Introduction
Invasive shrubs are an increasingly prevalent component of eastern deciduous forests in North America (Rejmánek Reference Rejmánek2014; Schulz and Gray Reference Schulz and Gray2013; Webster et al. Reference Webster, Jenkins and Jose2006). Some of the more common species include Amur honeysuckle [Lonicera maackii (Rupr.) Herder], Tatarian honeysuckle (Lonicera tatarica L.), privet species (Ligustrum spp.), and Japanese barberry (Berberis thunbergii DC.), which have been variously linked to declines in native species across taxa (e.g., herbaceous species and tree regeneration [Collier et al. Reference Collier, Vankat and Hughes2002; Hutchinson and Vankat Reference Hutchinson and Vankat1997; Link et al. Reference Link, Turnblacer, Snyder, Daugherty and Utz2018], pollinator services [McKinney and Goodell Reference McKinney and Goodell2010], amphibian diversity [Watling et al. Reference Watling, Hickman and Orrock2011], and insect communities [Hanula and Horn Reference Hanula and Horn2011a, Reference Hanula and Horn2011b]). Concern for native species and dramatic changes in vegetation structure brought about by shrub invasion have triggered interest in the restoration of forest understories through invasive shrub removal, with the expectation that removal will reverse the negative impacts of shrub invasion. However, management-relevant outcomes for the removal of a diverse invasive shrub community are lacking in the literature (but see Ward et al. Reference Ward, Williams and Linske2017).Reference Webster, Jenkins and Jose2001).
Management Implications
North America is the recipient of more invasive shrub species than any other region of the world, adding to the plethora of challenges natural resource managers already face in eastern deciduous forests. The research presented here builds on our understanding of the negative impacts of a diverse invasive shrub community and the benefits of its removal after 7 yr. Recurring management to remove a suite of invasive shrub species facilitates successful passive natural regeneration of native plants in a mature, eastern deciduous forest. Specifically, removal increases understory plant diversity, overstory tree species regeneration, and native plant abundance, including a herbaceous Species of Special Concern in the region. Of note, invasive herbs and native weedy species were not favored after 7 yr of treatment. This information helps build the case for invasive shrub removal. The passive natural regeneration observed here can be used as an estimate for potential regeneration in other, similar sites.
Our research also demonstrates that sampling the unmanaged abundance of invasive shrubs and native plants can underestimate the impacts that invasive shrubs have on native plant abundance. We found that 7 yr of invasive shrub removal facilitated passive natural regeneration of native plants that exceeded the abundance measured in unmanaged forest understories with ambient, naturally occurring, low levels of shrub invasion. This is important because several studies use the relationship between unmanaged, ambient abundances of invasive shrubs and native species to estimate the impacts of invasive shrubs. In this study, in which invasive shrubs have been prominent in the understory for over 20 yr, an ambient sampling approach underestimates the impact of invasive shrubs and the benefits of their removal.
Land managers face a complex and growing suite of challenges ranging from the uncertainty of a changing climate (Millar et al. Reference Millar, Stephenson and Stephens2007) to budget limitations restricting management options to the most effective and important. In eastern deciduous forests of the United States, challenges also include the decline and extirpation of overstory tree species from disease and/or pests (e.g., emerald ash borer [Agrilus planipennis Fairmaire], hemlock woolly adelgid [Adelges tsugae Annand], and gypsy moth [Lymantria dispar dispar Linnaeus, Aukema et al. 2011]), white-tailed deer (Odocoileus virginianus Zimmermann) overpopulation (Begley-Miller et al. Reference Begley-Miller, Hipp, Brown, Hahn and Rooney2014; Horsley et al. Reference Horsley, Stout and DeCalesta2003), and a growing number and abundance of invasive plant species (Schulz and Gray Reference Schulz and Gray2013; Webster et al. Reference Webster, Jenkins and Jose2006). The demands of managing multiple ecosystem stressors requires knowledge of the impacts of management actions so financial and institutional resources can be allocated effectively.
Invasive shrub removal experiments provide a practical approach to understanding the potential outcomes of management efforts. Largely used to quantify the impacts of invasive shrubs to native species, removal experiments have generally been applied on a small scale (≤5-m-diameter plots) and over a relatively short timeframe (five or fewer growing seasons) (e.g., Gould and Gorchov Reference Gould and Gorchov2000; Hartman and McCarthy Reference Hartman and McCarthy2004; Luken et al. Reference Luken, Kuddes and Tholemeier1997; Merriam and Feil Reference Merriam and Feil2002; Miller and Gorchov Reference Miller and Gorchov2004; Shields et al. Reference Shields, Saunders, Gibson, Zollner, Dunning and Jenkins2015; but see Hudson et al. Reference Hudson, Hanula and Horn2014; Ward et al. Reference Ward, Williams and Linske2017). In some cases, native species are planted to more quickly assess the impact of invasive shrub presence to survival (Hartman and McCarthy Reference Hartman and McCarthy2004) or to population demography (Gould and Gorchov Reference Gould and Gorchov2000; Miller and Gorchov Reference Miller and Gorchov2004). While such research has improved our understanding of the impacts of invasive shrubs, there remains a disconnect to natural regeneration following invasive shrub removal, especially on a larger scale as it would be carried out by land managers. Native plant communities take time to establish and may do so in successional stages in the absence of more targeted restoration efforts (Prach and Hobbs Reference Prach and Hobbs2008). For example, three growing seasons after invasive shrub removal (L. maackii) only frost grape (Vitis vulpina L.), an early successional species, had increased in abundance in the forest understory (Luken et al. Reference Luken, Kuddes and Tholemeier1997). Additionally, there is the potential for establishment to be delayed by soil legacy effects (Corbin and D’Antonio Reference Corbin and D’Antonio2012), the size of the removal treatments, and the removal treatment itself (Kettenring and Adams Reference Kettenring and Adams2011). Removal experiments at expanded spatial and temporal scales could account for these effects and provide a greater understanding of the potential outcomes of management efforts.
In this study, an invasive shrub removal experiment provides insight into passive natural regeneration after 7 yr of repeated invasive shrub removal. We paired an experimental approach comparing invasive shrub removal plots with data collection in nearby control (unmanipulated) plots to quantify the effect of removal treatments and provide an estimate of the impact of invasive shrub presence on the native plant community. We hypothesize that invasive shrub removal treatments will have a positive impact on understory plant species abundance, community diversity, and tree regeneration. In addition to the experimental approach, we systematically sampled the surrounding forest understory to establish a relationship between ambient, unmanaged invasive shrub and native species cover. We hypothesize a negative relationship between ambient invasive shrub abundance and native species abundances, as reported in other studies (e.g., Collier et al. Reference Collier, Vankat and Hughes2002; Greene and Blossey Reference Greene and Blossey2012; Hutchinson and Vankat Reference Hutchinson and Vankat1997; Link et al. Reference Link, Turnblacer, Snyder, Daugherty and Utz2018; Wilcox and Beck Reference Wilcox and Beck2007; Woods Reference Woods1993). By comparing the native community abundance in the removal experiment to areas in the ambient forest that naturally have low invasion, we can test the hypothesis that passive natural regeneration of native species can meet or exceed what is found in uninvaded portions of a forest. This will also help us to understand how accurately ambient sampling estimates the impact of invasive shrub removal treatments.
Materials and Methods
Study Site
The study took place in a 16-ha woodlot at the Arboretum at Penn State in central Pennsylvania, adjacent to State College (40.8°N, 77.9°W). The woodlot, named Hartley Wood, is a remnant, mature, mixed-hardwood forest dominated by oak (Quercus spp.), hickory (Carya spp.), and maple (Acer spp.) with an Opequon rock-outcrop complex (OxB) soil derived from limestone residuum. This forest escaped the widespread clearing for the iron industry between the late 18th century through the 19th century in the area, and so contains old-growth oak trees among the mature, mixed-age structure (Grinstead Reference Grinstead2007). The average January high from 1981 to 2010 was 1.2 C and the low was −6.6 C, and for July it was 27.6 C and 17 C (U.S. Climate Data 2018). Average annual precipitation is about 100 cm relatively evenly distributed throughout the year; however, during the year of sampling (2016), the central Pennsylvania region experienced the second year in a row of below-average rainfall (bottom one-third ranking of all records on the Palmer Drought Severity Index, PDSI=−2.2 [NOAA 2018]). The study site has experienced propagule pressure from invasive shrubs in the surrounding horticulture since at least 1929 from the Penn State Office of Physical Plant planting records, and by 2006 the forest was already heavily invaded by border privet (Ligustrum obtusifolium Siebold & Zucc.) and invasive honeysuckles (Lonicera spp.) with at least five other genera of invasive shrubs present (Grinstead Reference Grinstead2007). There are also some nonnative trees and invasive herbs and vines present in the forest (Supplementary Table S1). Vegetation sampling was carried out in two ways in 2016: (1) as part of an invasive shrub removal experiment and (2) as systematic sampling of the unmanaged, ambient forest understory. Each is described in detail in the following sections.
Experimental Removal Design and Sampling
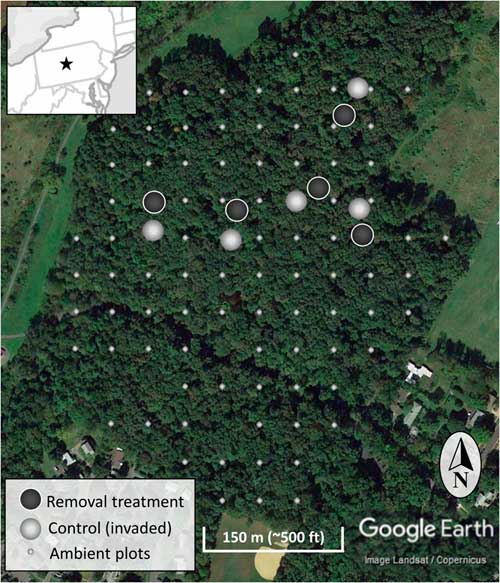
The study occurred in the year 7 of a long-term invasive shrub removal experiment underway in the forest (Figure 1) (Kaye and Hone Reference Kaye and Hone2016). The experiment consists of five sites randomly identified using GIS and located using hand-held GPS units, with paired invasive species removal (treatment) and control (invaded) plots each 20-m in diameter (circular; Figure 2). The pairs have plot centers approximately 30 m apart, and the removal treatment was randomly assigned to one of each pair in 2009 when invasive shrub removal was implemented. For the removal treatments, shrubs were cut at the base with hand clippers in April of 2009 and removed from the forest. During the 2009 growing season, emerging foliage from sprouting stumps and roots of the shrubs were treated with glyphosate (Roundup® Ready-To-Use Weed & Grass Killer III, 2% v/v solution, 12 g ae L−1, Monsanto, St Louis, MO). The removal treatments have been maintained by cutting new seedlings and the few surviving resprouts each spring since 2009. With much heavier propagule pressure than if implemented across the entire forest, manually maintaining the removal treatments conservatively ranged from 180 to 300 man-hours ha−1 (70 to 120 man-hours acre−1) each year. To evaluate the response of the native community to the removal treatments, all plants within the ten 20-m-diameter plots were identified to species, where possible. Sampling was done systematically in twenty-four 15° sectors, and the data were combined to the 20-m-diameter plot level afterward. Individual plants under 1-m tall were counted, and the cover of each species was estimated. An individual was counted based upon a shared root system. For sprawling species (e.g., vines) or those spreading by root system (e.g., clonal grasses), cover will more accurately represent abundance than count. To estimate cover, each taxon was considered separately, so any given plot could sum to greater than 100% cover if species or groups overlap in horizontal space. Individuals 1 to 4 m and those greater than 4 m were identified to species and counted. Additionally, the cover of invasive woody species and native woody species 1 to 3 m in height were estimated for a congruent comparison with the ambient forest plots. Native and invasive shrub species are vouchered in the Pennsylvania State University Herbarium.

Figure 1 Photographs of two of the pairs of removal treatment and control (invaded) plots 7 yr after the initial invasive shrub removal treatment.
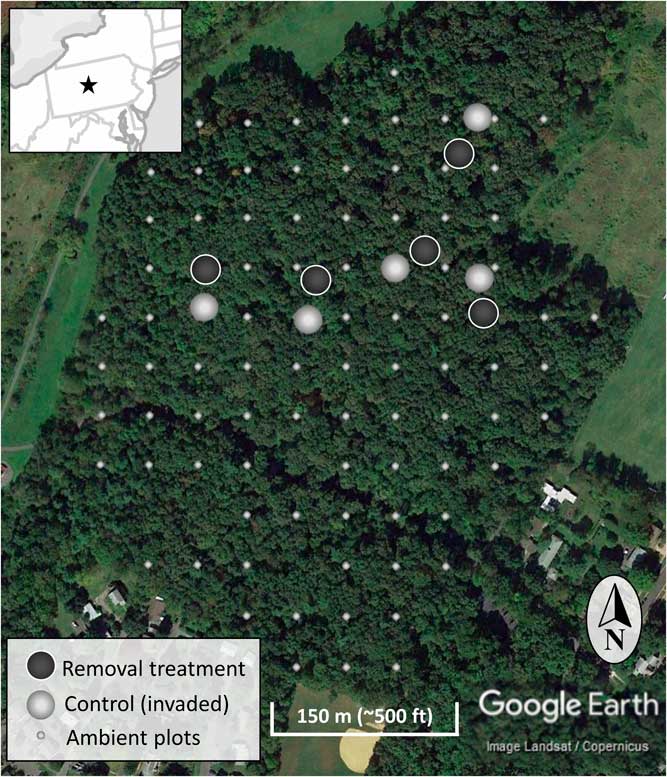
Figure 2 The location of the experimental invasive shrub removal treatment plots (dark gray), paired control plots (light gray), and ambient forest sampling plots (smaller light gray) within the forest. The inset map is a section of the northeastern United States centered on Pennsylvania and including portions of surrounding states. The star indicates the location of the research site.
Ambient Forest Sampling
Eighty-six 1.13-m-radius (1-milacre) plots were located in a 38 by 38 m (125 by 125 ft) grid (hereafter, ambient forest plots). The ambient forest plots were located in the 15-ha portion of the forest that overlaps with and is similar in overstory composition to the experimental plot area, and excluded an active restoration and management area in the northernmost hectare of the forest (Figure 2). Native herbaceous plant cover was estimated in three functional groups (gramminoids, forbs, ferns), but all other taxa were estimated at the species level or, when necessary, the genus level. Cover was estimated for all vegetation present within the plot regardless of where individuals were rooted, as was done for the experimental plots. Only individuals under approximately 3m (10ft) in height were considered. The ambient plots differ from the experimental control plots in their size and in that they span a larger range of invasive shrub cover, but cover data were collected to be comparable between these plot types.
Data Analyses
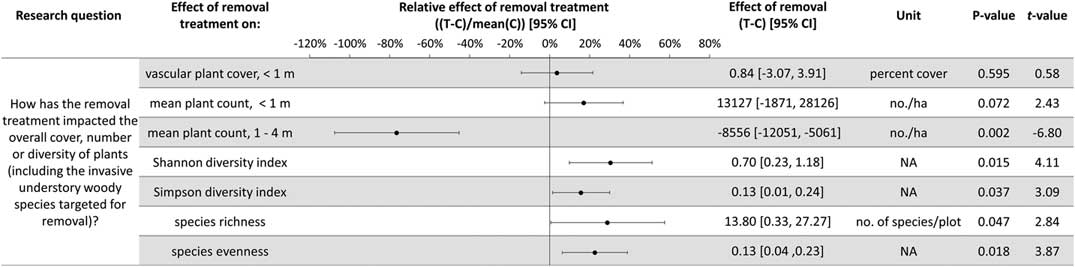
The experimental design using large plots (314 m2) required a trade-off for fewer plots (10), which prevented multivariate analysis of the community composition. As a result, several aspects of the understory plant community were examined separately to test and quantify the impact of invasive shrub removal. The abundance of all understory plants not targeted for removal was considered, as well as the following functional groups: native herbs (non-woody, seed-bearing plants, including perennials), understory woody species (shrubs, vines, and understory trees), and regenerating overstory tree species (seedlings and saplings). Abundance was quantified in three ways: cover less than 1 m in height, count of individuals less than 1 m in height, and count of individuals 1 to 4 m in height. Because of the potential for weedy, generalist species to be favored by a removal treatment that rapidly opens resources, the effect of repeated invasive shrub removal on invasive herb cover was tested. Along these lines, to test for a relative increase in weedy, generalist native forbs compared with woodland habitat-specific forbs in the removal treatments, the relative composition of native forbs was considered in terms of the ratio of woodland habitat–specific to generalist species cover. Species were categorized using habitat descriptions from Rhoads and Block (Reference Rhoads and Block2007): species assigned as “woodland habitat–specific” have only undisturbed forest types listed as habitat and “generalist” species have “disturbed” or another habitat type beyond forested listed (e.g., waste spaces, thicket, wet meadow). To assess the impact of the removal treatments to the entire plant community, the cover and diversity, including the species targeted for removal, were calculated and analyzed. This provides an overview of the changes to understory plant abundance with the removal treatment, as well as addressing the portion of the hypothesis relating to invasive shrub impacts to diversity. The diversity metrics calculated include the Shannon and Simpson diversity indices as well as species evenness and richness. For each of the response variables discussed above, two-way t-tests were used, and 95% confidence intervals (CIs) were estimated, to test whether the effect of removal (treatment plot value minus the paired control plot value) was significantly different from zero (α=0.05). In one case, the data did not meet the assumption of normality, so a Wilcoxon rank-sum test was performed for the effect of invasive shrub removal on invasive herbs. The effect of removal does not incorporate the original abundances, because this value is the difference between paired treatment and control plots. Therefore, the effect of the removal treatment relative to the local ambient levels was calculated by dividing the effect by the mean of the control plots (hereafter, the relative effect of the removal treatment). This simple conversion provides a percent change to relativize a given absolute effect and produces the same test statistics as the corresponding absolute effect tests (i.e., same t-values and P-values).
The hypothesis of a negative relationship between invasive shrub and native species abundance in the ambient forest was explored by summing the percent cover of all invasive shrubs and all native species within each ambient forest plot. Due to the left-skewed nature of most plant cover data, square-root, arcsin square-root, and cube-root transformations were each considered to best meet the assumptions of a linear relationship. A linear relationship was fit between the data, and the hypothesis of a negative relationship was tested by a significant model fit with a significant negative slope (α=0.05). This ambient forest relationship can be used to understand whether passive natural regeneration in the experimental plots meets or exceeds native understory cover in the unmanaged forest, and whether ambient sampling accurately estimates the impact of invasive shrubs.
The experimental plot abundances were compared with the ambient forest relationship using their respective 95% CIs to test for significant differences (α=0.05). To circumvent problems associated with parametric tests of small sample size (that tend to diverge from normality), bootstrapping with replacement (1,000 replicates) was used to estimate means of native species abundance and invasive shrub species abundance as well as their basic 95% CIs for both the removal treatment plots (n=5) and the control plots (n=5) (Davison and Hinkley Reference Davison and Hinkley1997). Analyses were performed using the ‘boot’ package in the programming environment R (Canty and Ripley Reference Canty and Ripley2017). All data analyses herein were performed using the programming environment R v. 3.4.3.
Results and Discussion
Invasion in the Forest
In the 86 ambient forest plots, the cover of invasive shrubs ranged from 1% to 162.5% cover, with a mean of 42.9 (SD=39.4). Invasive shrubs, in descending order of mean cover, include: L. obtusifolium (29%), bush honeysuckle (Lonicera morrowii A. Gray, 4%), linden arrowwood (Viburnum dilatatum Thunb., 4%), L. maackii (2%), winged burningbush [Euonymus alatus (Thunb.) Siebold, 1%], European cranberrybush (Viburnum opulus L., 1%), Hamilton’s spindletree (Euonymus hamiltonianus Wall., 1%), European buckthorn (Rhamnus cathartica L., 1%), autumn-olive (Elaeagnus umbellata Thunb., 1%), multiflora rose (Rosa multiflora Thunb., <1%), B. thunbergii (<1%), jetbead [Rhodotypos scandens (Thunb.) Makino, <1%]. Native species cover (under 3 m in height) ranged from 0% to 87% cover (mean=13.6, SD=17.3). The native species present consisted of: 31% herbs, 28% shrubs, 22% trees, and 19% vines by cover. Native shrubs were predominantly blackhaw (Viburnum prunifolium L.) and mapleleaf viburnum (Viburnum acerifolium L.), but seven genera were represented. Four woody vine genera were represented, but Virginia-creeper [Parthenocissus quinquefolia (L.) Planch] dominated. There were nine regenerating overstory tree genera. The woody species composition for the removal treatment and control plots is similar with some additional infrequent species represented: four native species (three additional genera) and six nonnative species (four additional genera). A complete species list, associated average abundances, functional groups, and constancy across the treatment and control plots is available in Supplementary Table S1.
Effect of Invasive Shrub Removal Treatments
Plant diversity, native understory species abundance, and overstory tree species regeneration have increased with invasive shrub removal (Figures 3 and 4). Plants under a meter in height that were not targeted for removal have increased by an average of 13.5% cover, which is a relative increase of about 250% (Figure 3). Despite the initial removal of more than 140,000 invasive shrub stems ha−1 (Kaye and Hone Reference Kaye and Hone2016), and the continued removal of new invasive shrub seedlings, the treatment and control plots do not differ in the cover and count of all plants under a meter in height (Figure 4). Plants not targeted for removal have filled the gap left by the removal treatments through passive natural regeneration, at least for plants under a meter in height. While the removal treatments also increase native shrubs 1 to 4 m in height (by 847 individuals ha−1; Figure 3), there remain on average 8,556 fewer individuals ha−1 in the removal treatment plots in this height class (Figure 4). This aligns with expectations for a mature, eastern deciduous forest, because invasive shrubs create a more closed understory than both recent (e.g., preinvasion [Woods Reference Woods1993]) and historical conditions (Braun [1916], cited in Collier et al. [Reference Collier, Vankat and Hughes2002]).
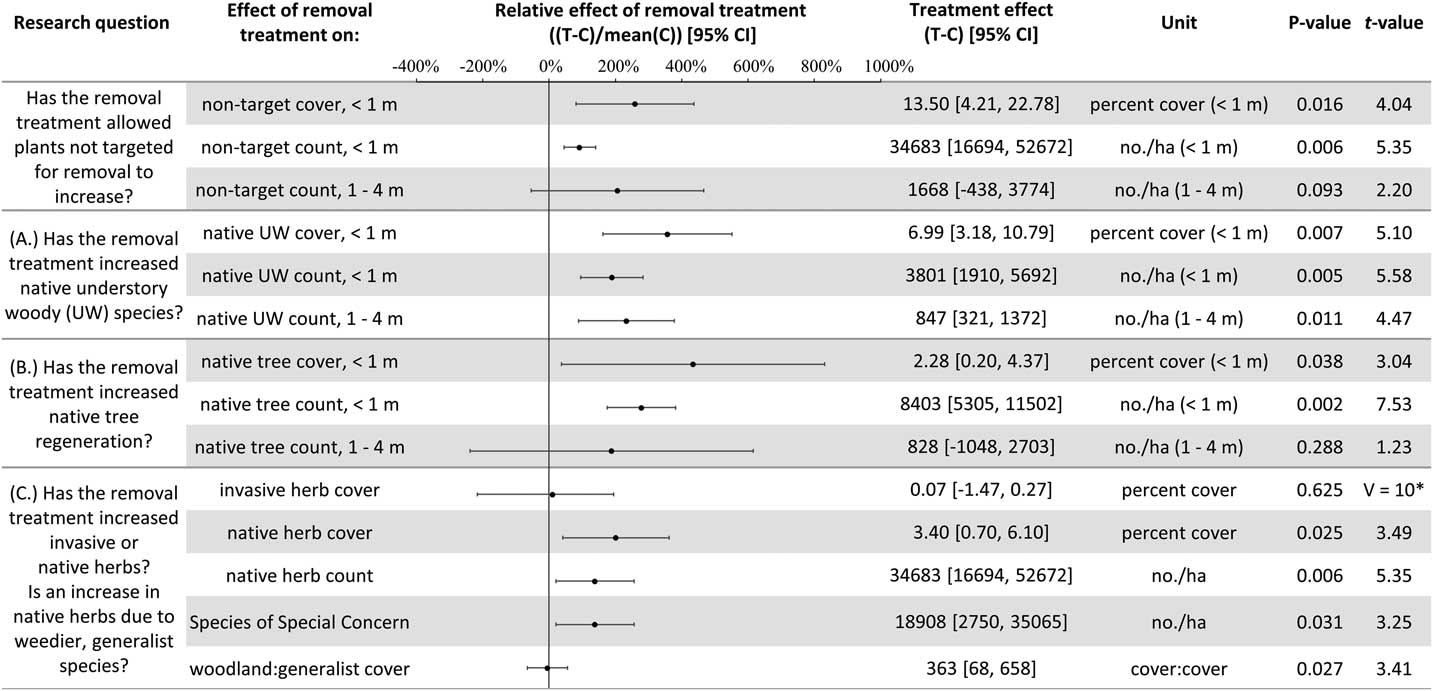
Figure 3 The effects of invasive shrub removal treatments on plant abundances. Plant abundance was measured as the percent cover of plants under 1 m in height, the count of individuals under 1 m, and the count of individuals 1 to 4 m in height. For example, the first line shows an average increase of 13.5% cover with the removal treatment for plants not targeted for removal and under 1 m in height, which constitutes about a 250% increase in cover in the removal treatment plots (T) compared with the control plots (C) (i.e., relative effect of removal treatment). The 95% confidence intervals (CIs) that do not overlap with zero are significant at the alpha cutoff level of 0.05 in the direction of the effect. *Wilcoxon rank-sum test was performed, because these data did not meet the assumptions of normality. The corresponding test statistic and the median value and 95% CI are displayed.
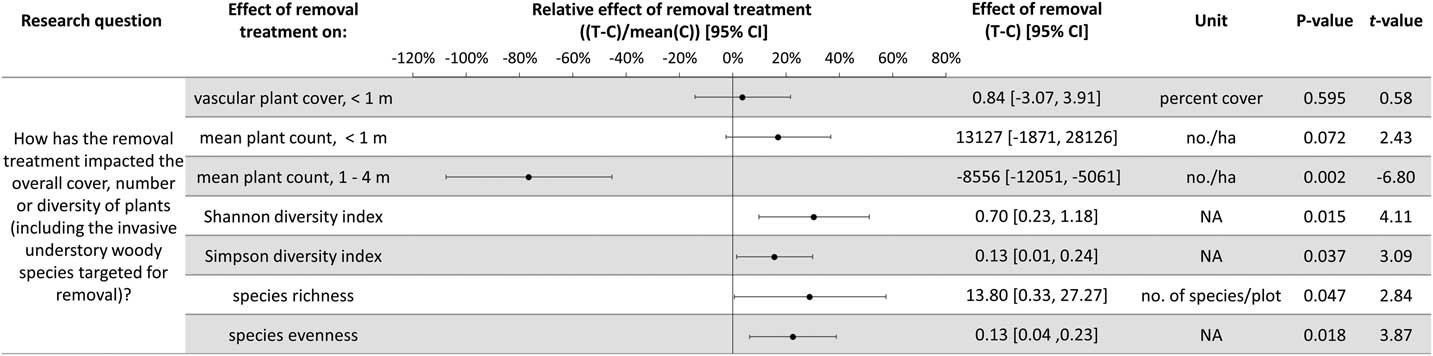
Figure 4 The effects of invasive shrub removal treatments on diversity and all plant abundances (including the invasive shrubs targeted for removal). Plant abundance was measured as the percent cover of plants under 1 m in height, the count of individuals under 1 m, and the count of individuals 1 to 4 m in height. For example, the first line indicates no difference with removal treatments (or between the removal treatment and control plots) in all plant cover under 1 m in height as indicated by 95% confidence intervals (CIs) that overlap with zero and the corresponding P-value exceeding the 0.05 cutoff. This indicates that passive natural regeneration in the removal treatment plots has replaced the large volume of stems under 1 m removed from the treatment.
In temperate deciduous forests, the majority of plant species diversity is found in the herbaceous layer (Gilliam Reference Gilliam2007). If biodiversity is a management priority, the herbaceous layer is an important consideration. Plant diversity (Figure 4) and the cover and count of native herbs increased with invasive shrub removal (Figure 3). Additionally, the removal treatment had a positive effect on a Species of Special Concern in the state and region and included new seedling emergence for this species (left unnamed here due to commercial value and poaching potential). Plant removal treatments generally increase resource availability (e.g., light [Kaye and Hone Reference Kaye and Hone2016]), which can favor fast-growing, weedy nonnative as well as native species [e.g., garlic mustard, Alliaria petiolata (M. Bieb.) Cavara & Grande] (Shields et al. Reference Shields, Saunders, Gibson, Zollner, Dunning and Jenkins2015). However, 7 yr after the initial removal treatments, there was no indication that invasive herbs were favored by the removal treatments (Wilcoxon signed-rank test, median difference=0.07, V=10, P-value=0.625; Figure 3). Furthermore, native, weedy, generalist herbs increased proportionally to woodland habitat–specific species in response to removal (mean difference=−0.107, t-value=−0.20588, df=4, P-value=0.8469; Figure 3). Both generalist and woodland habitat–specific native herbs were well represented (17 and 19 species, respectively).
Overstory tree species regeneration increased by an average of 8,403 stems ha−1 for individuals under a meter in height (Figure 3). However, overstory tree species 1 to 4 m in height have not benefited after 7 yr of removal. This could be the result of the suppressed growth expected for overstory tree species regenerating in the low-light conditions of the understory of a mature forest canopy (Bazzaz Reference Bazzaz1979; Canham Reference Canham1989). In addition, O. virginianus (white-tailed deer) could be contributing to limited overstory tree species regeneration above a meter in height (Ward et al. Reference Ward, Williams and Linske2017). While no browse damage was observed during sampling, O. virginianus have frequently been seen in the woodlot. In other studies, O. virginianus browse of regenerating trees has been found to increase with invasive shrub removal, presumably as access to preferred foods improves (Gorchov and Trisel Reference Gorchov and Trisel2003; Peebles-Spencer and Gorchov Reference Peebles-Spencer and Gorchov2017; Ward et al. Reference Ward, Williams and Linske2017). Odocoileus virginianus prefer to browse Quercus spp. and avoid black cherry (Prunus serotina Ehrh.) in mixed hardwood forests of the region (Horsley et al. Reference Horsley, Stout and DeCalesta2003). Much of the taller regeneration (1 to 4 m) across removal treatment and control plots is P. serotina (63%), while none was Quercus, despite its dominance in the forest canopy.
Under unmanaged, ambient forest conditions, there was a negative relationship between the cover of invasive shrubs and the cover of native species in the understory (<3 m in height; Figure 5). Where invasive shrubs have been removed, there is much greater native cover than in the ambient forest at the same level of very low invasion (47.3% compared with 17.5% native cover, respectively; Figure 5). Consequently, ambient forest sampling underestimated the impacts of invasive shrubs to the native plant community and the potential for passive natural regeneration with invasive shrub removal. This has implications for using a correlative sampling approach to estimate the impacts of invasive shrubs. There could be several explanations for this underestimation. First, our ambient sampling plots (1 milacre or 4.05 m2) were similar in area to other studies using this approach to estimate the impacts of invasive shrubs to native plants (e.g., ≤4 m2 in Collier et al. [Reference Collier, Vankat and Hughes2002]; Greene and Blossey [Reference Greene and Blossey2012]; Hutchinson and Vankat [Reference Hutchinson and Vankat1997]; Link et al. [Reference Link, Turnblacer, Snyder, Daugherty and Utz2018]; Woods [Reference Woods1993]). An invasive shrub could be near one of these relatively small ambient plots without aboveground cover inside of the plot. In this case, there is still the potential for native species to be negatively influenced through shading, belowground competition, or allelopathy. On the other hand, our experimental removal plots (314 m2) were created to approach management-relevant scales and have relatively less area near the edges where invasive shrubs could be influencing native species from outside the plots. If this is the case, studies that sample within larger patches of similarly invaded habitat (e.g., Link et al. Reference Link, Turnblacer, Snyder, Daugherty and Utz2018) should avoid any underestimation related to these edge effects. Another mechanism for the underestimation of the impacts of invasive shrubs using a correlative sampling approach could relate to the heterogeneity of resources common to mature, deciduous forest understories (Canham Reference Canham1989; Canham et al. Reference Canham, Finzi, Pacala and Burbank1994). Resource heterogeneity results in a mosaic of resource-rich to resource-poor microsites. For example, in the ambient forest plots, the percent of photosynthetically active radiation intercepted at 3 m varies both spatially and temporally throughout the growing season from a low of 33.5% to a high of 99.8% (unpublished data). Where invasive shrubs have been a component of the understory for many years, their bird-dispersed seeds can become ubiquitous (Greenberg and Walter Reference Greenberg and Walter2010). Widespread propagules provide the opportunity to establish and succeed in resource-rich microsites, especially if invasive shrubs are able to exclude native species as the removal treatment results indicate. Native species would be left to persist where resources become more limiting to both themselves and invasive shrubs, explaining the underestimation of the potential native response to invasive removal from the ambient forest sampling. Under this scenario, the impacts of invasive shrubs on native species could be accurately estimated with ambient sampling of more recently invaded forests than forests with a long history of invasive shrub presence.
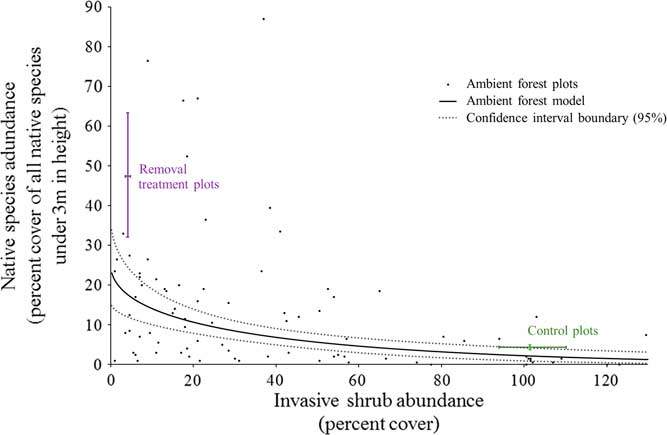
Figure 5 The relationships between invasive shrub and native species abundance (percent cover) under 3 m in height. The linear model using the cube root of native percent cover (response) and the square root of invasive shrub cover (predictor) was fit as follows: (sum of native species % cov.)-3=-0.16412(sum of invasive shrubs % cov.)-2 + 2.93032 The small black dots represent the ambient forest plots (n=86). The solid line is the fitted regression line for the ambient forest plots (back-transformed, square root–cube root linear relationship), and the dotted lines are the 95% confidence region boundary for the fit model. The adjusted R2 value for this relationship is 0.228 (slope, intercept, and model P-values << 0.0001). The bars labeled “Removal treatment plots” and “Control plots” represent the bootstrap-estimated 95% confidence intervals around the mean for both axes (n=5 for each). As expected, the control plots do not differ from the ambient forest model predictions. However, the native species abundance is greater in the removal treatment plots than what is expected from the ambient forest model. Ambient forest measurements underestimate the effects of invasive shrub presence in this forest.
A clear understanding of the benefits and impacts of forest management options is needed to warrant implementation, especially when funding and manpower are limited. After 7 yr, experimental shrub removal covering more than 1,800 m2 improves our understanding of the response of the native plant community to a naturally occurring suite of invasive shrubs and to invasive shrub removal treatments. Invasive shrub removal allowed passive natural regeneration of native forbs, shrubs, and regenerating trees that together exceeded native abundance in the unmanaged, ambient forest under minimal invasive shrub abundance.
Acknowledgments
We thank the Arboretum at Penn State for the use of their forest and for planning management activities around the experimental plot areas. We also thank Steve Bean, Nick Hugo, Teal Jordan, and Warren Reed for help with field sampling. This work was supported in part by the USDA National Institute of Food and Agriculture and McIntire-Stennis Appropriations under Project #PEN04685 and Accession #1018181. No funding was received from commercial or not-for-profit sectors. Work by EM-B was partially supported by Penn State’s Ecology Intercollege Graduate Degree Program and Shaver’s Creek Environmental Center. No conflicts of interest have been declared.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2018.35