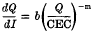Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Addiscott, T. M.
1970.
Potassium: calcium exchange in soils of the Broadbalk experiment at Rothamsted.
The Journal of Agricultural Science,
Vol. 75,
Issue. 3,
p.
451.
Addiscott, T. M.
1970.
The uptake of initially available soil potassium by ryegrass.
The Journal of Agricultural Science,
Vol. 74,
Issue. 1,
p.
123.
Johnston, A. E.
and
Addiscott, T. M.
1971.
Potassium in soils under different cropping systems:1. Behaviour of K remaining in soils from classical and rotation experiments at Rothamsted and Woburn and evaluation of methods of measuring soil potassium.
The Journal of Agricultural Science,
Vol. 76,
Issue. 3,
p.
539.
Addiscott, Thomas M.
1974.
Potassium and the absorption of calcium and magnesium by potato plants from soil.
Journal of the Science of Food and Agriculture,
Vol. 25,
Issue. 9,
p.
1165.
Singh, Bijay
Sharma, K. N.
and
Rana, D. S.
1978.
The quantity-intensity relations of potassium in soils from plots having nine fixed crop rotations for six years.
Plant and Soil,
Vol. 50,
Issue. 1-3,
p.
363.
Grimme, H.
and
Nair, P. K. R.
1979.
Q/I relations and electroultrafiltration of soils as measures of potassium availability to plants.
Zeitschrift für Pflanzenernährung und Bodenkunde,
Vol. 142,
Issue. 1,
p.
87.
Sinclair, A. H.
1982.
A comparison of electro-ultrafiltration and quantity/intensity measurements of soil potassium with its uptake by ryegrass in Scottish soils.
Plant and Soil,
Vol. 64,
Issue. 1,
p.
85.
Sinclair, A. H.
1982.
Application of Electro-Ultrafiltration (EUF) in Agricultural Production.
p.
85.
Ganeshamurthy, AN
and
Biswas, CR
1984.
Q/I relationship of potassium in two soils of long-term experiments.
Fertilizer Research,
Vol. 5,
Issue. 2,
p.
197.
Cervantes, C. E.
and
Hanson, R. G.
1991.
A potential method to incorporate quantity/intensity into routine soil test interpretations.
Communications in Soil Science and Plant Analysis,
Vol. 22,
Issue. 7-8,
p.
683.
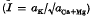 were measured in soil samples from manuring experiments at Rothamsted and Woburn. Within each experiment, Q/I curves for different K-manuring treatments were super-imposable on each other and on the curve relating exchangeable K to Io, the activity ratio at which the soil neither gains nor loses K. The distances on the Q axis between the curves were equal to the differences in exchangeable K.
were measured in soil samples from manuring experiments at Rothamsted and Woburn. Within each experiment, Q/I curves for different K-manuring treatments were super-imposable on each other and on the curve relating exchangeable K to Io, the activity ratio at which the soil neither gains nor loses K. The distances on the Q axis between the curves were equal to the differences in exchangeable K.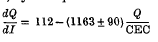 (Q and CEC in m-equiv/100 g)
(Q and CEC in m-equiv/100 g)