Management Implications
Mangroves are an invaluable component of coastal ecosystems in Florida. However, they are subject to invasion by species such as Schinus terebinthifolia (Brazilian peppertree). Management in these systems is extremely difficult due to difficulties with access and potential non-target issues for protected mangrove species. This study examined the efficacy of imazamox and carfentrazone-ethyl on S. terebinthifolia and the potential non-target impacts on four mangrove species in Florida. The selectivity exhibited by these herbicides in other systems warranted examination as a foliar application, which would be useful for aerial treatment options. In greenhouse studies on established saplings, we found that both imazamox and carfentrazone-ethyl provided significant defoliation, but incomplete kill of S. terebinthifolia. Additionally, both herbicides resulted in generally severe damage to all four mangrove species tested. These results do not immediately provide new management recommendations but inform us on the continuing struggle to find selective treatments for effective S. terebinthifolia control in mangrove communities. Future research should focus on enhancing other approaches, including more selective herbicide application methods and biological control.
Introduction
Brazilian peppertree (Schinus terebinthifolia Raddi) is the most abundant invasive shrub in Florida. It is widely distributed throughout peninsular Florida, where it has invaded numerous ecosystems including many freshwater and brackish wetlands. Currently, S. terebinthifolia occupies more than 12,000 ha of mangrove habitat in southwest Florida (Ewe and Sternberg Reference Ewe and Sternberg2007; Jones and Doren Reference Jones, Doren, Brock, Wade, Pysek and Green1997). The species exhibits a sprawling growth habit, forming dense, tangled thickets that exclude native species through competition and exclusion (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008). It is a prolific seed producer and is dispersed by water and frugivorous birds (Dlamini et al. Reference Dlamini, Zachariades and Downs2018; Donnelly and Walters Reference Donnelly and Walters2008). Additionally, sprouting is common from epicormic buds around the root collar and lateral roots (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008).
In Florida, S. terebinthifolia invasions have negatively impacted native mangrove communities. Mangroves (woody plants with strong tolerance to brackish water conditions) are a critical component of many tropical coastal ecosystems worldwide, providing essential ecosystem services such as coastal stabilization, wave action buffering, and wildlife habitat (Laegdsgaard and Johnson Reference Laegdsgaard and Johnson2001; Nagelkerken et al. Reference Nagelkerken, Blaber, Bouillon, Green, Haywood, Kirton, Meynecke, Pawlik, Penrose, Sasekumar and Somerfield2008; Thayer et al. Reference Thayer, Colby and Hettler1987; Tomlinson Reference Tomlinson1994). Management of S. terebinthifolia is particularly challenging within mangrove habitat, where high stem density, hidden pneumatophores, and overlapping brace roots in standing water make access extremely difficult for ground crews. Aerial herbicide treatments would allow managers to avoid many of these challenges; however, mangroves are generally highly sensitive to herbicides (Doren and Jones Reference Doren, Jones, Simberloff, Schmitz and Brown1997; Enloe et al. Reference Enloe, Leary, Prince, Sperry and Lauer2020a; Westing Reference Westing1971). Given a lack of other effective tools, there is a need for more selective herbicide options that would make aerial treatments feasible in mangrove communities.
Imazamox and carfentrazone-ethyl are two herbicides labeled for use in upland and aquatic systems that have provided selective control of other invasive plants. In aquatic systems, imazamox alone or in combination with carfentrazone-ethyl controls emergent invasive plants, including Uruguay water primrose [Ludwigia grandiflora (Michx.) Greuter & Burdet ssp. hexapetala (Hook. & Arn.) G.L. Nesom & Kartesz; syn: Ludwigia hexapetala (Hook. & Arn.) Zardini, Gu, & Raven] (Enloe and Lauer Reference Enloe and Lauer2017; Enloe et al. Reference Enloe, Prince, Banu and Lauer2020b). Imazamox has been shown to selectively control cattail (Typha domingensis Pers.) with no measurable impact on several native emergent species in a sawgrass (Cladium sp.) marsh (Rodgers and Black Reference Rodgers and Black2012). Carfentrazone-ethyl alone controlled water hyacinth [Eichhornia crassipes (Mart.) Solms], water lettuce (Pistia stratiotes L.), and water fern (Salvinia minima Baker), but was selective for landoltia [Landoltia punctata (G. Mey.) D. H. Les & D. J. Crawford] (Koschnick et al. Reference Koschnick, Haller and Chen2004). Carfentrazone-ethyl has also been useful in combination with other modes of action to broaden the spectrum of weed control in both aquatic and upland conditions (Brosnan et al. Reference Brosnan, Breeden, Henry and Walls2012; Gray et al. Reference Gray, Madsen, Wersal and Getsinger2007; Sharma and Singh Reference Sharma and Singh2007).
Imazamox also selectively controls certain woody species, including Chinese tallowtree [Triadica sebifera (L.) Small] in bottomland hardwood sites (Enloe et al. Reference Enloe, Loewenstein, Streett and Lauer2015) and hen’s eyes (Ardisia crenata Sims) in hardwood hammocks (Cristan et al. Reference Cristan, Minogue, Enloe, Sellers and Osiecka2019). Oaks (Quercus spp.) are generally tolerant to imazamox (Vasic et al. Reference Vasic, Konstantinovic and Orlovic2014). However, there are limited data on the impact of carfentrazone-ethyl on woody species in general, given that its primary uses have been for herbaceous weed control in agricultural, turf, and aquatic settings. It is possible that these herbicides will also provide effective, selective control of S. terebinthifolia without damaging mangroves.
Given the difficulties of S. terebinthifolia management within mangrove communities, there is a need for selective aquatic-labeled herbicide treatments that can be used for aerial applications. Here, we evaluated the sensitivity of S. terebinthifolia and four mangrove species to imazamox and carfentrazone-ethyl. If successful, this would be the first selective foliar treatment option for woody invasive plant control in mangroves.
Materials and Methods
A greenhouse study was conducted from July 2018 through January 2019 at the University of Florida’s Center for Aquatic and Invasive Plants in Gainesville, FL (29.721542°N, 82.417300°W). The study was conducted in parallel with other mangrove herbicide studies and used a similar approach (Enloe et al. Reference Enloe, Leary, Prince, Sperry and Lauer2020a). Schinus terebinthifolia plants were propagated from seed collected in Largo, FL (27.882833°N, 82.811725°W). Four mangrove species, all native to Florida, were evaluated. These included black mangrove [Avicennia germinans (L.) L.], button mangrove (Conocarpus erectus L.), white mangrove [Laguncularia racemosa (L.) C.F. Gaertn.], and red mangrove (Rhizophora mangle L.). All mangroves were purchased from a commercial nursery (Florida Native Plants Nursery, Sarasota, FL, USA).
Saplings of all five species were grown out to approximately 30 cm in height and transplanted into 11.3-L pots containing a commercial potting mix (Sun Gro® Metro-Mix® 510, Sun Gro Horticulture, Agawam, MA, USA) and slow-release fertilizer (Osmocote® Plus, Scotts, Maryville, OH, USA). Pots were placed in 51-L tubs and subirrigated with well water (0.2 ppt salinity). Subirrigation levels varied by species in tubs. Schinus terebinthifolia, C. erectus, and L. racemosa were submersed to 3-cm depth while R. mangle and A. germinans were submersed at a slightly greater depth of 5 cm to simulate the commonly wetter, more inundated conditions that they grow within. Water levels were evaluated weekly and adjusted as needed to maintain depth. Plants were maintained under these conditions until they reached 80 cm in height and approximately 1.7 cm in root collar diameter.
Two experimental runs were conducted on July 18 and 24, 2018. Plants were treated with foliar applications of imazamox (Clearcast®, SePRO, Carmel, IN, USA) alone at 0, 0.28, or 0.56 kg ai ha−1 or in combination with carfentrazone-ethyl (Stingray®, SePRO) at 0 or 0.1 kg ai ha−1. For imazamox, these rates were based on rate recommendations for aquatic and woody species (Anonymous 2016). For carfentrazone-ethyl, the rate selected was based upon recommendations for several aquatic species (Anonymous 2017). All herbicide treatments included a methylated seed oil (MSO Concentrate with Leci-Tech, Helena, Collierville, TN, USA) at 2.3 L ha−1. Treatments were applied over the foliar canopy of each plant as a single directional pass, 45 cm above the highest point of the canopy. We used a CO2-pressurized backpack sprayer equipped with three TeeJet® 11002 DG nozzles (TeeJet Technologies, Spraying Systems, Wheaton, IL, USA) calibrated to deliver 187 L ha−1 at 276 kPa. To prevent drift injury in the confined greenhouse, all plants were briefly removed from their tubs, treated outside, and allowed to air-dry for several minutes before being returned to the greenhouse. Daily greenhouse temperatures were maintained between 29/24 C (maximum/minimum) throughout both experimental runs, with supplemental heating during the winter months.
Visual estimations of percent defoliation were conducted at 30 and 90 d after treatment (DAT) for all species, as well as at 180 DAT for S. terebinthifolia. A percentile scale was used, where 0% indicated no defoliation, while 100% indicated complete defoliation. Additional quantitative estimates of plant health were made for all species at 180 DAT to determine percent cambium kill. Percent cambium kill was determined for each species by gently scraping the bark with a razor blade on one side of the main stem, along the whole length between the terminal bud and the root collar. The length of live cambium starting from the collar was recorded as a proportion of the total stem length. For all species, live cambium was distinguished as green and hydrated, while dead cambium was identified as being dry and brown. Plant survival was determined using cambium data and the visual percent defoliation data; plants with no live green cambium to the soil line and 100% defoliation were recorded as dead, and all others were recorded as alive. For the four mangrove species, total leaf area and leaf counts were also taken at 180 DAT. Leaf area was measured with a leaf area meter LI-3100C (Li-Cor Biosciences, Lincoln, NE, USA).
Statistical Analysis
A completely randomized design was used, and there were four replicate pots per treatment with two experimental runs. Data were subjected to a mixed-model ANOVA, in which imazamox rate and carfentrazone inclusion were considered fixed effects, while experimental run and replicate (nested in experimental run) were considered random effects. The efficacy of imazamox and carfentrazone-ethyl herbicides were compared within species. The analysis partitioned treatment comparisons to that of a 2 by 2 factorial part for imazamox level (0.28 or 0.56 kg ha−1) with or without carfentrazone-ethyl and a comparison of treatments to the appropriate controls (nontreated or carfentrazone only). Treatment means and main effect means were compared with both the nontreated control and the carfentrazone-ethyl only reference treatment via Bonferroni adjustment for multiplicity at the 5% level.
Analysis was performed using the PROC GLIMMIX (Littell et al. Reference Littell, Milliken, Stroup, Wolfinger and Schabenberger2006) package of SAS® v. 9.4 software (SAS Institute, Cary, NC, USA). All percent data utilized the arcsine square-root transformation, and the count data (leaf count, leaf area) utilized the square-root transformation to improve normality and homogeneity of variance (Snedecor and Cochran Reference Snedecor and Cochran1989). Further nonhomogeneity of variance was addressed by grouping variation within run by treatment, when appropriate, based on Akaike’s information criteria and residual graphs. Treatment means were compared at the 5% significance level using Tukey’s adjustment for multiplicity, and the comparison of main effect means and treatment means to the appropriate controls (nontreated or carfentrazone only) were performed using Bonferroni’s adjustment for multiplicity.
Results and Discussion
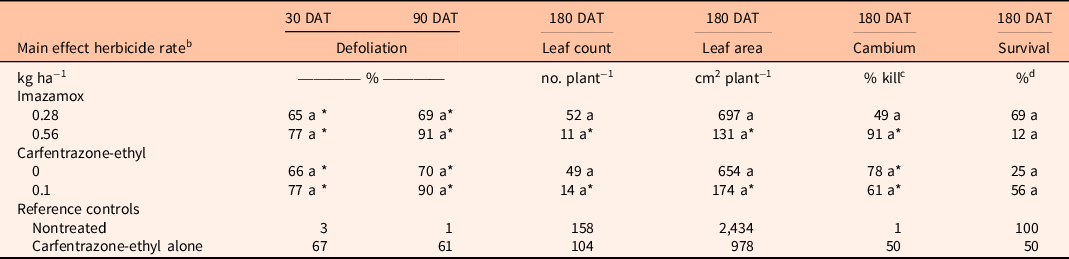
There were no significant interactions between imazamox rate and carfentrazone-ethyl on S. terebinthifolia response at any sample date (P > 0.05); however, main effects were detected for both herbicides (Table 1). Schinus terebinthifolia defoliation did not differ between imazamox rates at any sample date. Additionally, S. terebinthifolia defoliation was not different between either imazamox rates or the carfentrazone-ethyl reference treatment at any sample date. However, defoliation with imazamox at either rate was always greater than for the nontreated control. Inclusion of carfentrazone-ethyl resulted in 23% greater S. terebinthifolia defoliation at 30 DAT, although defoliation at 90 and 180 DAT was similar among treatments with and without carfentrazone-ethyl. Likewise, 37% greater S. terebinthifolia defoliation was observed at 30 DAT when carfentrazone-ethyl plus imazamox at either rate was applied compared with carfentrazone-ethyl alone.
Table 1. Comparison of imazamox and carfentrazone-ethyl main effects on Schinus terebinthifolia percent defoliation at 30 and 90 d after treatment (DAT) and percent cambium kill and percent survival at 180 DAT. a
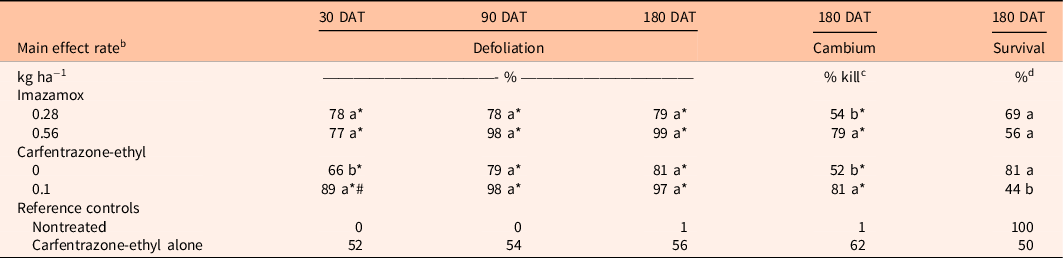
a Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by the same letter are not different according to Tukey’s adjustment for multiplicity (α = 0.05). Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by an asterisk (*) are different from the nontreated reference at the 5% level using the Bonferroni adjustment for multiplicity. Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by a number sign (#) are different from the carfentrazone-ethyl only reference at the 5% level using the Bonferroni adjustment for multiplicity.
b A methylated seed oil was added to each herbicide treatment at 2.3 L ha−1.
c Percent cambium kill was calculated from the length of live cambium measured in relation to the total stem length.
d Plant survival was determined as follows: plants with 100% defoliation and no live green cambium from the tallest shoot tip to the soil line were recorded as dead, and all others were recorded as alive.
Schinus terebinthifolia cambium kill was 25% greater in treatments containing imazamox at 0.56 kg ha−1 compared with imazamox at 0.28 kg ha−1 (Table 1). Similarly, S. terebinthifolia cambium kill increased 29% when carfentrazone-ethyl was included in imazamox treatments compared with imazamox alone. However, S. terebinthifolia cambium kill in all treatments containing imazamox was similar to carfentrazone-ethyl alone. Schinus terebinthifolia survival did not differ between imazamox rates or among imazamox treatments and the nontreated control or carfentrazone-ethyl alone (Table 1). However, S. terebinthifolia survival decreased 37% when carfentrazone-ethyl was included in imazamox treatments. Regardless, S. terebinthifolia survival did not differ from the nontreated control in any herbicide treatment.
For A. germinans, the interaction between imazamox rate and carfentrazone-ethyl and the imazamox main effect were not significant for any variables tested. By 90 DAT, imazamox at both rates resulted in 58% to 59% defoliation, which was different from the nontreated control (Table 2). At 180 DAT, a similar pattern was found for leaf count, leaf area, and percent cambium kill, as imazamox significantly reduced the first two variables and increased the third compared with the nontreated controls. Survival at 180 DAT was not different from that of the nontreated control. However, the defoliation data, when coupled with the negative impact on leaves and live cambium, eliminate the possibility of operational treatment selectivity for this species.
Table 2. Comparison of imazamox and carfentrazone-ethyl main effects on Avicennia germinans percent defoliation at 30 and 90 d after treatment (DAT) and leaf count, leaf area, cambium percent kill, and percent survival at 180 DAT. a
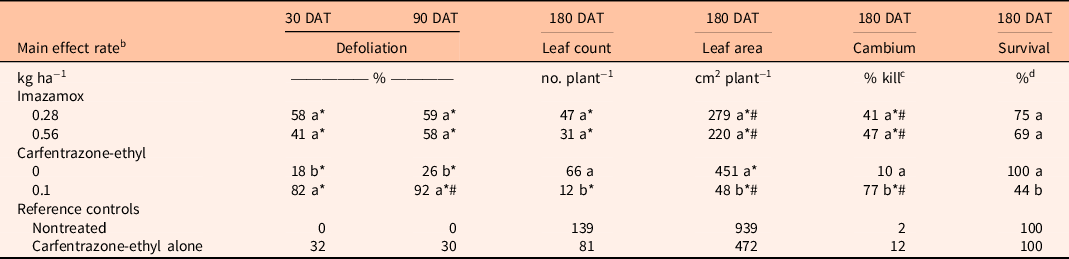
a Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by the same letter are not significantly different at the 5% level (LSD). Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by an asterisk (*) are different from the nontreated reference at the 5% level using the Bonferroni adjustment for multiplicity. Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by a number sign (#) are different from the carfentrazone-ethyl alone reference at the 5% level using the Bonferroni adjustment for multiplicity.
b A methylated seed oil was added to each herbicide treatment at 2.3 L ha−1.
c Percent cambium kill was calculated from the length of live cambium measured in relation to the total stem length.
d Plant survival was determined as follows: plants with 100% defoliation and no live green cambium from the tallest shoot tip to the soil line were recorded as dead, and all others were recorded as alive.
As a main effect, carfentrazone-ethyl significantly increased percent defoliation of A. germinans from 26% to 92% at 90 DAT (Table 2). This level of defoliation was greater than in the nontreated controls and the reference carfentrazone-ethyl control. By 180 DAT, carfentrazone-ethyl reduced leaf count and leaf area and increased percent cambium kill compared with the nontreated control. The severity of these reductions would also preclude the use of carfentrazone-ethyl as a selective treatment when A. germinans is present.
For C. erectus, there were no significant interactions between imazamox and carfentrazone-ethyl for any variables tested or significant main effects for either of the two herbicides except percent survival for imazamox. Conocarpus erectus was very sensitive to both herbicides, as imazamox and carfentrazone-ethyl resulted in ≥73% defoliation at 90 DAT (Table 3). At 180 DAT, the high imazamox rate significantly reduced leaf count and leaf area and increased percent cambium kill compared with the nontreated control. The high rate of imazamox also reduced survival from 81% to 19% compared with the low rate. Carfentrazone-ethyl also reduced C. erectus leaf count and leaf area compared with the nontreated control.
Table 3. Comparison of imazamox and carfentrazone-ethyl main effects on Conocarpus erectus percent defoliation at 30 and 90 d after treatment (DAT) and leaf count, leaf area, cambium percent kill, and percent survival at 180 DAT. a
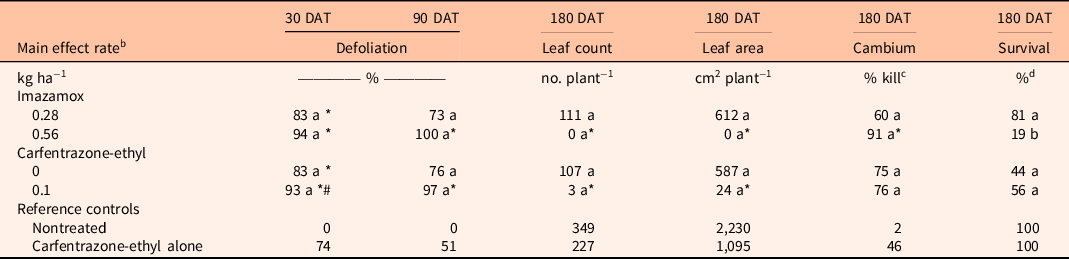
a Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by the same letter are not significantly different at the 5% level (LSD). Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by an asterisk (*) are different from the nontreated reference at the 5% level using the Bonferroni adjustment for multiplicity. Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by a number sign (#) are different from the carfentrazone-ethyl alone reference at the 5% level using the Bonferroni adjustment for multiplicity.
b A methylated seed oil was added to each herbicide treatment at 2.3 L ha−1.
c Percent cambium kill was calculated from the length of live cambium measured in relation to the total stem length.
d Plant survival was determined as follows: plants with 100% defoliation and no live green cambium from the tallest shoot tip to the soil line were recorded as dead, and all others were recorded as alive.
For R. mangle, there were no significant interactions between herbicides for all variables tested, and the impact of both herbicides was generally severe. At 90 DAT, percent defoliation was 75% to 100% for both herbicides (Table 4). At 180 DAT, the high rate of imazamox reduced leaf count and leaf area and increased percent cambium kill and percent survival compared with the nontreated control. Carfentrazone-ethyl also reduced leaf count, leaf area, and live cambium of R. mangle compared with the nontreated control.
Table 4. Comparison of imazamox and carfentrazone-ethyl main effects on Rhizophora mangle percent defoliation at 30 and 90 d after treatment (DAT) and leaf count, leaf area, cambium percent kill, and percent survival at 180 DAT. a
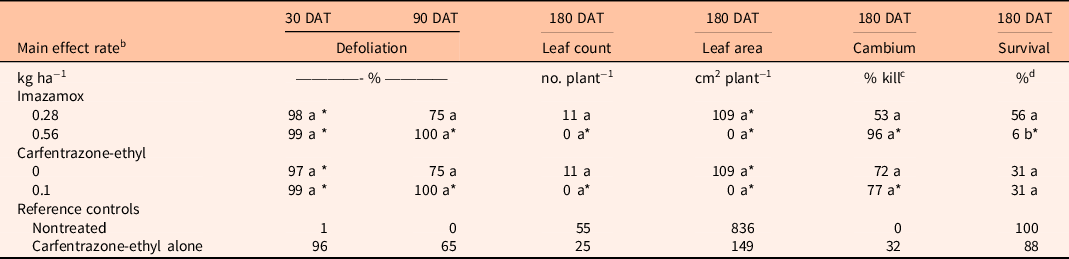
a Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by the same letter are not significantly different at the 5% level (LSD). Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by an asterisk (*) are different from the nontreated reference at the 5% level using the Bonferroni adjustment for multiplicity. Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by a number sign (#) are different from the carfentrazone-ethyl alone reference at the 5% level using the Bonferroni adjustment for multiplicity.
b A methylated seed oil was added to each herbicide treatment at 2.3 L ha−1.
c Percent cambium kill was calculated from the length of live cambium measured in relation to the total stem length.
d Plant survival was determined as follows: plants with 100% defoliation and no live green cambium from the tallest shoot tip to the soil line were recorded as dead, and all others were recorded as alive.
For L. racemosa, results were quite similar to R. mangle and C. erectus. There were no significant interactions between herbicides for all variables tested. Both imazamox and carfentrazone-ethyl resulted in high levels of defoliation at 90 DAT and reduced leaf count and leaf area and increased percent cambium kill at 180 DAT compared with the nontreated control (Table 5).
Table 5. Comparison of imazamox and carfentrazone-ethyl main effects on Laguncularia racemosa percent defoliation at 30 and 90 d after treatment (DAT) and leaf count, leaf area, cambium percent kill, and percent survival at 180 DAT. a
a Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by the same letter are not significantly different at the 5% level (LSD). Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by an asterisk (*) are different from the nontreated reference at the 5% level using the Bonferroni adjustment for multiplicity. Means within a column and main effect (imazamox or carfentrazone-ethyl) followed by a number sign (#) are different from the carfentrazone-ethyl alone reference at the 5% level using the Bonferroni adjustment for multiplicity.
b A methylated seed oil was added to each herbicide treatment at 2.3 L ha−1.
c Percent cambium kill was calculated from the length of live cambium measured in relation to the total stem length.
d Plant survival was determined as follows: plants with 100% defoliation and no live green cambium from the tallest shoot tip to the soil line were recorded as dead, and all others were recorded as alive.
The results from this study are highly informative concerning the impact of two aquatic herbicides on S. terebinthifolia and the four non-target mangrove species. The data indicate that both imazamox and carfentrazone-ethyl provided good defoliation of S. terebinthifolia. However, the presence of live green cambium at 180 DAT following either herbicide alone indicated a lack of effective long-term control. Furthermore, the lack of significant interaction between imazamox and carfentrazone-ethyl does not support any concept of useful synergism between the two herbicides on the target species. Although the tank mix with the lower rate of imazamox and carfentrazone-ethyl appeared promising at 30 DAT, the results were not significant at later evaluation dates. This agrees with other reports on fast-acting, protoporphyrinogen oxidase–inhibiting herbicides increasing the speed of defoliation of other lignified species in aquatic systems but offering no long-term complementation with slower-acting amino acid inhibitors (Enloe and Lauer Reference Enloe and Lauer2017).
Additionally, these data indicate high levels of injury to all four mangrove species from both herbicides. Early injury, as defoliation, was high for each species. This injury was also expressed in total reduction in leaf number and area and significant percent cambium kill at 180 DAT. The mangrove species tested have been previously documented to be highly sensitive to auxin-type herbicides (Enloe et al. Reference Enloe, Leary, Prince, Sperry and Lauer2020a; Teas Reference Teas1976; Westing Reference Westing1971). The imidazolinone herbicide imazapyr was reported to be highly injurious to R. mangle in eradication efforts in Hawaii, where it is nonnative (MacKenzie and Kryss Reference MacKenzie and Kryss2013). However, this is the first documented evidence of sensitivity to imazamox or carfentrazone-ethyl. Although not germane to mangrove protection, the high level of activity exhibited on these mangrove species warrants testing on invasive plants in the same families, including Mexican petunia (Ruellia simplex C. Wright; Acanthaceae), West Indian almond (Terminalia catappa L.; Combretaceae), and large-leafed orange mangrove [Bruguiera gymnorhiza (L.) Lam. ex Savigny; Rhizophoraceae]. All of these are locally problematic in Florida and additional management tools would be useful for each one (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008).
In conclusion, given that all four mangrove species are protected in Florida, these herbicides would not be useful for selectively eliminating S. terebinthifolia from mangrove communities. Currently, there are several herbicide active ingredients known to be highly effective, albeit without selectivity. While the search for a selective broadcast application is not exhausted, we have learned that mangroves are sensitive to a broader range of herbicides than previously known. Schinus terebinthifolia invasion has not reached its apex and will continue to alter the structure and function of mangrove ecosystems without action. Future research should revisit known effective chemistries to determine optimal thresholds that maintain efficacy on S. terebinthifolia while minimizing non-target damage.
Acknowledgments
The authors would like to thank Colette Jacono for assistance in mangrove propagation and maintenance as well as Jessie Solomon, Kenzie Bell, and Matt Shinego for assistance in treatment and data collection. This project was funded by the U.S. Department of Defense through the U.S. Army Corps of Engineers under agreement number W9126G-18-2-0065. The authors declare no conflicts of interest.








