older and younger brothers and sisters from the same biological mother. Information regarding the sexual orientation of transsexual patients was obtained during semi-structured interviews with a psychiatrist (patients attended group and/or individual medical appointments on a biweekly basis). On the basis of this clinical information, all transsexuals were categorized as sexually attracted towards men. For the comparison group of men, they were asked to self-report their sexual orientation identity. All men self-reported a heterosexual sexual orientation identity (i.e. gynephilia, sexual attraction towards women).
Ethics statement
This research was approved by the institutional research ethics review board at the Hospital de Clínicas de Porto Alegre (HCPA).
Results
Table 1 shows descriptive statistics regarding age, year of birth and numbers of older brothers and other siblings by group. Male-to-female transsexuals were significantly younger (Levene’s test for equality of variances: F=35.53, p<0.001, two-tailed independent samples t-test, t(231.71)=−5.48, p<0.001) and had significantly later years of birth (Levene’s test for equality of variances: F=39.18, p<0.001, two-tailed independent samples t-test, t(227.64)=3.54, p<0.001). The correlation between age and year of birth was near perfect (two-tailed Pearson’s r=−0.99, p<0.001), indicating that these variables were redundant with respect to the information they provided. As such, only age was retained as a control variable when comparing groups on sibship composition.
Table 1 Descriptive statistics

a Number of older sisters+younger brothers+younger sisters.
Table 2 summarizes the results of a logistic regression examining group differences in sibship composition. Group membership was the criterion variable with the male-to-female transsexuals coded as 1 and the control men coded as 0. Predictors in the model included: age, number of older brothers, number of other siblings, the interaction between age and number of older brothers, and the interaction between age and number of other siblings. All predictors were first centred to reduce multicollinearity and then entered in the model simultaneously to identify the unique contribution of each variable to predicting group membership (i.e. male-to-female transsexuals vs men). Male-to-female transsexuals had significantly more older brothers and significantly more other siblings. In addition, there was a significant interaction between age and number of other siblings such that probands in the present sample who were younger and had larger numbers of other siblings were more likely to be male-to-female transsexuals.
Table 2 Logistic regression predicting group membership
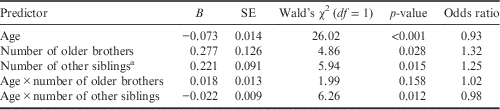
Group is the criterion variable with men coded as 0 and male-to-female transsexuals coded as 1.
a Number of older sisters+younger brothers+younger sisters.
Discussion
The present study examined the fraternal birth order effect in Brazil, a previously unexamined population. Consistent with the maternal immune hypothesis and numerous previous studies conducted in other populations, Brazilian male-to-female transsexuals who reported sexual attraction towards men had significantly greater numbers of older brothers than a comparison group of gynephilic non-transsexual men. Importantly, this effect was independent of numbers of other siblings, thus providing further evidence of the unique contribution of older brothers to the development of same-sex sexual orientation among males.
In addition to documenting the fraternal birth order effect in the present sample, the odds ratio associated with this effect is noteworthy. Each additional older brother increased the odds of being in the male-to-female transsexual group by 32% (see Table 2). This value falls in the middle of the range of 15–50% reported previously (Blanchard & Bogaert, Reference Blanchard and Bogaert1996; Blanchard et al., Reference Blanchard, Zucker, Siegelman, Dickey and Klassen1998; Blanchard & Lippa, Reference Blanchard and Lippa2007) and is remarkably similar to the values of 33% reported for a Canadian sample (Cantor et al., Reference Cantor, Blanchard, Paterson and Bogaert2002) and 34% reported for a Samoan sample (VanderLaan & Vasey, Reference VanderLaan and Vasey2011). Thus, across diverse populations, the fraternal birth order effect has been documented and each additional older brother contributes similarly to the odds of developing an androphilic sexual orientation in natal males. These patterns are consistent with the maternal immune hypothesis and suggest that the influence of older brothers on male sexual orientation development is ubiquitous.
It is less clear, however, whether older brothers, via maternal immune mechanisms, have a more general influence on male psychosexual development that includes the domains of gender behaviour and identity in addition to sexual orientation. Gay men tend to exhibit elevated cross-gender behaviour and identity during childhood (Bailey & Zucker, Reference Bailey and Zucker1995; Rieger et al., Reference Rieger, Linsenmeier, Gygax and Bailey2008) and report some female-typical characteristics during adulthood (for review, see Lippa, Reference Lippa2005). Also, as was the case with the present study, many studies documenting the fraternal birth order effect examined samples of natal males who exhibited marked cross-gender behaviour and identity (for review, see VanderLaan et al., Reference VanderLaan, Blanchard, Wood and Zucker2014). As such, some have suggested that fraternal birth order may not only relate to sexual orientation, but also to female-typical gender expression and identity, among androphilic natal males (Wampold, Reference Wampold2013; VanderLaan et al., Reference VanderLaan, Blanchard, Wood, Garzon and Zucker2015).
To date, data bearing on this issue are limited. Two studies did not find associations between numbers of older brothers and female-typical characteristics among gay men (Bogaert, Reference Bogaert2003; Rahman, Reference Rahman2005; although see VanderLaan et al., Reference VanderLaan, Blanchard, Wood, Garzon and Zucker2015, for recent insights into why these studies may have not found such an effect). In four other studies, there was no fraternal birth order effect among clinical samples of natal males who exhibited marked cross-gender behaviour and identity but did not report predominant sexual attraction towards males (Blanchard & Sheridan, Reference Blanchard and Sheridan1992; Blanchard et al., Reference Blanchard, Zucker, Cohen-Kettenis, Gooren and Bailey1996; Green, Reference Green2000; VanderLaan et al., Reference VanderLaan, Blanchard, Wood and Zucker2014). Thus, although further data are needed to address this issue, it appears that if the fraternal birth order effect and maternal immune hypothesis apply to variation in natal male gender expression as well as sexual orientation, then they probably only apply to natal males who exhibit both cross-gender characteristics and androphilic sexual orientation.
Apart from an older brother effect, two additional effects were observed in the present study. First, male-to-female transsexuals had greater numbers of siblings other than older brothers. It is important to note that this effect was independent of the older brother effect discussed above. Furthermore, it is not uncommon for other sibling category effects to be observed in studies of birth order and male sexual orientation, although they are observed with less regularity than older brother effects (for review, see Blanchard & VanderLaan, Reference Blanchard and VanderLaan2015). As such, the presence of the other sibling effect in the present sample (Table 2) is not inconsistent with research on this topic, the fraternal birth order effect or the maternal immune hypothesis. Second, there was an interaction between age and number of other siblings in the prediction of group such that the transsexual probands were more likely to be younger and to have more other siblings. Such a finding has not been reported in the literature previously and there is no a priori reason to expect such a pattern. Unless this pattern is replicated in future studies, the most reasonable explanation is that this interaction effect was due to some form of sampling bias and is, therefore, unlikely to be theoretically meaningful.
Acknowledgments
DPV was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship and by the University of Toronto Mississauga.




