Introduction
California is the world's leading producer of almond nuts with a production of 1.02 billion kg in 2017. Field weight yields of almonds (Prunus dulcis) at harvest are 23% meats (nuts), 13.0% debris, 14.0% shells and 50.0% hulls (EPA, 1995). Based on EPA (1995) values, there were 2.22 billion kg of almond hulls (AH) produced as a by-product of the hulling industry in 2017. Almond hulls contain high sugar and fibre but low crude protein (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020). Because of their readily available carbohydrate composition, AH are a by-product feedstuff fed to ruminants.
In California, commercial feed laws and regulations (California Department of Food and Agriculture validation 2773.5) define AH as: ‘They shall not contain more than 13.0 percent moisture, nor more than 15.0 percent crude fiber, and not more than 9 percent ash.’ This definition attempts to restrict the amount of non-hull material in commercial AH.
Harvesting practices for almonds involve shaking the tree so the fruit drops to the ground. The almond fruit is swept into rows between the trees to allow the fruit to dry. The almond fruit is then swept from the orchard ground for transport to a huller. The process of tree shaking and ground harvesting contributes a debris component, which is predominately sticks (tree) and dirt (ground). At the almond huller the nuts, hulls, shells and debris are separated. However, it is not possible with current methods to remove all of the shells and the sticks of short length from the hulls. In this paper, debris is composed of stick, dirt and shell material although there were no dirt clumps present in any samples used in this study. The hulling process yields commercial AH (≤15.0% crude fibre) that are predominately hulls, with the amount of debris (sticks and shells) varying, at least, for the variety of almonds (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020). The contribution of debris greatly impacts the chemical composition of the AH (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020). As the proportion of debris decreased, the fibre, lignin and ash contents decreased and the sugar content increased (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020).
The few in vitro studies with AH did not adequately describe the AH used (Arosemena et al., Reference Arosemena, DePeters and Fadel1995; Jafari et al., Reference Jafari, Alizadeh and Imani2011; Elahi et al., Reference Elahi, Kargar, Dindarlou, Kholif, Elghandour, Rojas Hernández, Odongo and Salem2017). In the case of Arosemena et al. (Reference Arosemena, DePeters and Fadel1995), the AH were commercial AH and therefore contained debris. Based on the lignin and fibre content, it is unlikely that the AH used in other studies (Jafari et al., Reference Jafari, Alizadeh and Imani2011, Reference Jafari, Alizadeh, Imani, Meng, Rajion and Ebrahimi2015; Elahi et al., Reference Elahi, Kargar, Dindarlou, Kholif, Elghandour, Rojas Hernández, Odongo and Salem2017) were pure AH. To the best of our knowledge, there are no reports in the literature for the in vitro fermentability of pure AH.
The aim of this study was to investigate the impact of debris on the in vitro and in sacco rumen fermentability of AH by evaluating 12 samples of commercial AH (Total almond hulls; TAH) of which a portion of each was hand-sorted to create pure almond hulls (PAH) and Debris (non-hull material). The authors hypothesized that the debris and TAH would be lower in digestibility than PAH indicating that reducing the proportion of debris will improve the nutritive value of AH for livestock.
Materials and methods
Twelve different samples of AH were obtained from five hullers throughout California. Samples contained five Nonpareil, two Butte/Padre pollinator mixes, one Butte/Mission pollinator mix and four pollinators that had no variety designation. Each huller supplied a sample of Nonpareil hulls as well as 1–2 samples of ‘other’ varieties. Samples were designated either Nonpareil or Other Variety. Each sample of AH was thoroughly mixed and divided into two samples. One sample represented TAH while the other half was hand sorted to separate AH from debris to create samples of PAH and Debris (wood sticks and shells). Three samples from one of the hullers did not have enough Debris to be used for in vitro analysis, so only the PAH and TAH samples were used from that huller. This resulted in 12 PAH samples, 12 TAH samples and nine Debris samples. The 33 samples of TAH, PAH and Debris were coarsely ground in a hammermill using a 3 mm screen prior to analysis, subsampled, and eventually ground through a 1 mm screen for analysis.
In vitro gas production
In vitro gas production was measured by incubating 33 samples using the syringe method (Menke and Steingass, Reference Menke and Steingass1988). Approximately 200 mg of each ground sample were placed in 100 mL glass syringes. Rumen fluid was collected into pre-warmed insulated thermoses using a polyvinyl chloride pipe with holes and a 600mL drenching syringe as previously described (Getachew et al., Reference Getachew, Laca, Putnam, Witte, McCaslin, Ortega and DePeters2018). Fluid was collected after the morning feeding from two rumen-fistulated nonlactating Holstein cows that were both consuming a high forage diet as described by Getachew et al. (Reference Getachew, Laca, Putnam, Witte, McCaslin, Ortega and DePeters2018). The management and care of the rumen-fistulated cows were according to the guidelines and standard operating procedures of an animal care protocol that was approved by the Institutional Animal Care and Use Committee at the University of California at Davis. At the laboratory, the rumen fluid from the two cows was mixed, filtered through cheesecloth and flushed with carbon dioxide (CO2). This mixture was then added to a buffered mineral solution (1: 2, v/v) and maintained in a water bath at 39°C with a continuous bubbling of CO2. The buffered mineral solution was prepared according to the protocol described by Menke and Steingass (Reference Menke and Steingass1988). The buffered rumen fluid (30 mL) was pipetted into each syringe, and each syringe was placed in a water bath maintained at 39°C. Gas production was recorded by hand every 2 h during a 10-hour period daily and incubation continued for 72 h. Individual gas readings at each time point were determined by physically reading the value on the syringe at the red line on the bottom of the piston. The incubation run was repeated three times for three independent and separate runs. In addition, total gas produced at 24 h was used to calculate metabolizable energy (ME) values with the following equation from Melesse et al. (Reference Melesse, Steingass, Schollenberger and Rodehutscord2018):

In vitro true digestibility on a dry matter basis and NDF digestibility (Ankom Daisy)
In vitro true digestibility on a dry matter (DM) basis (IVTD) and neutral detergent fibre digestibility (NDFD) determinations were carried out using multilayer polyethylene polyester cloth bags (F57 filter bag, ANKOM Technology, Macedon, NY). Before weighing the ground samples into bags, the bags were rinsed with acetone and dried at air temperature overnight. A subsample of each of the ground AH samples for TAH, PAH and Debris (0.25 ± 0.02 g per bag) was weighed in duplicate into the bags so that there would be two bags of each sample at each of the four timepoints for three independent runs, for a total of 24 bags of each sample. The bags with samples were heat-sealed, and placed in digestion jars containing a rumen fluid and buffer mixture (Getachew et al., Reference Getachew, Ibáñez, Pittroff, Dandekar, McCaslin, Goyal, Reisen, DePeters and Putnam2011). The buffer mixture used contained potassium phosphate, urea, sodium chloride, calcium chloride dihydrate, magnesium sulphate heptahydrate, sodium carbonate and sodium sulphide nonahydrate and was mixed according to the protocol outlined by ANKOM Technology (https://www.ankom.com/technical-support/daisy-incubator). At the end of each incubation period (12 h, 24 h, 48 h and 72 h), the bags were removed from the chamber and were rinsed with deionized water until the rinse water ran clear. Bags were then placed in an ANKOM fibre analyser, along with heat-stable alpha-amylase and sodium sulphite, and boiled in a neutral detergent solution for 75 min to determine the amount of neutral detergent fibre remaining in the presence of amylase (aNDF). After 75 min, the bags were removed, soaked in acetone in a beaker for 5 min, dried at 100°C for 24 h, and weighed to determine IVTD. The NDFD was calculated by dividing the amount of aNDF residue remaining after the incubation by the amount of aNDF (% aNDF of sample multiplied by DM g of sample) in the sample before incubation.
In sacco DM and NDF disappearance
Two nonlactating, nonpregnant Holstein cows had been previously surgically fitted with 4-inch ruminal cannulas with a rolled inner flange (#1C, Bar Diamond, Parma, ID). Each cow was individually fed and consumed 12.4 ± 0.54 and 10.9 ± 0.35 kg daily (as-fed basis) of a high forage diet as previously described (Getachew et al., Reference Getachew, Laca, Putnam, Witte, McCaslin, Ortega and DePeters2018).
Monofilament nylon bags, 5 × 10 cm in size with a pore size of 50 ± 10.0 μm (Ankom Technology, Macedon, NY), were used in a series of in sacco ruminal incubations. In each bag, 1 ± 0.2 g of either a PAH (n = 12) or TAH (n = 12) sample was weighed and added before sealing each bag with a Nyclone impulse heat sealer (Lorvic Corp., St. Louis, MO). Two series of in sacco incubations were conducted with bags of TAH or PAH incubated in the rumen for 0, 1, 2, 4, 8, 16, 32 and 64 h as described by Nocek (Reference Nocek1988). After removing all bags from the rumen, they were immediately submerged and rinsed through cool water to stop microbial activity. Each bag was then uniformly rinsed with tap water until the rinse water ran clear. Bags were then dried for 12 h at 55°C in a forced-air oven before being placed in desiccators to be subsequently weighed for DM disappearance calculations. Digestibility of NDF was calculated after refluxing the monofilament bags in NDF solution for 1 h as described by DePeters et al. (Reference DePeters, Fadel and Arosemena1997).
Chemical analysis
The chemical composition of the TAH, PAH and Debris was reported previously (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020). The wet chemistry composition analysis for all samples was completed by Cumberland Valley Analytical Services (Waynesboro, PA). Briefly, DM was determined by drying to a constant weight at 100°C for 5 h followed by equilibration in a desiccator (AOAC 2000). The concentration of aNDF was determined by refluxing the sample in a neutral detergent solution in the presence of both sodium sulphite and α-amylase (Van Soest et al., Reference Van Soest, Robertson and Lewis1991).
Statistical analysis
All statistical analysis was completed using R version 3.6.2 (R core team, 2019). For the in vitro gas production data, a nonlinear mixed-effects model, using the nlme package (Pinheiro et al., Reference Pinheiro, Bates, DebRoy and Sarkar2019), was used:
where P ij = the gas produced (ml/g) for the ith syringe (i = 1,…, 398) at the jth sampling time j (j = 1,…,n i), t ij is the corresponding sample time, and e ij is the error. The parameter A i represents the asymptotic potential gas production (ml/g) and k i represents the rate of gas production (/h). The treatment effects of Type (TAH, PAH and Debris) and Variety (Nonpareil and Other) were incorporated directly into the parameters A i and k i such that each parameter had a random error variance associated with each parameter as well as covariance between parameters. (Pinheiro and Bates, Reference Pinheiro and Bates2000). Individual contrasts were analysed for the main effects using the emmeans package and P values were adjusted using the Tukey method (Lenth, Reference Lenth2020).
For the ME data, a linear mixed-effects model using the lme4 package was used and P values were calculated using a Type III Analysis of Variance with Satterthwaite's method (Bates et al., Reference Bates, Maechler, Bolker and Walker2015). Individual contrasts were analysed for the main effects using the emmeans package and P values were adjusted using the Tukey method (Lenth, Reference Lenth2020). The model used as follows:
where yijkm is the ME (MJ/kg) of the ith AH type for the jth sample variety during kth run for mth sample ID, μ is a constant and eijkm is residual error. Ti is the fixed effect of ith almond hull type (i = PAH, TAH, Debris); Vj is the fixed effect of jth variety of the sample (j = Nonpareil or Other); TVij is the interaction term of the ith almond hull type with the jth variety of sample; Rk is the random effect of the kth run (k = 1,…,3); Sm is the random effect of the mth sample ID (m = 1,…,12).
For the Daisy digestibility data, a linear mixed-effects model was used with the lme4 package (Bates et al., Reference Bates, Maechler, Bolker and Walker2015). Individual contrasts were analysed for the interaction effects using the emmeans package and P values were adjusted using the Tukey method (Lenth, Reference Lenth2020). The Daisy model was:
where yijkm is the coefficient of the disappearance of g of starting material of the ith AH type at the jth hour during run kth for sample ID mth, μ is a constant and eijkm is residual error. Ti is the fixed effect of ith almond hull type (i = PAH, TAH, Debris); Hj is the fixed effect of jth hour of sampling (j = 12, 24, 48, 72 h); THij is the interaction term of the ith almond hull type with the jth hour of sampling; Rk is the random effect of the kth run (k = 1,…3); Sm is the random effect of the mth sample ID (m = 1,…12). Variety was not included in this model due to the lack of degrees of freedom necessary for that many contrasts. All two-way and three-way interactions were not significant except the TH and were removed from the model using log-likelihood ratio test (Pinheiro and Bates, Reference Pinheiro and Bates2000),
A similar nonlinear mixed-effects model to that of the gas production, using the nlme package (Pinheiro et al., Reference Pinheiro, Bates, DebRoy and Sarkar2019), was used for the in sacco data:
where Pij = the coefficient of the disappearance of g of starting material for the ith bag (i = 1,…, 144) at the jth sampling time j (j = 1,…,ni), tij is the corresponding sample time and eij is the error. The parameter Int represents the intercept of time 0 with the coefficients of disappearance on the y-axis, A represents the asymptotic potential coefficient of disappearance, while k represents the fractional rate of disappearance (/h). The treatment effect of Type (PAH or TAH) was incorporated directly into the parameters A, k and Int such that each parameter had a random error variance associated with each parameter as well as covariance between parameters. (Pinheiro and Bates, Reference Pinheiro and Bates2000). There were no differences due to Variety, so it was not included in the final model. The emmeans package was used to analyse Type contrasts and P values were adjusted using the Tukey method (Lenth, Reference Lenth2020).
For all data analysis, a P value of <0.05 was considered significant while a P value of <0.1 but >0.05 was considered a trend.
Results
In vitro gas production and digestibility
In vitro total estimated gas production (Table 1) was overall significantly (P = 0.009 and <0.001 respectively) higher for PAH (270 ml/g) compared with both TAH and Debris (261 and 79 ml/g respectively). The rate of gas production was significantly higher for PAH and TAH (0.10 and 0.10 /h respectively) compared with Debris (0.07 /h), but there was no difference between PAH and TAH. Estimated gas production was significantly greater for PAH (283 ml/g) than TAH (267 ml/g) for Nonpareil but the difference was not significant for the Other Variety. As anticipated, total estimated gas production was significantly lower for Debris (94 ml/g Nonpareil and 69 ml/g Other) compared with both PAH and TAH for both varieties. The estimated rate of gas production was similar for PAH and TAH for both varieties but was significantly lower for Debris compared with TAH. There was a greater numerical difference between Nonpareil and Other Variety for PAH (283 ml/g and 261 ml/g, respectively) than for TAH (267 ml/g and 257 ml/g, respectively). A similar pattern was observed with the estimated rate of gas production, with the Nonpareil Debris having a numerically greater estimated rate (0.09 /h) than Other Variety (0.06/h).
Table 1. Estimated potential gas production (ml/g) of almond hulls (AH) for each Type (Total AH, Pure AH, Debris) and Variety (Nonpareil or Other).

Total AH = contains AH and Debris; Pure AH = sorted to contain only hulls; Debris = sticks and shells sorted from TAH.
The effects of Type (Total AH, Pure AH, Debris) on parameters of the gas production function are shown. The estimate is the asymptote or total volumes (ml/g) of gas produced for each Type and Variety from the model. The corresponding rate constants for each Type and Variety are expressed as /h.
a Pure v. Debris = contrast between Pure almond hulls and Debris samples; Pure v. Total = contrast between Pure almond hulls and Total almond hulls; Debris v. Total = contrast between Debris and Total almond hulls.
The calculated ME concentration was numerically greater for PAH (9.3 MJ/kg and 8.7 MJ/kg) than TAH (9.0 MJ/kg and 8.5 MJ/kg) while both were significantly greater (P < 0.001) than Debris at approximately half the energy content (4.7 MJ/kg and 3.8 MJ/kg) for both Nonpareil and Other Variety respectively. There were small numerical differences within Variety between TAH and PAH, but over all Types, Nonpareil had significantly higher (P = 0.003) calculated ME content compared with the Other Variety (Table 2). The Other Variety had a greater numerical proportion of Debris (6.8%) compared with Nonpareil (4.7%) (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020).
Table 2. Calculated metabolizable energy (ME) estimates from the model for each Type (Total AH, Pure AH, Debris) and Variety (Nonpareil or Other Variety) of almond hulls (AH).

Total AH = contains AH and Debris; Pure AH = sorted to contain only hulls; Debris = sticks and shells sorted from TAH.
a Type = main effect of PAH, TAH, or Debris; Variety = main effect of Nonpareil and Other; Type × Variety = interaction of all Types over both Varieties.
b ME calculated by following equation: [1.68 + (0.1418 × GP at 24 h in ml/200 mg) + (0.0073 × %CP) + (0.0217 × %EE) – (0.0028 × %Ash)] (Melesse et al., Reference Melesse, Steingass, Schollenberger and Rodehutscord2018).
c Across both varieties, TAH and PAH had significantly higher (P < 0.05) ME averages compared with Debris, but there were no differences between PAH and TAH.
The IVTD and NDF digestibility were measured at 12, 24, 48 and 72 h (Table 3). The IVTD and NDFD were significantly greater for PAH than TAH (P < 0.001) at 48 and 72 h and Debris was significantly lower in digestibility than both TAH and PAH (P < 0.001) for IVTD at every time point and for NDFD at 24, 48 and 72 h.
Table 3. Daisy in vitro true digestibility on a dry matter (DM) basis (IVTD) and neutral detergent fibre digestibility (NDFD) of almond hulls (AH).

Total AH = contains AH and Debris; Pure AH = sorted to contain only hulls; Debris = sticks and shells sorted from TAH.
The effects of Type (Total AH, Pure AH, Debris) on IVTD and NDFD coefficients at each timepoint measured in vitro are shown. All main effects and interactions of Type and Hour (Hours of incubation) were significant (P < 0.05).
a Pure v. Total = contrast between Pure almond hulls and Total almond hulls; Debris v. Total = contrast between Debris and Total almond hulls; Pure v. Debris = contrast between Pure almond hulls and Debris samples.
In sacco digestibility
In sacco disappearance only included TAH and PAH (Fig. 1). The estimated asymptote for the coefficient of DM disappearance was significantly greater (P = 0.018 for PAH (0.35 and TAH (0.34; Table 4). The estimated fractional rate of in sacco DM disappearance was 0.06/h for TAH and 0.07/h for PAH, which was not significantly different (P = 0.195. The intercept of the coefficient of DM disappearance with the y-axis was significantly greater for PAH (0.58) compared with TAH (0.55; P < 0.001). The calculated coefficient for the proportion of DM disappearance (P) was numerically higher for PAH (0.93) than TAH (0.89). A similar response was observed for estimated potential disappearance of NDF (Table 4). The estimated asymptote for the proportion of NDF disappearance was 0.80 for TAH and 0.89 for PAH (P < 0.001). The estimated rate of NDF disappearance was also greater for PAH (0.06 /h) compared with TAH (0.05 /h; P = 0.007). The intercept of the coefficient of NDF disappearance with the y-axis was not significantly different for PAH (−0.15) and TAH (−0.14; P = 0.071). The calculated coefficient for the proportion of NDF disappearance (P) was numerically higher for PAH (0.74) than TAH (0.66).
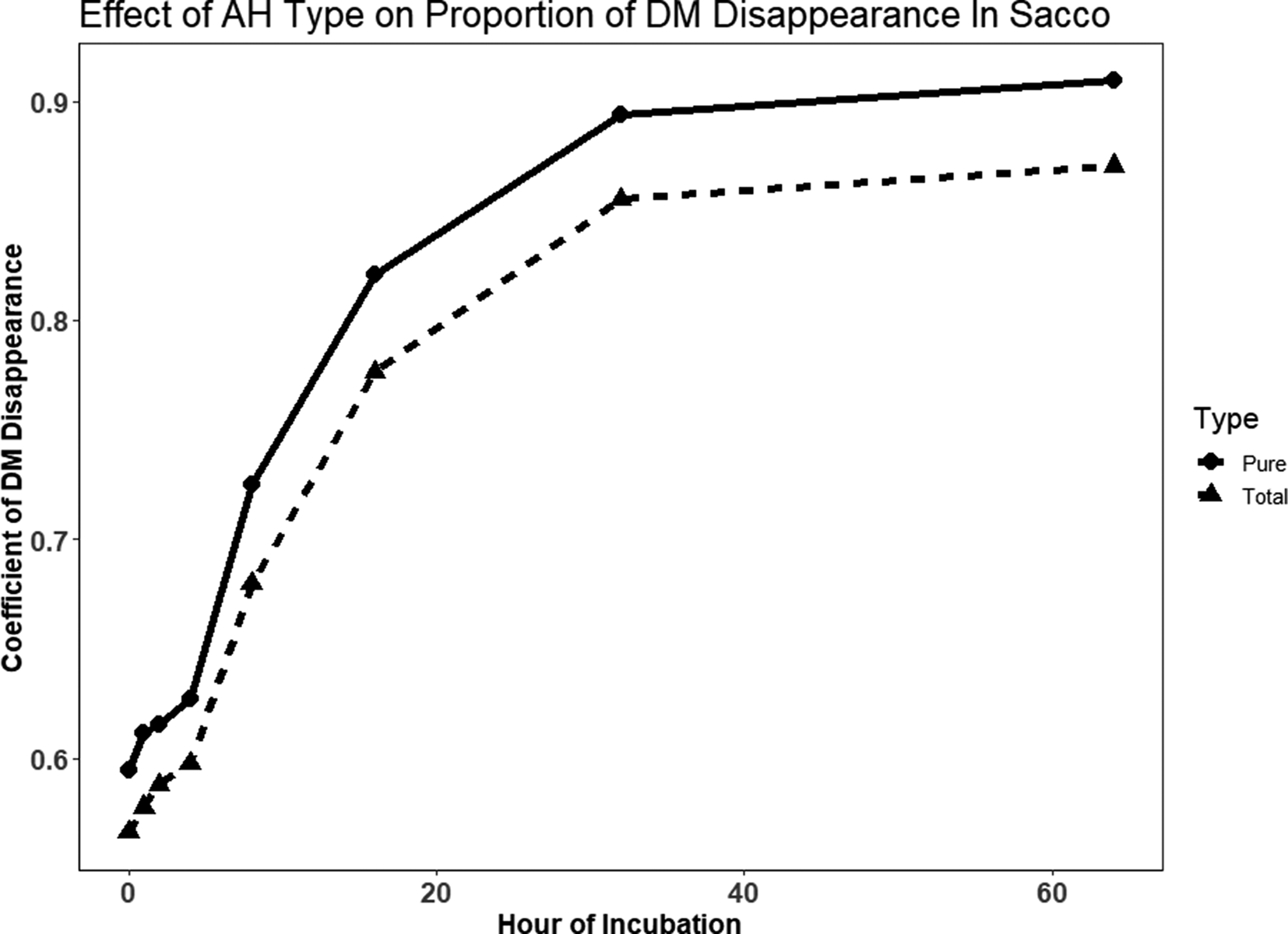
Fig. 1. Raw data for in sacco dry matter (DM) proportion of disappearance (digestibility coefficient) for each Type (Pure AH or Total AH) where Total AH contains AH and Debris and Pure AH contains only hulls and no Debris (Debris is sticks and shells).
Table 4. In sacco dry matter (DM) and neutral detergent fibre (NDF) disappearance for each Type (Pure almond hulls (AH) or Total AH).
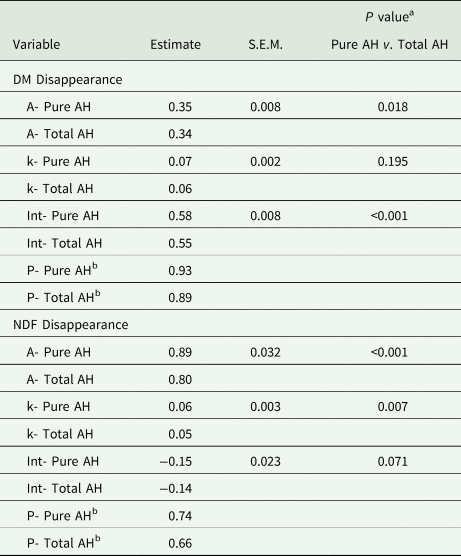
Total AH = contains AH and Debris; Pure AH = sorted to contain only hulls.
Effects of Type (Pure AH or Total AH) on coefficients of the in sacco disappearance function. The estimates were determined from the model. A are the estimated asymptote for the coefficient of disappearance for each Type. k are the corresponding rate constants (/h) for each Type. Int are the corresponding intercepts of time 0 with the coefficient of disappearance on the y-axis for each Type. P are the estimated Asymptote (A) + Intercept (Int) or total estimated proportion of disappearance (Eq. 4) for each Type.
a Pure v. Total = contrast between Pure almond hulls and Total almond hulls.
b These are calculated values: A + Int = P (proportion of disappearance).
Discussion
In vitro gas production and digestibility
The contribution of debris was reflected in the significantly lower amount of estimated total gas produced for TAH compared with PAH even though the overall estimated rate was not significantly different. Elahi et al. (Reference Elahi, Kargar, Dindarlou, Kholif, Elghandour, Rojas Hernández, Odongo and Salem2017) using rumen fluid from sheep reported a rate of gas production for dry AH of 0.09/h, which is similar to the current findings. Jafari et al. (Reference Jafari, Alizadeh and Imani2011) evaluated the impact of AH variety on in vitro rumen fermentation. Total gas produced (ml/g DM), rate of gas production (ml/h) and organic matter coefficient of digestibility differed by variety as seen with Rabei (79.5, 0.13, 0.823), Mamaei (78.9, 0.13, 0.815), Shahroud15 (63.1, 0.11, 0.68) and Shokoufe (70.1, 0.12, 0.715) respectively. Similar to the results in this study, the Rabei variety that had the greatest gas production also had the highest non-fibre carbohydrates (NFC) and lowest acid detergent lignin (ADL) concentrations (Jafari et al., Reference Jafari, Alizadeh and Imani2011; DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020). The Nonpareil variety in our study also had numerically the highest estimated amount and rate of gas production along with greater NFC and lower lignin content for all types when compared with the Other Variety (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020). Offeman et al. (Reference Offeman, Holtman, Covello and Orts2014) also found that Nonpareil AH had the highest fermentable sugar content when compared with other varieties grown in California. Rumen microorganisms are able to easily break down and ferment NFC, while lignin is mostly undegradable, so greater NFC content could lead to an overall increase in fermentation and improved digestibility (Nocek and Russell, Reference Nocek and Russell1988).
The lower ME concentration of Debris contributed to the numerically lower energy content of TAH compared with PAH. The larger difference in estimated ME content for Nonpareil compared with the Other Variety was likely due to the differences in aNDF, lignin, ash and NFC content. As reported previously (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020), Nonpareil TAH contained 21.4% aNDF, 8.6% lignin, 7.0% ash and 65.4% NFC compared to the Other Variety TAH that were 25.5% aNDF, 9.7% lignin, 7.6% ash and 60.7% NFC. Similar trends were observed for PAH and Debris for Nonpareil and Other Varieties (DePeters et al., Reference DePeters, Swanson, Bill, Asmus and Heguy2020). These differences in composition would account for the lower ME concentration of Other Variety compared with Nonpareil across all Types.
The lower IVTD and NDFD for Debris contributed to the lower digestibility of TAH compared with PAH at 24, 48 and 72 h for IVTD and 48 and 72 h for NDFD. The digestibility of aNDF for PAH compared with TAH at 12 h of does not agree with changes in IVTD, but could be linked to the greater amount of aNDF due to the presence of sticks and shells in TAH compared with PAH. Lignin, a fraction of aNDF, was measured as ADL in this study. The ADL does not include soluble lignin, such as Klason lignin, which is usually measured at greater amounts than ADL (Hatfield et al., Reference Hatfield, Jung, Ralph, Buxton and Weimer1994). Queirós et al. (Reference Queirós, Cardoso, Lourenço, Ferreira, Miranda, Lourenço and Pereira2020) found that almond shells had between 5–9% soluble lignin and 27.9–30.5% Klason lignin. Hall (Reference Hall2000) reported that AH have 16.9% soluble fibre, which would include soluble lignin. It is possible that some of the greater aNDF (24.3% TAH; 20.9% PAH) observed in TAH in this study was soluble lignin, which quickly solubilized within the first 12 h of incubation. This would lead to a deceptively high aNDF digestibility amount at 12 h for TAH compared with PAH. At this time more research still needs to be done on the type and amount of lignin in AH.
In sacco digestibility
In this study, PAH had greater (P = 0.018 and P < 0.001 respectively) overall estimated asymptotes for DM and NDF disappearance, along with a greater estimated rate of disappearance for NDF. The calculated total proportion of disappearance was numerically greater for PAH for both DM and NDF. The significant difference in PAH and TAH DM coefficient of disappearance intercepts describes the difference in the amount of material that washed out of the monofilament bags during the rinse. Yalchi and Kargar (Reference Yalchi and Kargar2010) compared stone shell and paper shell (similar to soft shell of Nonpareil and the hard shell of Other Variety) AH in the rumens of four sheep. The degradation rate of DM for stone shell (0.067/h) and paper shell (0.063/h) differed. The proportion of degradation of DM was also greater for stone AH (0.81) compared with paper AH (0.77). The degradation rate of NDF was 0.054 and 0.046/h and degradation coefficients were 0.56 and 0.52 for stone shell and paper shell AH, respectively. Yalchi (Reference Yalchi2011) evaluated PAH in the rumen of three sheep at seven-time points ranging from 2 to 96 h. Digestibility coefficients of DM for PAH were 0.47 at 2 h and 0.77 at 96 h compared with 0.24 at 2 h and 0.67 at 96 h for alfalfa hay. Interestingly, the digestibility of NDF in PAH was lower than in alfalfa hay at all times points except for the 96 h time point. The perception is that the fibre fraction of AH is highly digestible. However, the findings of Yalchi (Reference Yalchi2011) question this view. In fact, earlier work by DePeters et al. (Reference DePeters, Fadel and Arosemena1997) reported that for three samples of AH, the proportion of NDF remaining after 72 h of in situ digestion averaged 0.14, for a digestibility coefficient of 0.86. In contrast, the proportion of NDF remaining for beet pulp was 0.036 and 0.042 for soy hulls. The NDF digestibility of AH deserves further study.
Conclusions
Overall, this study found that Debris was not as digestible as PAH and TAH, and contributed to TAH having significantly lower IVTD and NDF digestibility at 48 and 72 h, along with numerically lower calculated ME and significantly lower gas production when compared with PAH. This is important for dairy producers in California who need high quality, digestible feeds to support milk production. Reducing the amount of Debris contamination in commercial AH is one important approach to improving the nutritive value of AH for ruminants and to improving the overall monetary value of the hulls for almond hullers.
Acknowledgements
The authors thank the Almond Board of California, Biomass Workgroup for funding this research and for supplying almond hull samples from various hullers. The authors acknowledge the advice of K. Lapsley and G. Huang from the Almond Board of California. We thank the dairy staff (D. Gisi, M. Patino, J. Hernandez and P. Domer) and the student research assistants (K. Kennicutt and S. Anderson) for their support. We acknowledge the input of the CA Chapter of ARPAS. Research was supported by the CA Agricultural Experiment Station at U.C. Davis through Multistate NC-2042.
Financial support
This research was funded by the Almond Board of California, Biomass Workgroup.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
All animal protocols were approved by the Institutional Animal Care and Use Committee.








