Introduction
The seasonality in forage production occurs due to the variability of climatic conditions throughout the year, leading to a reduction in the quantity and quality of forage and, consequently, animal performance (Souza et al., Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello, Muir, Ribeiro and Dubeux2021; Tlahig et al., Reference Tlahig, Nejj, Atoui, Seddik, Dbara, Yahia, Nagaz, Najari, Khorchani and Loumerem2024). In Brazil, the genus Urochloa spp. occupies around 138 million ha (Corrêa et al., Reference Corrêa, Bonetti, Barrios, Valle, Torres and Techio2020). The species U. decumbens (Stapf) R. Webster (signal grass) is widely cultivated for its high persistence, productivity, tolerance to acidic and low fertility soils, and adaptability to a wide range of Brazilian soil and climate conditions (Machado et al., Reference Machado, Fonseca, Lima, Martuscello, Paciullo and Chizzotti2020; Lara et al., Reference Lara, Silva, Sollenberger and Pedreira2021).
Urochloa decumbens has been widely used in extensive pasture systems, generally, the increase in production is obtained through the expansion of areas. These extensive pasture systems often display poor management, including overgrazing and insufficient fertilization, associated with the absence or inadequate cattle supplementation. This increases the risk of pasture degradation, leading to lower animal performance and reduced gains per area, especially in U. decumbens monocultures. It is estimated that 50–70% of Brazilian pastures are in different stages of degradation, mainly due to poor establishment and inadequate pasture management practices (Lapig, 2022).
The agricultural sector has been challenged over the years to adopt new technologies or other agronomic practices to maximize forage accumulation (FA), whether through the association of organic fertilization and mulch (Izidro et al., Reference Izidro, Souza, Vieira Leite, Simões, Silva and Tabosa2024), intercropping and mulch (Araújo Jr et al., Reference Araújo, Morais, Steidle Neto, Souza, Alves, Silva, Leite, Silva, Jardim, Montenegre and Silva2023), mainly grasses with legumes (Carvalho et al., Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a; Pessoa et al., Reference Pessoa, Cunha, Mello, Santos, Soares, Camelo, Apolinário, Dubeux Júnior and Coêlho2024; Santos et al., Reference Santos, Dubeux, Santos, Costa, Coêlho, Santos, Silva, Oliveira, Apolinário and Coêlho2024) to establish production systems capable of maintaining or improving the environment, through sustainable intensification (Dubeux Jr et al., Reference Dubeux, Muir, Apolinário, Nair, Lira and Sollenberger2017). Pursuing these objectives, several studies have focused on exploring agroforestry systems (Kumar et al., Reference Kumar, Roy, Kumar, Gautam, Singh, Ghosh, Singh and Koli2022; Moreno-Galván et al., Reference Moreno-Galván, Romero-Perdomo, Pardo-Díaz, Dávilla-Mora, Castro-Rincón, Rojas-Tapias and Estrada-Bonilla2023; Simões et al., Reference Simões, Souza, Martins, Tiecher, Bremm, Ramos, Farias and Carvalho2023; Monteiro et al., Reference Monteiro, Barreto-Mendes, Fanchone, Morgavi, Pedreira, Magalhães, Abdalla and Eugène2024). Currently, silvopasture systems stand out (Herrera et al., Reference Herrera, Mello, Apolinário, Dubeux Júnior, Silva, Santos and Cunha2020; Carvalho et al., Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a, Reference Carvalho, Mello, Cunha, Santos, Apolinário, Dubeux Júnior, Pessoa, Oliveira Neto and Silva2022b; Pessoa et al., Reference Pessoa, Cunha, Mello, Santos, Soares, Camelo, Apolinário, Dubeux Júnior and Coêlho2024; Izidro et al., Reference Izidro, Souza, Vieira Leite, Simões, Silva and Tabosa2024), an agroforestry practice that integrates livestock with trees (e.g., timber, cellulose, fruits), and increase the efficiency of land use sustainably (Monteiro et al., Reference Monteiro, Barreto-Mendes, Fanchone, Morgavi, Pedreira, Magalhães, Abdalla and Eugène2024).
Silvopasture combines arboreal species, herbaceous plants and animals in the same area (Tonucci et al., Reference Tonucci, Nair, Nair, Garcia and Bernardino2011). The inclusion of legume trees as a forestry component of the system can increase N2-fixation, and provide timber and forage with higher protein content than warm-season grass species, additionally providing several ecosystem services (Dubeux Jr et al., Reference Dubeux, Muir, Apolinário, Nair, Lira and Sollenberger2017; Zhong et al., Reference Zhong, Tian, Li and Liao2023; Santos et al., Reference Santos, Dubeux, Santos, Costa, Coêlho, Santos, Silva, Oliveira, Apolinário and Coêlho2024). The legume tree Mimosa caesalpiniifolia Benth. (‘sabiá’), native to Brazil, has been studied in silvopasture systems. It has shown potential to accelerate litter decomposition due to its low C:N ratio, enhancing nutrient cycling. Its inclusion also improves the FA and nutritive value of the herbaceous component by supplying nutrients to the soil, such as N from N2-fixation and other nutrients uptake from deeper layers of the soil (Lira Jr et al., Reference Lira, Fracetto, Da Silva Ferreira, Silva and Fracetto2020; Herrera et al., Reference Herrera, Mello, Apolinário, Dubeux Júnior, Cunha and Santos2021; Pessoa et al., Reference Pessoa, Cunha, Mello, Santos, Soares, Camelo, Apolinário, Dubeux Júnior and Coêlho2024).
On the other hand, the shading of the herbaceous layer by trees can influence the morphology (Gomes et al., Reference Gomes, Pedreira, Santos, Bosi, Lulu and Pedreira2020), physiology (Nascimento et al., Reference Nascimento, Pedreira, Sollenberger, Pereira, Magalhães and Chizzotti2019) and FA (Silva et al., Reference Silva, Dubeux, Santos, Mello, Cunha, Apolinário and Freitas2021b). The impact of shading may be strong or absent depending on the type of trees and herbaceous species, planting arrangement and spacings, density and age of the tree component in the silvopasture among other factors. Silva et al. (Reference Silva, Dubeux, Santos, Mello, Cunha, Apolinário and Freitas2021b) evaluated a silvopasture system with a tree density of 2500 trees/ha of M. caesalpiniifolia under 15 m spacing between double rows and reported reductions of 37% in the green dry forage mass of signal grass. However, in the study by Carvalho et al. (Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a) in an environment with a hot sub-humid tropical climate, the silvopasture system with a tree density of 600 trees/ha and a spacing of 25 m between double rows had no effect of M. caesalpiniifolia trees on signal grass productivity of signal grass. Even with wider spacing and reduced tree density, M. caesalpiniifolia is a species with a high degree of competition (Costa et al., Reference Costa, Mello, Dubeux, Santos, Lira, Oliveira and Apolinário2016; Silva et al., Reference Silva, Dubeux, Mello, Cunha, Santos, Apolinário and Freitas2021a, Reference Silva, Dubeux, Santos, Mello, Cunha, Apolinário and Freitas2021b). In this context, it is important to understand the impacts of the silvopasture system with M. caesalpiniifolia on the structural, morphological and productive characteristics of signal grass.
This study hypothesized that the signal grass modifies its structural and productive characteristics under a silvopasture system with legume trees. The objective was to evaluate the productive and structural characteristics of signal grass under monoculture and silvopasture systems.
Materials and methods
Site description
The experiment was carried out at the Experimental Farm Prof. Antônio de Pádua Maranhão Fernandes, of the Universidade Federal Rural de Pernambuco (UFRPE), located in Garanhuns-PE (8°53′30″ S 36°30′00″ W), at 842 m altitude (Fig. 1). The experimental period was from August/2020 to July/2021 (first grazing season GS1) and from October/2021 to September/2022 (second GS2). According to the Köppen–Geiger classification, the climate is tropical wet and dry (Aw). The average annual temperature is 22.8°C, with an average annual precipitation of 866 mm (Barbosa et al., Reference Barbosa, Souza, Galvíncio and Costa2016).

Figure 1. Map of the experimental area (Garanhuns city, Pernambuco state, Brazil).
The sequential water balance (Fig. 2) was calculated using the method proposed by Thornthwaite and Mather (Reference Thornthwaite and Mather1955) in Excel spreadsheets (Rolim et al., Reference Rolim, Sentelhas and Barbieri1998), with the months that presented a positive or zero water balance being considered a rainy season, corresponding April to July 2021 and April to August 2022. The term dry period was attributed to the months with a negative water balance, corresponding August 2020 to March 2021, October 2021 to March 2022, and August and September 2022. Accumulated precipitation during the entire experimental period was 1720 mm.
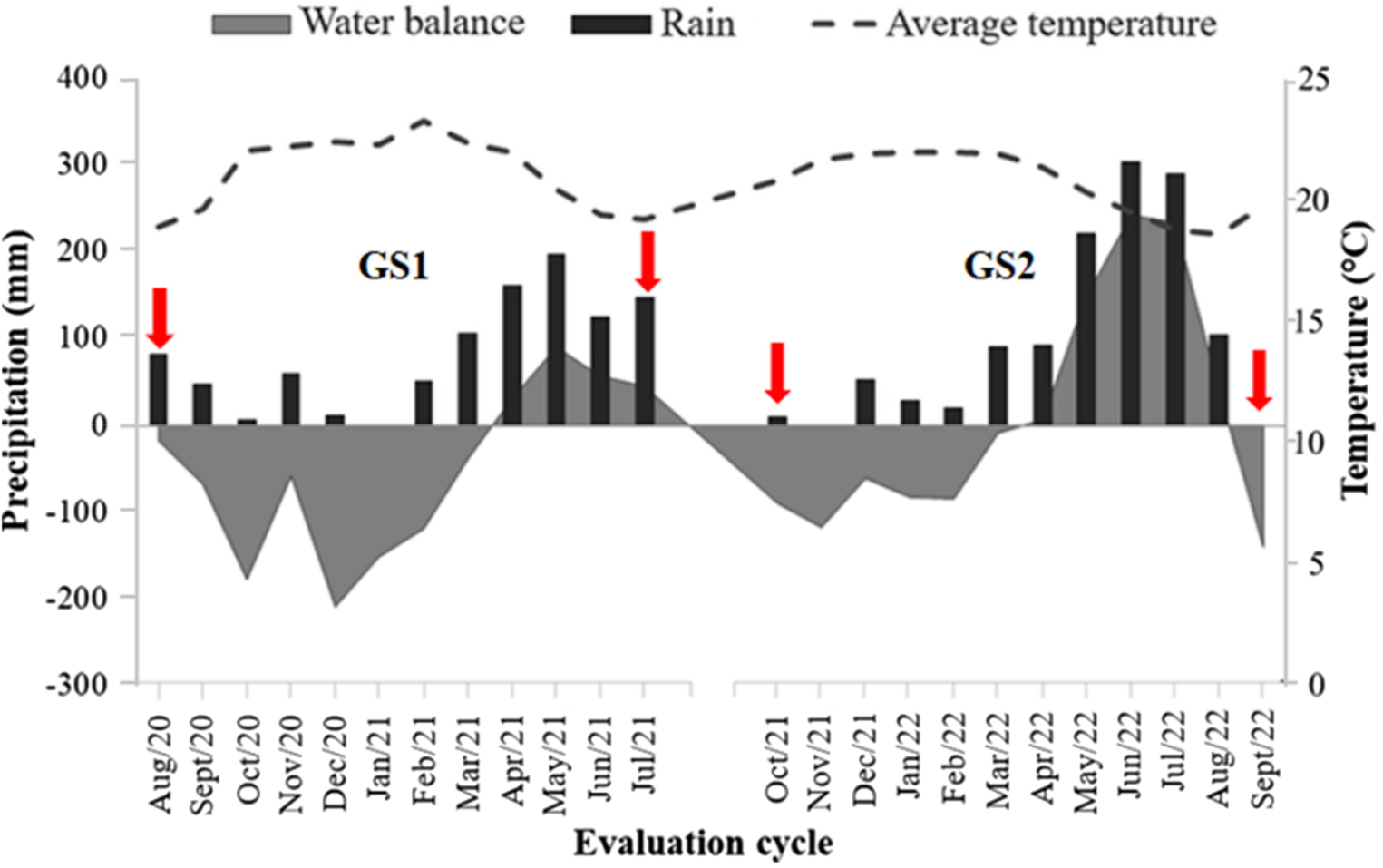
Figure 2. Water balance, amount of rain and average temperature from August 2020 to September 2022, Experimental Farm of the Universidade Federal Rural de Pernambuco, Garanhuns-PE, Brazil. Red arrows indicate the beginning and end of each grazing season (GS1 and GS2).
The soil in the experimental area is classified as Yellow Argisol, with a clay loam texture, this soil classification is equivalent to Yellow Argisol by the Food and Agriculture Organization (FAO) (IUSS working group, 2014). Soil sample was carried out at a depth of 0–20 cm, during the establishment of the experiment (2017). The soil had the following chemical characteristics before liming application: pH in water (1:2.5) = 5.3; P (Mehlich-I) = 2.0 mg/dm3; Na = 0.006 cmolcdm−3; K = 0.19 cmolc/dm3; Ca = 0.35 cmolc/dm3; Mg = 0.53 cmolc/dm3; Al = 0.95 cmolc/dm3; H = 4.95 cmolc/dm3; sum of bases = 1.15 cmolc/dm3; cation exchange capacity = 7.05 cmolc/dm3; V = 16%; organic matter = 4.47% and m = 46.5%. Soil analysis followed the methods recommended by the Brazilian Agricultural Research Corporation (EMBRAPA) (Teixeira et al., Reference Teixeira, Donagemma, Fontana and Teixeira2017).
Liming was performed 60 days before planting the double rows of legumes (August/2017), using dolomitic limestone (54.3% calcium carbonate [CaCO3] and 45.7% magnesium carbonate [MgCO3], PRNT = 90%). During the establishment, fertilization was carried out for M. caesalpiniifolia, using 30 kg of K2O/ha (potassium chloride [83% K]) and 60 kg of P2O5/ha (single superphosphate [44% P]), following the recommendations of Cavalcanti (Reference Cavalcanti2008), for Leucaena leucocephala (Lam.) de Wit.
Experimental design
The experiment was established in areas of signal grass (cultivar Basilisk) already existing on the experimental farm (established in the year of 1998). The treatments consisted of a monoculture system of U. decumbens and a silvopastoral system (signal grass + M. caesalpiniifolia) arranged in a completely randomized block design, with three replications. The experimental plots consisted of 1 ha paddocks.
In the silvopasture system, M. caesalpiniifolia seedlings were planted in October 2017. The seedlings were placed in 15 × 15 cm furrows, in an east–west direction, and the double rows were spaced 25 × 2 × 1 m, with some plant replanting carried out in May 2018 (replaced failures), establishing a population of 600 plants/ha. The experimental area was deferred before the first grazing season, the pastures were kept without the presence of grazing animals for 170 days, from March to July/2020; and, before the start of the second grazing season, for 83 days (July to September/2021).
Area management
When the animals were introduced into the area, at the beginning of the grazing trial (August/2020), the trees were already on average 2.6 m tall with considerable canopy projection (Izidro et al., Reference Izidro, Souza, Vieira Leite, Simões, Silva and Tabosa2024). The grazing method used was continuous stocking, with a variable stocking rate, according to Mott and Lucas (Reference Mott and Lucas1952), with adjustment of the stocking rate carried out following the recommendation of Sollenberger et al. (Reference Sollenberger, Moore, Allen and Pedreira2005). The paddocks were grazed across two seasons using two uncastrated crossbred male cattle (Holstein × Zebu) with an average initial body weight (BW) of 186 ± 26 kg. These cattle served as test animals. ‘Put and take’ animals of varying body weights were added to the pasture as needed to maintain a forage allowance of 3 kg of dry green forage mass per kg of BW per hectare (Carvalho et al., Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a, Reference Carvalho, Mello, Cunha, Santos, Apolinário, Dubeux Júnior, Pessoa, Oliveira Neto and Silva2022b). The study was certified by the Ethics Committee on the Use of Animals (CEUA/UFRPE), number 3695240820 (ID 000457).
Measured variables
The forage mass (FM) of signal grass was estimated by adapting the double sampling method (Haydock and Shaw, Reference Haydock and Shaw1975), measured every 28 days. The direct measurement consisted of harvesting six points, with two reference points of maximum, two intermediate and two minimum in terms of FM. The FM was harvested 5 cm from the ground level, using a 0.5 × 0.5 m square. At these sampling points, the canopy height was measured with a graduated ruler from ground level to the base of the last fully expanded leaf of signal grass. The average of 50 height measurements was used in the regression equation to estimate FM (Pedreira, Reference Pedreira2002). The regression equations for green forage mass (GFM; dry matter basis) were developed similarly to that described for total forage mass (TFM). The GFM corresponds to the FM excluding the senescent material.
The FM of the M. caesalpiniifolia was estimated in 12 plants in each silvopasture system plot, every 56 days. The samples were grouped according to the months of signal grass FM assessments in GS1 (2020/2021: February to June 2021) and GS2 (2021/2022: January to September 2022). Tender branches with a diameter of up to 5 mm and leaves up to a height of 1.5 m were considered potential forage material in the legume trees (Ydoyaga-Santana et al., Reference Ydoyaga-Santana, Lira, Santos, Ferreira, Silva, Marques, Mello and Santos2011; Oliveira et al., Reference Oliveira, Santos, Cunha, Mello, Lira and Barros2015). Total forage production (TFP) was the sum of the estimated FM of the legume plus the FM values of signal grass in the same months that the assessments coincided, totalling eight cycles of combined forage production (grass + legume), characterizing the TFP of the silvopasture system.
The samples harvested to calibrate the mass estimates were weighed in paper bags and then separated into green material (leaf and stem + sheath) and senescent material. Following, they were placed in a forced ventilation oven, at 55°C, until constant weight. Dry weight was used to determine TFM (kg DM/ha), GFM (kg DM of green leaves/ha) and M. caesalpiniifolia FM in the silvopastoral system. The leaf:stem ratio was obtained by dividing the dry weight of the leaf blade fraction by the dry weight of the stem fraction. Total forage density (kg/cm/ha) was estimated by dividing the TFM (kg DM/ha) by the average canopy height (cm). The morphological parts of signal grass: leaf blade, stem (sheath + stem) and senescent material were calculated considering the TFM.
The FA was estimated using six 1 × 1 m exclusion cages located in each paddock, totalling 36 cages in the experiment. The criterion adopted in choosing the points was the average condition of the 50 height measurements taken in the paddock. The cages were relocated every 14 days. The differences between the estimated values at the end and beginning of the 14 days represented the FA in each month of evaluation (Sollenberger and Cherney, Reference Sollenberger and Cherney1995), and when divided by the growth period, the forage accumulation rate (FAR) values were obtained (kg DM/ha/day). The total FA was obtained by summing the accumulations from each evaluation cycle, per treatment, in each grazing season.
Statistical analysis
The data were subjected to normality (Shapiro–Wilk) and homoscedasticity (Bartlett) tests for residuals. Data that did not present normal distribution and homoscedasticity were transformed to square root (√x). After meeting the assumptions, the data were subjected to analysis of variance, using PROC MIXED from SAS (SAS on Demand software). The treatments (cropping systems), the periods of the year (rainy and dry), the GS and the interaction between them were considered fixed effects, while the blocks were considered random effects. The means were compared using PDIFF, adjusted for the Tukey test (P ⩽ 0.05). The statistical model used was:
where Yijk is the dependent variable; μ is the general average; αi is the fixed effect of the treatments; βj is the fixed effect of the periods of the year; Ik is the effect of the GS; (βI)jk is the effect of the interaction between periods of the year and grazing season; γk is the random effect of the block; and eijk is the residual error.
Results
There was no effect of the cropping system (P ⩾ 0.05) on any structural, morphological and productive trait of the signal grass (Table 1). Canopy height (P ⩽ 0.001), TFM (P = 0.006), forage density (P ⩽ 0.001) and leaf:stem ratio (P ⩽ 0.001) were influenced by the interaction between the period of the year and GS (Table 2). The highest canopy height occurred in the dry period compared to the rainy period in GS1. However, there was a reduction (P ⩽ 0.05) in canopy height (54 v. 24 cm) in the dry period of GS2.
Table 1. Canopy height, total forage mass, green forage mass, forage density, morphological composition, forage accumulation, forage accumulation rate and forage accumulation total of Urochloa decumbens in monoculture and silvopastoral systems, during the experimental period
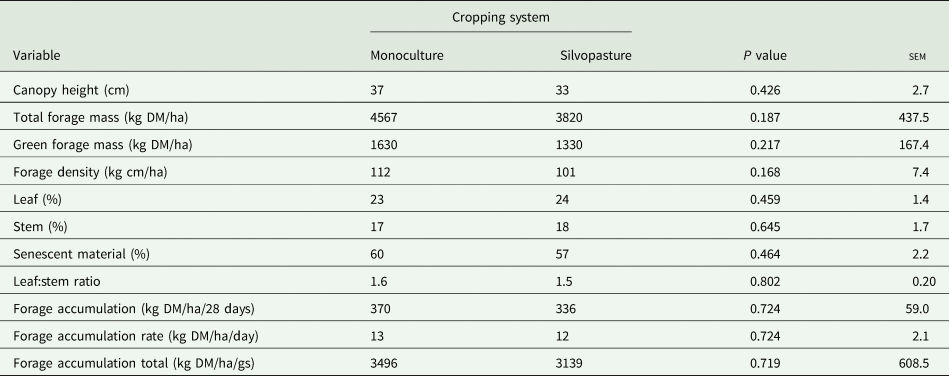
sem, standard error of the mean; gs, grazing season.
Table 2. Season and grazing season interaction in structural, morphological and productive characteristics of signal grass
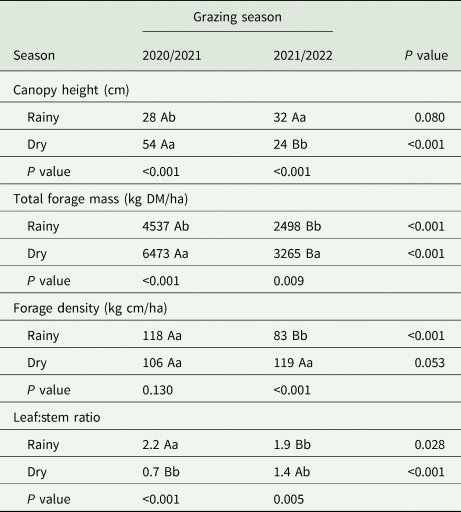
Means followed by different uppercase letters in the row and lowercase letters in the column differ from each other using the Tukey test (P⩽0.05).
There was a higher (P ⩽ 0.05) TFM (6473 v. 4537 kg DM/ha) in the dry period compared to the rainy period, respectively, in GS1. Furthermore, during the rainy season, in GS1 the TFM was 55% greater when compared to GS2. However, there was a 49.6% reduction in TFM in GS2 in the dry period compared to GS1. There was a greater TFM (3265 v. 2498 kg DM/ha) in the dry period compared to the rainy season in GS2 (Table 2). In GS1 during the rainy period there was a greater forage density, while in the rainy period of GS2, there was a lower forage density. During the rainy season of the GS2, there was an increase of 36 kg cm/ha (Table 2). The highest (P ⩽ 0.05) leaf:stem ratio in the rainy period (2.2) of GS1, while in the dry period of GS2, it was 1.4. There was a 214% increase in leaf:stem ratio during the rainy season v. dry of GS1.
A seasonal effect was observed on FA and FAR (P ⩽ 0.01) of signal grass (Fig. 3). Greater FA occurred in the rainy season (515 kg DM/ha/day), compared to the dry period (191 kg DM/ha/day) (Fig. 3(a)). The FAR was also greater (18 v. 7 kg DM/ha/day) in the rainy season compared to the dry season, respectively (Fig. 3(b)).
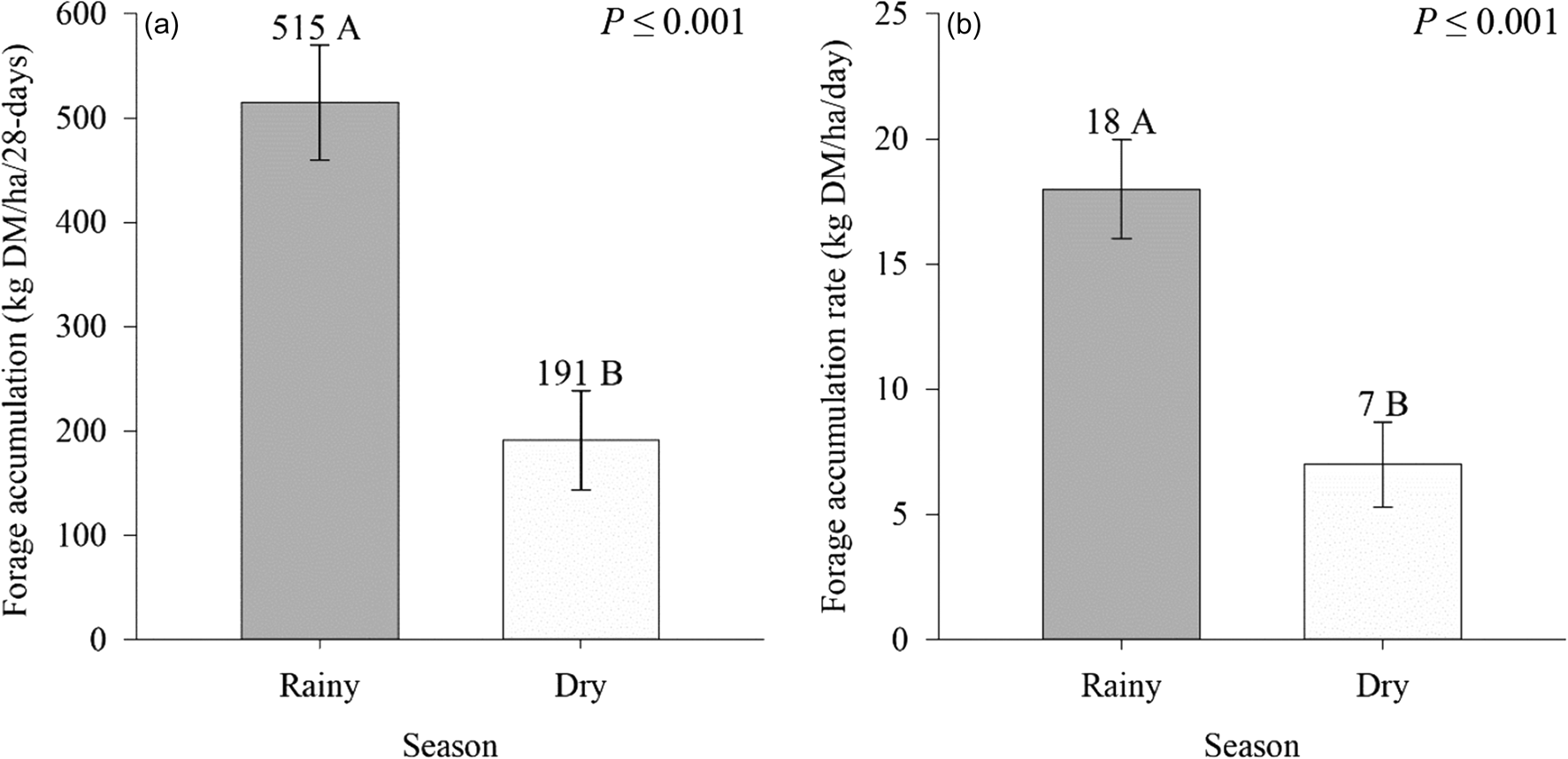
Figure 3. Effect of season on forage accumulation (a) and forage accumulation rate (b) of Urochloa decumbens Stapf RD Webster in monoculture and silvopastoral systems. Means with distinct letters in bars differ by F test (P⩽0.05). Vertical bars in columns indicate mean standard error (sem = 60.8, 2.2).
No effect of the period of the year (P = 0.6329) was observed on the GFM (Fig. 4) of signal grass. Regardless of the period of the year, the average GFM value was 1481 kg DM/ha. The greatest proportion of leaves occurred in the rainy season (Fig. 5(a)), concerning the dry season, on the other hand, the proportion of senescent material was greater in the dry season (68%) (Fig. 5(b)). The highest proportion of leaf (29%) occurred in GS2, compared to GS1 (18%) (Fig. 6(a)), the opposing occurred for stem proportions (15% in GS1 and 20% in GS2) (Fig. 6(b)). The highest proportion of senescent material (67%) was observed in the GS1, compared to 51% observed in the GS2 (Fig. 6(c)).

Figure 4. Effect of season on green forage mass of Urochloa decumbens Stapf RD Webster in monoculture and in silvopastoral systems. Means with distinct letters in bars differ by F test (P⩽0.05). Vertical bars in columns indicate mean standard error (sem = 169).

Figure 5. Effect of season on the proportions of the leaf (a) and senescent material (b) of Urochloa decumbens Stapf RD Webster. Means with distinct letters in bars differ by F test (P ⩽ 0.05). Vertical bars in columns indicate mean standard error (sem = 1.9, 3.1).

Figure 6. Effect of grazing season on the proportions of leaf (a), stem (b) and senescent material (c) of Urochloa decumbens Stapf RD Webster in the Agreste of Pernambuco. Means with distinct letters in bars differ by F test (P ⩽ 0.05). Vertical bars in columns indicate mean standard error (sem = 1.9, 1.5, 3.1).
The TFP of the silvopastoral system (signalgrass + M. caesalpiniifolia) was influenced seasonally (Fig. 7(a)), the greatest TFP occurred in the dry period (3948 kg DM/ha) concerning the rainy season (3143 kg DM/ha). Greater TFP (4583 kg DM/ha) was obtained in GS1 compared to 2508 kg DM/ha obtained in GS2.

Figure 7. Effect of season (a) and grazing season (b) on total forage production of Urochloa decumbens Stapf RD Webster in monoculture and silvopasture (signal grass + M. caesalpiniifolia). Means with distinct letters in bars differ by F test (P ⩽ 0.05). Vertical bars in columns indicate mean standard error (sem = 351.4, 351.4).
Discussion
The lack of impact of different cropping systems on the productive characteristics of signal grass can be explained by the good performance and persistence of the grass pasture under moderate shading conditions in the silvopasture system plots. Some studies conducted with the same species and in the same location (Carvalho et al., Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a; Oliveira Neto, Reference Oliveira Neto2022) also did not verify the effect of trees on signal grass in silvopasture systems. On the other hand, Costa et al. (Reference Costa, Mello, Dubeux, Santos, Lira, Oliveira and Apolinário2016) and Silva et al. (Reference Silva, Dubeux, Santos, Mello, Cunha, Apolinário and Freitas2021b) reported that trees competed with herbaceous vegetation, especially in silvopasture systems with M. caesalpiniifolia, and this can reduce forage production and animal performance over the years (Santos et al., Reference Santos, Dubeux Junior, Santos, Lira, Apolinário, Costa, Coêlho, Peixôto and Santos2020). However, at denser planting spacings, such as that adopted (15 m between rows of trees) by the previous authors mentioned, there is an increase in wood production in counterpart. According to Dubeux Jr. et al. (Reference Dubeux, Muir, Apolinário, Nair, Lira and Sollenberger2017), this can generate extra income for the system by the revenue of the timber produced.
The results of the present study indicate that the spacing of 25 m between the double rows of M. caesalpiniifolia can be considered adequate, from the point of view of competition between the trees and the signal grass pasture, corroborating results from Oliveira et al. (Reference Oliveira, Menezes, Gonçalves, Araújo, Ramirez, Guimarães Júnior, Jayme and Lana2023), who worked with Urochloa brizantha A. (Rich.) Stapf and Eucalyptus sp. in silvopasture system, as well as the results of Nascimento et al. (Reference Nascimento, Pedreira, Sollenberger, Pereira, Magalhães and Chizzotti2019), who carried out a systematic review on the effect of shading on tropical grasses used in silvopasture systems. Both studies indicated that spacing wider than 30 m between trees does not cause a significant reduction in the accumulation of forage in the grass of the genus Urochloa sp. (Syn. Brachiaria sp) and also in animal performance. It is necessary to consider the tree species used in the silvopasture system, because if the purpose is the herbaceous component and the tree is M. caesalpiniifolia, under the edaphoclimatic conditions of the present study, the distance between tree rows should be above 25 m.
The highest canopy height in the dry season in the GS1 was due to a 170-day grazing deferral period, before starting assessments. This delay occurred due to the outbreak of the COVID-19 pandemic. In the rainy period of both grazing seasons, the lower canopy height may be related to local climatic conditions, since, during this period, the Garanhuns region presents high cloudiness, which probably limits the photosynthetic rates of signal grass in function of the lower quantity and quality of incident radiation (Taiz et al., Reference Taiz, Zeiger, Møller and Murphy2015). However, the reduction in canopy height in the dry period (54 v. 24 cm) from the GS1 to the GS2 was a result of the grazing action by animals. In signal grass pastures, the ideal canopy height usually ranges between 15 and 25 cm under continuous stocking (Santos et al., Reference Santos, Silveira, Gomes, Fonseca, Sousa and Santos2013), considering the canopy height values observed in the present study, only in the dry period of the GS2, it was within this range (15–25 cm) of recommendation.
The greater FA was due to the higher accumulation of all parts of the plant, and the increased senescence rate (Silva et al., Reference Silva, Pedreira, Sollenberger, Silva, Yasuoka and Almeida2016). Water deficit can cause a reduction in basal and aerial tillering, as well as in the leaf:stem ratio, TFM and density forage in forage grasses (Mattos et al., Reference Mattos, Gomide and Martinez y Huaman2005; Ramos et al., Reference Ramos, Pedreira, Santos, Cruz, Pezenti, Silva, Diavão, Morenz, Pitta and Fries2022). Furthermore, the FA and FAR results were considered lower when compared to the results obtained in the same experimental area by Carvalho et al. (Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a), when they evaluated the productive characteristics of signal grass during the first year after establishment. Pedreira and Mattos (Reference Pedreira and Mattos1981) evaluated the seasonal growth of 25 species of forage grasses, including U. decumbens, and reported marked seasonality in production with an average annual production of 87% in the rainy season and 13% in the dry season. The lower FA during the dry season can be justified by the limited growth of signal grass due to the reduction in soil moisture.
FA is driven by favourable environmental conditions that allow the plant to express its genetic potential (Souza Sobrinho et al., Reference Souza Sobrinho, Lédo and Kopp2011). In the present study, the low average temperature (18°C) and reduced light during the rainy season (Carvalho et al., Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a) were factors that possibly limited the accumulation of forage in signal grass. Temperatures between 30 and 35°C are considered optimal for the growth of C4 grasses, and decreases from 30 to 25°C reduce CO2 assimilation by the leaves (Massad et al., Reference Massad, Tuzet and Bethenod2007). In a study with U. decumbens, Rabêlo et al. (Reference Rabêlo, Daneluzzi, Santos, Colzato, Montanha, Nakamura, Carvalho, Lavres and Alleoni2022) observed that there was a reduction in CO2 assimilation of more than 35% at an average temperature of 26.1°C, consequently, forage production was compromised.
The lack of nutrient replenishment in the soil may have limited FA, as suggested by Oliveira Neto (Reference Oliveira Neto2022), who observed a reduction in phosphorus levels from 2.0 to 0.60 mg/dm3 in the plots of the present experiment. This highlights the importance of nutrient replenishment in silvopastoral systems, especially other nutrients apart from the N that can be fixed. Herrera et al. (Reference Herrera, Mello, Apolinário, Dubeux, Mora and Freitas2023) also reported reductions in soil fertility in silvopastoral systems composed of signal grass + M. caesalpiniifolia in double rows over the years, along with an increased nutrient demand as the trees grew. Larsen (Reference Larsen, Weil and Brady2017) emphasized that phosphorus levels in the soil are low, and part of it is unavailable to plants. Phosphorus is crucial for stimulating root growth and tillering, its deficiency affects plant development, seed production and N2 fixation (Dubeux Jr. et al., Reference Dubeux, Lira, Santos, Muir, Silva, Teixeira and Mello2014; Zhong et al., Reference Zhong, Tian, Li and Liao2023). Phosphorus fertilization improves nodulation and N2 fixation in legumes (Mitran et al., Reference Mitran, Meena, Lal, Layek, Kumar and Datta2018; Chen et al., Reference Chen, Qin, Zhou, Xinxin, Chen, Sol, Wang, Lin, Zhao, Yamaj, Ma, Gu, Xu and Liao2019).
The long period of deferment (170 days – March to August 2021) in the rainy–dry transition period due to the start of COVID-19 also influenced the TFM. Deferment is one of the pasture management strategies and can be an efficient method to mitigate the effect of seasonality on forage production, requiring the evaluation of the regrowth period and the grazing intensity of the grass (Silva et al., Reference Silva, Carvalho, Malafaia, Garcia, Barbero and Ferreira2019). However, it is important to mention that the deferment was not a planned management in the present study, but a situation necessary due to the social isolation imposed by the COVID-19 pandemic.
The variation in results in TFM also occurred due to the effect of some biotic (grazing) and abiotic (precipitation, temperature, and radiation) factors. Additionally, the reduction in soil moisture during the dry period led to changes in pasture structure and FM throughout the grazing seasons. When allowed to grow freely for extended periods, tropical grasses are characterized by intense stem elongation, with an increased contribution of stems to the upper stratum of the canopy (Silveira et al., Reference Silveira, Nascimento, Rodrigues, Pena, Souza, Barbeiro, Limao, Euclides and Silva2016; Ramos et al., Reference Ramos, Pedreira, Santos, Cruz, Pezenti, Silva, Diavão, Morenz, Pitta and Fries2022). Reduced soil moisture during the dry period accelerates grass maturation, thickening the cell walls, reducing the leaf-to-stem ratio and increasing the proportion of senescent material (Ferreira et al., Reference Ferreira, Cardoso, Andrade, Brito and Ruggieri2023), directly affecting the quantity and quality of TFM in signal grass (Silva et al., Reference Silva, Dubeux, Santos, Mello, Cunha, Apolinário and Freitas2021b).
Silva et al. (Reference Silva, Dubeux, Santos, Mello, Cunha, Apolinário and Freitas2021b) reported a reduction in TFM (2266 kg DM/ha) in a silvopasture system with a 15 m spacing between double rows of trees and a tree density of 2500 trees/ha, compared to grass monoculture areas (TFM of 3496 kg DM/ha), in a study conducted in another tropical pasture. These authors reported that the reduction in TFM was associated with the degree of competition between M. caesalpiniifolia with signal grass under a higher tree density, this fact was not observed in the present study with a 25 m spacing between double rows, and a tree density of 600 trees/ha. In silvopasture systems that prioritize wood production, reduced spacing (10–15 m between double rows) is recommended (Apolinário et al., Reference Apolinário, Dubeux Junior, Lira, Sampaio, Amorim, Silva and Muir2016; Silva et al., Reference Silva, Dubeux, Mello, Cunha, Santos, Apolinário and Freitas2021a, Reference Silva, Dubeux, Santos, Mello, Cunha, Apolinário and Freitas2021b). However, if the purpose is to produce forage, it is necessary to adopt tree planting spacings of ⩾25 m, this allows more unshaded areas for grass growth and might reduce below-ground competition for soil nutrients.
The GFM results align with the results reported by Aroeira et al. (Reference Aroeira, Paciullo, Lopes, Morenz, Saliba, Silva and Ducatti2005), who found that GFM in signal grass pastures in the silvopastoral system can range from 800 to 1800 kg DM/ha, with the lowest and highest values obtained in the dry and rainy seasons, respectively. However, the lower forage density can reduce forage consumption by grazing animals, due to its negative effects on bite size and, consequently, animal performance (Lima et al., Reference Lima, Paciullo, Morenz, Gomide, Rodrigues and Chizzotti2019; Silva et al., Reference Silva, Dubeux, Mello, Cunha, Santos, Apolinário and Freitas2021a). Additionally, during dry periods with reduced forage density, grazing time increases (Selemani et al., Reference Selemani, Eik, Holand, Ådnøy, Mtengeti and Mushi2013), which raises the animals' energy expenditure; this associated with the reduced FA explains the low weight gain during this season year (GS1). The results are similar to those reported by Carvalho et al. (Reference Carvalho, Mello, Cunha, Apolinário, Silva, Costa, Carvalho and Santos2022a), who evaluated the productive potential of signal grass in the same experimental area.
It was observed that the dry period of GS2 had less effect on the leaf:stem ratio, as the average value during this season was twice as high compared to the GS1. The higher proportion of leaves during the rainy season contributed to an increased leaf-to-stem ratio in GS1. The formation and maintenance of living plant tissues depend on various genetic and environmental factors. Among environmental variables, photosynthetically active radiation and soil moisture content are key factors in plant growth (Taiz et al., Reference Taiz, Zeiger, Møller and Murphy2015). The water deficit during the dry period likely impaired the formation and maintenance of living plant tissues, reducing the leaf:stem ratio in both GSs (Sousa et al., Reference Sousa, Moreira, Lemos Filho, Paciullo, Vendramini, Luna and Maurício2023).
Changes in the proportions of morphological constituents can be influenced by seasonal variations, fertilization and cropping systems (Rojas et al., Reference Rojas, Hernández, Quero, Guerrero, Ayala, Zaragoza and Trejo2016; Maldonado Peralta et al., Reference Maldonado Peralta, Rojas Garcia, Magadan Olmedo, Martinez, Aguirre and Perez2020). Juskiw et al. (Reference Juskiw, Helm and Salmon2000) stated that in advanced physiological stages, stem, senescent material and inflorescence production increases, reducing the leaf biomass. This occurs partly due to the translocation of nutrients from the leaves, leading to their senescence and death during the reproductive phase. Geremia et al. (Reference Geremia, Crestani, Mascheroni, Carnevalli, Mourão and Silva2018) reported that increasing the proportion of leaves and reducing senescent material should be a management goal in silvopastoral systems, to enhance the supply of forage with superior nutritional quality for the grazing animals.
These results were influenced by the lower canopy height and high leaf:stem ratio during GS2. Leaves are a key structural component of forage, as they are the most digestible part of the plant for grazing animals (Tesk et al., Reference Tesk, Pedreira, Pereira, Pina, Ramos and Mombach2018). Signal grass typically accumulates more stems than leaves (Maldonado Peralta et al., Reference Maldonado Peralta, Rojas Garcia, Magadan Olmedo, Martinez, Aguirre and Perez2020; Lara et al., Reference Lara, Silva, Sollenberger and Pedreira2021). In another study, Silveira et al. (Reference Silveira, Nascimento Júnior, Silva, Euclides, Montagner, Sbrissia, Rodrigues, Sousa, Pena and Vilela2010) compared morphogenetic and structural responses of several tropical grasses, including U. decumbens, and reported greater daily stem elongation for Arapoty, Basilisk and Marandu (1.18, 1.49 and 1.33 cm/tiller/day, respectively). The higher proportion of stem in forage species is associated with a higher concentration of fibres and lignified tissues, which reduces their digestibility (Nave et al., Reference Nave, Pedreira and Pedreira2010). This outcome was due to the advanced physiological maturity of signal grass in GS1, leading to an accumulation of senescent material (Ferreira et al., Reference Ferreira, Cardoso, Andrade, Brito and Ruggieri2023).
The temperature strongly interacts with the level of incident radiation on the plant and has an immediate and significant influence on leaf growth. For example, low temperatures can limit cell division and elongation, reduce photosynthetic efficiency and restrict the growth of tropical forages (Hodgson, Reference Hodgson1990; Taiz et al., Reference Taiz, Zeiger, Møller and Murphy2017). Signal grass experiences a marked decline in growth rate at lower temperatures (15°C) compared to higher ones (30°C), indicating that temperature is a key growth factor (Matos et al., Reference Matos, Cole, Whitney, MacKinnon, Kay and Hazen2014). Approximately 83% of its dry matter is produced during the summer, showing that warmer temperatures favour its development, while forage production decreases in colder seasons (Mislevy and Everett, Reference Mislevy and Everett1981).
In the dry season, there was greater participation of FM from signal grass v. M. caesalpiniifolia, since during this season this deciduous legume tree loses its leaves to minimize water loss (Castro Filho et al., Reference Castro Filho, Muniz, Rangel, Santos, Santana and Araújo2016). Reductions in the FM of M. caesalpiniifolia generally occur during periods of low rainfall (Herrera et al., Reference Herrera, Mello, Apolinário, Dubeux Júnior, Cunha and Santos2021). It is important to note that the FM of M. caesalpiniifolia was only measured up to 1.5 m, taking into account browsing by cattle (Ydoyaga-Santana et al., Reference Ydoyaga-Santana, Lira, Santos, Ferreira, Silva, Marques, Mello and Santos2011; Mello et al., Reference Mello, Costa, Dubeux, Santos, Apolinário, Tenório Filho, Meirelles and Pereira2014).
The reduction in TFP in the GS2 occurred due to the consumption of forage by animals throughout this period, as signal grass increased TFP by more than 95%, mainly in the silvopastoral system. It is important to consider the TFP of the system to assist in decision-making in the management practices of the components present in the system. The adoption of intercropping generally increases the net productivity of the system, and the conservation of natural resources (Araújo Jr. et al., Reference Araújo, Morais, Steidle Neto, Souza, Alves, Silva, Leite, Silva, Jardim, Montenegre and Silva2023; Simões et al., Reference Simões, Souza, Martins, Tiecher, Bremm, Ramos, Farias and Carvalho2023).
Conclusion
The silvopasture with M. caesalpiniifolia did not affect the growth, structural and productive traits of signal grass, considering that the trees were spaced double rows spaced 25 m apart, and with a tree density of 600 plants/ha. FA, morphological components and TFP in the silvopastoral system varied seasonally throughout the year and across grazing seasons.
Acknowledgements
The authors thank the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for funding the project (APQ-1176-5.04/14) and also for the post-doctoral scholarship (BFP-0072-5.04/34) to J. L. P. S. Izidro. The authors also thank the Universidade Federal Rural de Pernambuco (UFRPE) for the experimental area, laboratories and equipment; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the doctoral scholarship to J. L. P. S. Izidro; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for the productivity scholarship to A. C. L. de Mello, M. V. da Cunha and M. V. F. dos Santos (CNPq scholarship); and the PET/SESU/MEC for the scholarship to V. J. da Silva (PET scholarship).
Author contributions
A. C. L. de Mello, M. V. da Cunha, V. J. da Silva, M. V. F. dos Santos, V. P. da Silva: supervision, conceptualization, funding acquisition, project administrator; J. L. P. S. Izidro: investigation, sampling, data curation, formal writing; S. B. de M. Costa, C. B. de. M. Carvalho, D. V. Pessoa, P. M. de Oliveira Neto: sampling and laboratory; J. J. Coêlho: writing review.
Funding statement
This work was financed by the Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE) (APQ-1176-5.04/14).
Competing interests
None.
Ethical standards
The study was certified by the Ethics Committee on the Use of Animals (CEUA/UFRPE), number 3695240820 (ID 000457).












