Management Implications
The sagebrush steppe rangelands of the American West are threatened by infestations of annual nonnative plant species, and an effective approach to controlling these species is through seedbank depletion. Indaziflam is a preemergent herbicide that can effectively deplete the soil seedbank of invasive annual grasses. This herbicide shows great promise for control of these annual invaders due to its residual soil activity of up to 3 yr. There have been no documented negative effects to established perennial vegetation; however, non-target impacts to the native seedbank are expected. A field study was conducted to examine the impacts of indaziflam on nontarget species aboveground, and a greenhouse study addressed impacts to the soil seedbank. The mountain big sagebrush (Artemisia tridentata ssp. vaseyana) sites had localized infestations of nonnative annual mustards (Alyssum spp.) and high levels of species richness and diversity. Overall indaziflam controlled Alyssum spp. for 2 yr but also reduced richness and diversity of the nontarget forb community. Annual forbs were the most impacted, both aboveground—with reductions of up to 50% cover—and in the soil. Perennial forbs were also prevented from regenerating from the soil seedbank. Native annual forb species are of particular concern, as they rely on annual regeneration from the seedbank, whereas established perennial species can repopulate after the herbicide has degraded. The soil seedbank is often relied upon for passive restoration efforts, and broadscale use of indaziflam may not be appropriate in areas with minimal infestations. Our findings highlight the importance of considering nontarget impacts when using indaziflam in areas with diverse native plant communities and low infestations of annual nonnative species.
Introduction
Nonnative plant invasions can have devastating impacts on native plant communities (Elton Reference Elton1958; Mack and D’Antonio Reference Mack and D’Antonio1998; Tilman Reference Tilman1999; Vilà et al. Reference Vilà, Espinar, Hejda, Hulme, Jarošík, Maron, Pergl, Schaffner, Sun and Pyšek2011). However, chemical efforts to control nonnative plants can also damage co-occurring native species and communities (Crone et al. Reference Crone, Marler and Pearson2009; Kettenring and Adams Reference Kettenring and Adams2011; Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009; Wagner and Nelson Reference Wagner and Nelson2014), sometimes causing more harm than the invader itself (Ortega and Pearson Reference Ortega and Pearson2011; Skurski et al. Reference Skurski, Maxwell and Rew2013). Understanding the efficacy of herbicides on target species and potential adverse impacts on nontarget desirable species is key to developing effective management strategies.
Controlling invasive plants in semiarid grasslands is critical to maintaining the essential functions and services this ecosystem provides to humans around the world (Lund Reference Lund2007; O’Mara Reference O’Mara2012). The sagebrush steppe in the western United States is a diverse ecological community that provides forage for livestock operations and habitat for wildlife species (Beck et al. Reference Beck, Connelly and Wambolt2012). This semiarid ecosystem is threatened by land use change, climate change, and nonnative plant invasions (DiTomaso Reference DiTomaso2000; Knapp Reference Knapp1996; Vasquez et al. Reference Vasquez, James, Monaco and Cummings2010), particularly winter annual grass species that can alter fire regimes and disrupt ecosystem functions (Balch et al. Reference Balch, Bradley, D’Antonio and Gómez-Dans2013; Billings Reference Billings, Monsen and Kitchen1994; Young and Fay Reference Young and Fay1997), creating novel plant communities far less diverse than the native communities they replaced (Allen and Knight Reference Allen and Knight1984).
The preemergent herbicide indaziflam is promoted as an option to control invasive annual grasses in sagebrush steppe (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2020; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016). Indaziflam is a nonselective herbicide that inhibits cellulose biosynthesis (Brabham et al. Reference Brabham, Lei, Gu, Stork, Barrett and DeBolt2014) and provides residual control in the top few centimeters of soil (0 to 2.5 cm). Originally developed for turf and orchard use, indaziflam was recently approved for use in natural areas and grazed rangeland (Bayer 2020) and may be used to provide long-term control of invasive annual grasses by depleting the soil seedbank (Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a, Reference Sebastian, Nissen, Sebastian and Beck2017b). Indaziflam shows great promise to reduce target species, as this herbicide remains active in the soil for up to 3 yr (Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016) and would potentially require fewer applications than other less persistent herbicides (Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a).
The efficacy of indaziflam in sagebrush rangeland has been evaluated primarily in highly disturbed areas dominated by invasive annual grasses with little remaining native vegetation (Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). The control of invasive annual grasses results in an increase in growth of existing perennial plants due to reduced competition (Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a, Reference Sebastian, Swanson, Sauer, Clark and Sebastian2020). Established perennial vegetation is largely unaffected by indaziflam (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019), likely because roots of perennial plants often extend below the zone of herbicide activity. However, new recruitment of a nonnative perennial species was inhibited by indaziflam (Sebastian et al. Reference Sebastian, Nissen, Sebastian, Meiman and Beck2017c), and it is likely that any germinating seeds in the zone of herbicide activity will not emerge, as indaziflam inhibits cellulose biosynthesis in the radicle and is nonselective (Brabham et al. Reference Brabham, Lei, Gu, Stork, Barrett and DeBolt2014). Species with annual life cycles and short-lived seedbanks will be more impacted by indaziflam than either perennial species that do not rely on annual germination or species with long-term seedbanks. Only a couple of indaziflam studies have examined impacts to annual forbs (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019; Sebastian et al. Reference Sebastian, Swanson, Sauer, Clark and Sebastian2020), and one of those found a slight increase in native annual cover after indaziflam treatment (Sebastian et al. Reference Sebastian, Swanson, Sauer, Clark and Sebastian2020).
The forb desert alyssum or desert madwort (Alyssum desertorum Stapf) is a nonnative winter annual species common to the sagebrush steppe (Noack Reference Noack, Pokorny and Jacobs2020) and is purported to displace native vegetation (Mosley Reference Mosley2014), though quantitative evidence of its impacts is lacking. Two other nonnative annual mustards [pale madwort (Alyssum alyssoides (L.) L.) and alyssum (Alyssum simplex Rudolphi)], can co-occur but receive less attention. In Yellowstone National Park (YNP), A. desertorum has recently been found above its documented elevational range, primarily in areas disturbed by wildlife, tourists walking off designated routes, or recent construction (H Anderson, personal communication). This spread has prompted YNP land managers to consider controlling localized infestations of A. desertorum to maintain the native diversity of these areas. Managers are controlling infestations of A. desertorum with indaziflam in one area of YNP that has experienced over a century of intense human land use. Using this herbicide to control Alyssum spp. in less disturbed and more species-diverse areas of YNP needs to be evaluated. Previous indaziflam studies in other semiarid grasslands have primarily focused on the response of perennial forbs and perennial grasses, as they are desirable components of rangeland plant communities; however, annual ephemeral forbs are also key components of rangelands (Pokorny et al. Reference Pokorny, Sheley, Svejear and Engel2004). They provide critical spring forage for many wildlife species (Drut et al. Reference Drut, Pyle and Crawford1994; Luna et al. Reference Luna, Mousseaux and Dumroese2018) and occupy an early successional niche. We sought to assess the impacts of indaziflam on the native plant community with a primary focus on annual forbs.
Our study examined the efficacy of preemergent indaziflam to control nonnative Alyssum spp. and evaluated impacts to the diversity of the nontarget plant community. The objectives were: (1) to evaluate the efficacy of indaziflam to control the target species; (2) to assess the effect of indaziflam on the richness and diversity of the whole plant community, distinguishing between perennial and annual species; and (3) to evaluate the effect of indaziflam on perennial and annual forb germination from the soil seedbank.
Materials and Methods
Study Area and Experimental Design
Six field sites were established along an elevational gradient (1,615 m to 2,347 m) in the northern range of YNP, USA (Table 1). Sites were randomly selected from a roadside survey of A. desertorum populations conducted with aid of the park botanist (H Anderson). However, further evaluation found a mix of A. desertorum with A. alyssoides and A. simplex at these sites (Alyssum spp. hereafter). The plant communities at all sites were characterized as mountain big sagebrush steppe (dominated by Artemisia tridentata Nutt. ssp. vaseyana).
Table 1. Site environmental characteristics for the six sites along an elevation gradient within Yellowstone National Park.

a High: areas with >10 Alyssum spp. individuals m−2; Low: areas with <10 Alyssum spp. individuals m−2.
We defined high and low invasion levels of Alyssum spp. at each site, with high levels equal to or above 10 individuals m−2, and adjacent low levels with fewer than 10 individuals m−2. Before spray application we assessed Alyssum spp. in each plot, using density classes (1: 0; 2: <10; 3: 10 to 100; and 4: >100 individuals m−2). High-invasion plots were mainly in the high (4) category (75%, 78 plots) with the rest in the medium (3) level (25%, 26 plots [104 plots total]). Most of the low-invasion plots had no Alyssum spp. (94%, 96 of 102 plots [2 plots lost to the study]); only 6 had between 1 and 10 Alyssum spp. At five of the sites, eighteen 1-m2 plots were established in each of the high- and low-invasion areas. In each of the invasion treatments, 12 plots were sprayed with indaziflam (Esplanade®, Bayer CropScience, Cary, NC, USA, 27513) and six plots were left unsprayed to serve as controls. At the sixth and highest site, fourteen 1-m2 plots were established in each high- and low-invasion area, with seven of the plots in each invasion treatment sprayed with indaziflam and seven plots left unsprayed as controls. We had a high level of replication and chose our plot size to ensure we had a high detection rate for small ephemeral annual species, allowing adequate representation of the plant community. Plots were randomly assigned to the spray or control treatment. All spray plots were treated in August 2018 with 63 g ai ha−1 indaziflam using a backpack sprayer fitted with a XR11002 flat spray nozzle (TeeJet® Spraying Systems, P.O. Box 7900, Wheaton, IL 60187) at 195 L ha−1 at 138 kPa. Ocular estimates of foliar cover (to the nearest 1%) for each species and ground cover were conducted 1 and 2 yr after treatment during peak vegetative season, for the central 0.75 m2 of each plot to account for potential edge effects of the herbicide. Species with “trace” (<0.5%) cover were recorded and analyzed as 0.1% cover. Estimates were allowed to exceed 100% to account for understory canopy structure. The sites were sampled from low to high elevation with the aim of sampling each site at comparable growth phases. Half of the spray plots were sampled 1 mo after the other plots at each site; this enabled us to us to determine whether we had missed any species and provided a better understanding of the plant community (Pokorny et al. Reference Pokorny, Sheley, Svejear and Engel2004).
Evaluating the Effect of Herbicide on the Soil Seedbank
To assess the effect of herbicide on the seedbank, soil samples were collected at the two lowest sites in April 2019. At each site, soil cores were taken in each of six spray plots and six control plots in both areas of invasion treatments (n = 48). Six soil cores (10-cm diameter by 6-cm deep) were taken per plot in the area outside the vegetation quadrat but within the 1-m2 plot and combined into one soil sample. Soil samples (2,832 cm3) were stored in a cold storeroom at 5 C for 2 wk, then spread out in trays (28 by 13 cm) on top of 2.5 cm of sterilized soil (1:1:1 ratio of mineral soil, sphagnum peat moss, and washed concrete sand) in the Montana State University Plant Growth Center (Bozeman, MT, 59717). Trays were placed in a greenhouse (22 to 18 C, 16-h photoperiod) and watered twice each day for 5 min using a drip irrigation system. Seedling emergence above the soil surface was recorded at least once per week. Seedlings that emerged were identified, counted, and then removed from the tray. Seedlings that were unidentifiable at the 1- to 2-leaf stage were repotted and grown until they were identifiable. Emerging graminoids were removed and not counted. There was no germination for the first 45 d; therefore, trays were moved to a cold, wet, and dark stratification chamber (4 C) for 30 d to break dormancy, after which the trays were returned to the greenhouse. After germination diminished again (6 mo) the trays were returned to the stratification chamber for 56 d, and then returned to the greenhouse. Any additional germinants were identified and removed. The experiment was terminated after 17 mo.
Statistical Analysis
All analyses were completed using R v. 3.6.1 (R Core Team 2019) and the packages BiodiversityR (Kindt and Coe Reference Kindt and Coe2005), lme4 (Bates et al. Reference Bates, Mächler, Bolker and Walker2015), lmerTest (Kuznetsova et al. Reference Kuznetsova, Brockhoff and Christensen2017), MuMIN (Bartoń Reference Bartoń2019), and vegan (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O’Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019).
To evaluate the effects of indaziflam on the abundance of Alyssum spp. (objective 1), the high invasion data were subset, and a linear mixed model following a Gaussian distribution was fit. The saturated model included herbicide treatment (spray, control), elevation, and year (2019, 2020) as main effects. To account for repeated measures of plots between years, the unique tag number for each plot was included as a random effect. For objective 2, indaziflam effects on the plant community, the full data set was used, including both levels of Alyssum spp. invasion. Species richness and Shannon’s diversity index were assessed for the whole community, perennial graminoids, perennial forbs, and annual native forbs. Percent cover estimates were used to calculate Shannon’s diversity index for each plot. Shannon’s diversity index was calculated as
where p i is the proportion of species i, S is the number of species, and logb is the logarithm base 10. Species richness was evaluated with generalized linear mixed-effects models following a Poisson distribution, and Shannon’s diversity was evaluated following a Gaussian distribution. A quasi-Poisson distribution was used when overdispersion was present. Saturated models with all possible interactions were fit with main and random effects as noted earlier and including invasion (high, low) as a main effect. Any significant interactions were maintained in the final models. Normality and heteroscedasticity assumptions were assessed, and no transformations were necessary. The model intercept was the lowest elevation site, control, and low invasion. A type II ANOVA was used to evaluate treatment effects.
For objective 3, to evaluate impacts to perennial and annual forbs in the soil seedbank, the abundance, richness, and Shannon’s diversity were analyzed with linear models similar to those previously described. Abundance and Shannon’s diversity responses were fit following a Gaussian distribution, and the richness responses were fit with a quasi-Poisson distribution to account for overdispersion; treatment effects were evaluated with a type II ANOVA.
Results and Discussion
Indaziflam Efficacy on Alyssum spp
Indaziflam provided excellent control of Alyssum spp. 1 and 2 yr after treatment (P < 0.01). There was no difference by elevation (P = 0.47); however, there was a difference by year (P < 0.01) and a significant interaction between year and indaziflam (P < 0.01) (Table 2). This is likely explained by natural variation of Alyssum spp. abundance in the control plots (Figure 1). The mean (±SE) cover of Alyssum spp. in spray plots was 0.1% (±0.04%) in 2019 and 0.2% (±0.1%) in 2020; in control plots, mean cover was 3.7% (±0.43%) in 2019 and 2.5% (±0.32%) in 2020. This reduction in sprayed plots equates to mean Alyssum spp. cover of 97% less in 2019 and 91% less in 2020 compared with control plots.
Table 2. Cover (%) of Alyssum spp. (ALSPP) in high-invasion plots in response to indaziflam treatment (control, sprayed), elevation, and year (2019, 2020). a
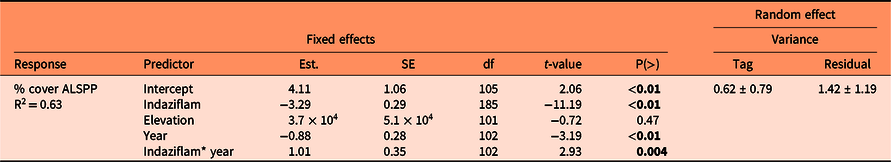
a Intercept is unsprayed control, 2019. Results are from a linear mixed model regression. P-values in bold indicate statistically significant difference (P < 0.05).
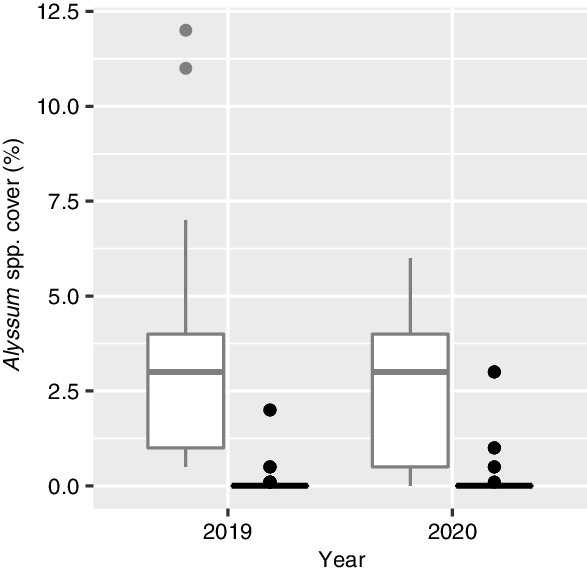
Figure 1. Alyssum spp. cover after indaziflam treatment (gray, control; black, sprayed) at sites in Yellowstone National Park along an elevational gradient, in 2019 and 2020. Data shown are from plots with high levels of Alyssum spp. invasion.
Impacts of Indaziflam on the Whole Plant Community
There were 160 species across all sites: 100 perennial forbs, 32 perennial graminoids, 1 annual graminoid, 16 annual forbs, and 11 shrubs (Supplementary Table S1). There were some co-occurring nonnative species: 4 perennial graminoids, 1 annual graminoid, 7 perennial forbs, and 2 annual forbs (Supplementary Table S1). Mean (±SE) cover of nonnative species across all elevations was 3.9% (±0.86), which included the targeted Alyssum spp. populations. Total species richness at each site ranged from 38 to 76 species (Table 1). The mean species richness of the whole plant community was lower in sprayed plots (P < 0.01) and high Alyssum spp. invasion plots (P = 0.05), decreased as elevation increased (P < 0.01) but did not differ by year (P = 0.26) (Table 3; Figure 2). Sprayed plots also had lower Shannon’s diversity than control plots (P < 0.01), and diversity decreased as elevation increased (P < 0.01) but did not differ between levels of invasion (P = 0.39) or by year (P = 0.18) (Table 4). Species richness has shown a decrease or hump-shaped response along elevation gradients in mountain habitats globally (Haider et al. Reference Haider, Kueffer, Bruelheide, Seipel, Alexander, Rew, Arévalo, Cavieres, McDougall, Milbau, Naylor, Speziale and Pauchard2018; Pauchard et al. Reference Pauchard, Kueffer, Dietz, Daehler, Alexander, Edwards, Arévalo, Cavieres, Guisan, Haider, Jakobs, McDougall, Millar, Naylor and Parks2009).
Table 3. Effects of indaziflam herbicide on species richness of the whole plant community, perennial graminoids, perennial forbs, and annual forbs, controlling for level of Alyssum spp. invasion (high, low), elevation, and year (2019, 2020). a
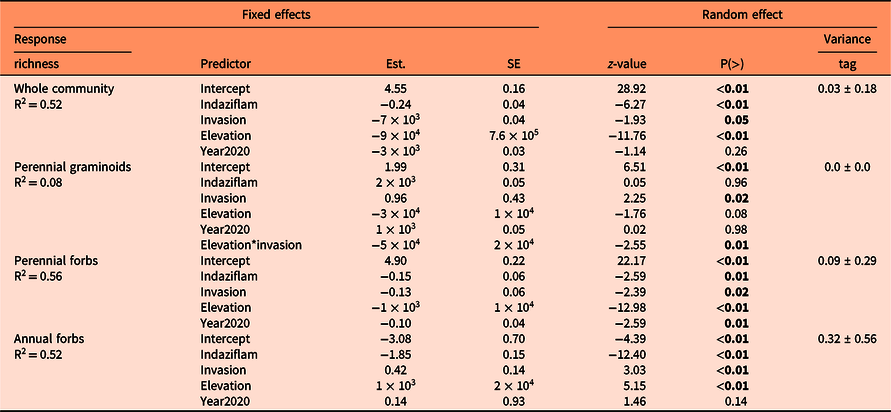
a Intercept is unsprayed control, low invasion, 2019. Results are from mixed-effects models with a Poisson distribution. Values in bold indicate statistically significant differences (P < 0.05).

Figure 2. All species and annual forb mean (±SE) richness after indaziflam treatment (gray, control; black, sprayed) in two levels of Alyssum spp. invasion (Δ solid, low; • dashed, high) along an elevation gradient in Yellowstone National Park.
Table 4. Effects of indaziflam (spray, control) on Shannon’s diversity of the whole plant community, perennial graminoids, perennial forbs, and annual forbs, controlling for level of Alyssum spp. invasion (high, low), elevation, and year (2019, 2020). a

a Intercept is control, low invasion, 2019. Results are from mixed-effects models. Values in bold indicate statistically significant differences (P < 0.05).
The significant difference in total species richness between high- and low-invasion treatments was generally fewer than two species and mainly related to the differences in richness between invasion treatments at the two highest elevations (Figure 2). To evaluate the impacts of an invader on richness and diversity, the values for the invader should be removed to avoid artificially inflating the response (Thomsen et al. Reference Thomsen, Wernberg, South and Schiel2016). This additional analysis was performed and found similar patterns (data not shown). However, when annual forb richness and diversity were evaluated, neither differed between the invasion treatments (Meyer-Morey Reference Meyer-Morey2021). These results suggest that Alyssum spp. is a weak invader in the mountain sagebrush plant community. However, further evaluation of Alyssum spp. impact is necessary to determine whether and which populations to prioritize for management (Rew et al. Reference Rew, Lehnhoff and Maxwell2007).
Our findings that richness and diversity decreased with herbicide application is in contrast with other rangeland studies that found increased richness and abundance 1 yr after treatment (Sebastian et al. Reference Sebastian, Swanson, Sauer, Clark and Sebastian2020) and no effects on species richness after 2 yr (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019) for the whole community. These studies were conducted in areas with dense infestations of downy brome (Bromus tectorum L.) (between 30% and 70%) and another four to five nonnative species, and the increased abundance and richness was attributed to release from competition. Our study sites were in relatively undisturbed mountain big sagebrush plant communities that are more resilient to disturbance and resistant to annual species invasion than lower-elevation sagebrush communities (Chambers et al. Reference Chambers, Bradley, Brown, D’Antonio, Germino, Grace, Hardegree, Miller and Pyke2014).
Richness was also higher at our sites than in the study sites of Clark et al. (Reference Clark, Sebastian, Nissen and Sebastian2019; 33 to 35 native species), and we only observed B. tectorum at one site, where its cover was very low (0.5%). To better understand the differences we observed, richness and diversity of perennial graminoids, perennial forbs, and annual forbs were analyzed separately.
Perennial Species Response
Established perennial graminoid richness and Shannon’s diversity were largely unaffected by indaziflam (P = 0.96 and P = 0.50, respectively) (Tables 2 and 3, Supplementary Figure S1), as expected from previous studies (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019, Reference Clark, Sebastian, Nissen and Sebastian2020). Perennial forb mean species richness was lower in sprayed plots (P < 0.01) and under high invasion (P = 0.02), decreased with elevation (P < 0.01), and differed between years (P = 0.01; Table 3, Supplementary Figure S1). Shannon’s diversity of perennial forbs was lower in sprayed (P = 0.04) and high-invasion plots (P = 0.05), decreased with elevation (P < 0.01), and was lower in 2020 than 2019 (P = 0.01). Other studies have shown no injury to or reduction in abundance of existing perennial vegetation (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019; Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a); therefore, we theorize that reduced recruitment from the seedbank may have been the cause of perennial forb reduction, which our seedbank study results corroborate. Sebastian et al. (Reference Sebastian, Nissen, Sebastian, Meiman and Beck2017c) also concluded that indaziflam reduced recruitment of a perennial nonnative forb at their field study site.
Annual Forb Response
Annual forbs were greatly impacted by indaziflam. Mean species richness of annual native forbs was lower in sprayed plots (P < 0.01) by at least 50% (Figure 2), increased with elevation (P < 0.01), and was greater in high-invasion areas (P < 0.01) due to the presence of Alyssum spp., but did not differ between years (P = 0.14) (Table 3; Figure 2). Shannon’s diversity of annual forbs was reduced by indaziflam (P < 0.01), increased significantly with elevation (P < 0.01), and did not differ by year (P = 0.08) or level of invasion (P = 0.17; Table 4). The increase with elevation was explained by blackfoot groundsmoke (Gayophytum racemosum Torr. & A. Gray) and northern linanthus [Leptosiphon septentrionalis (H. Mason) J.M. Porter & L.A. Johnson] at the highest elevation. The reduced richness and diversity of annual forbs after herbicide application is in contrast with another study that found no effect of indaziflam on annual forbs (Sebastian et al. Reference Sebastian, Swanson, Sauer, Clark and Sebastian2020) and suggests that individual species of annual forbs may have differing sensitivities to indaziflam. There were 12 species of annual forbs plus Alyssum spp. at our sites, six were observed in both control and spray plots: Alyssum spp., tiny trumpet (Collomia linearis Nutt.), woodland draba (Draba nemorosa L.), L. septentrionalis, slender phlox [Microsteris gracilis (Hook.) Greene], and Douglas’ knotweed (Polygonum douglasii Greene). The other six were only observed in the control plots: pygmyflower rockjasmine (Androsace septentrionalis L.), maiden blue-eyed Mary (Collinsia parviflora Lindl.), tall annual willowherb (Epilobium brachycarpum C. Presl.), pinyon groundsmoke (Gayophytum ramosissimum Torr. & A. Gray), G. racemosum, dwarf purple monkeyflower (Mimulus nanus Hook. & Arn.), and Suksdorf’s monkeyflower (Mimulus suksdorfii A. Gray). Because annual species rely on annual regeneration from the seedbank, long-lived residual preemergent herbicides like indaziflam may have long-term impacts on all these species, depending on the species’ seed decay rates, which are poorly quantified. We did not evaluate the relative fecundity of the species that emerged in control and sprayed plots, but they did produce seeds in both. Our results suggest the species only observed in the control plots are likely to be most affected, because for these species to remain in a community after herbicide application will require recolonization via seed movement from adjacent unsprayed areas.
Impacts of Indaziflam on Soil Seedbank Recruitment
Indaziflam greatly suppressed germination of both perennial and annual forbs from the soil seedbank (Figure 3). There were 29 forb species that emerged: 18 perennials and 11 annuals (Supplementary Table S1). Mean abundance of perennial forbs in the control soils was 23 (±15) individuals and there were no perennial forbs that emerged in the sprayed soils (Figure 3). This supports the results of our field study, which showed reduced richness and diversity of perennial forbs in sprayed plots, and the results of Sebastian et al. (Reference Sebastian, Nissen, Sebastian, Meiman and Beck2017c), which documented reduced recruitment of seven perennial nonnative forbs in their seedbank study.

Figure 3. Soil seedbank total abundance by life form (light gray, annual forb; dark gray, perennial forb) after indaziflam treatment (sprayed, control) from all samples (2.26 m2) collected at two sites (M, B) in Yellowstone National Park.
The few species that emerged in the sprayed soils were all annual forbs: herb sophia [Descurainia sophia (L.) Webb ex Prantl], C. linearis, D. nemorosa, and M. gracilis. These were very low in abundance compared with the control soils; mean annual forb abundance was 1 (±0.83) individual in sprayed soils compared with 33 (±11.3) individuals in the controls (Figure 4A). The latter three species (C. linearis, D. nemorosa, and M. gracilis) were also found in the sprayed plots in the field experiment but also occurred at greater abundance in the control plots. Annual forb richness was lower in the sprayed soils (P < 0.01) and differed between the two sites (P < 0.01) and levels of invasion (P = 0.03). Mean annual richness in the controls was 1.5 (±0.3) species at the lower site (M) and 3.6 (±0.4) species at the higher site (B) and, in sprayed soils, was reduced to 0 (±0) and 0.5 (±0.34), respectively (Figure 4B). Shannon’s diversity was also lower in sprayed soils (P < 0.01) and differed between sites (P < 0.01), though it did not differ between levels of invasion (Table 5). The soil seedbank at our sites had a high diversity of species (29 species) despite Alyssum spp. infestation, and these nontarget species, particularly the native annual forbs, were shown to be negatively impacted by indaziflam.

Figure 4. Mean annual forb soil seedbank (A) abundance and (B) richness after indaziflam treatment (gray, control; black, sprayed) from samples (471 cm2) at two sites (M, B) in Yellowstone National Park and by level of Alyssum spp. invasion (low, high).
Table 5. Effects of indaziflam on the abundance, species richness, and Shannon’s diversity of annual forb seedlings emerged from the soil seedbank, controlling for site and level of Alyssum spp. invasion. a

a Intercept is control (no indaziflam treatment), site M, and low invasion. Results are from linear models fit with Gaussian (G) and quasi-Poisson (QP) distributions. Values in bold indicate statistically significant difference (P < 0.05).
Previous indaziflam studies have primarily focused on the response of perennial forbs and perennial grasses, as they are desirable components of rangeland plant communities; however annual forbs are also key components of rangelands (Pokorny et al. Reference Pokorny, Sheley, Svejear and Engel2004). They occupy an early successional niche and provide critical spring forage for many wildlife species (Drut et al. Reference Drut, Pyle and Crawford1994; Luna et al. Reference Luna, Mousseaux and Dumroese2018). Additionally, native annual forbs are phenologically similar to nonnative winter annual grasses and likely use similar resource pools (Forbis Reference Forbis2010). The annual forbs in our study area are early-season and early seral species; early seral annual forbs can compete with nonnative winter annual grasses, specifically B. tectorum (Uselman et al. Reference Uselman, Snyder, Leger and Duke2015). Therefore, knowing which annual species are tolerant to indaziflam is useful for creating restoration seed mixes for use after indaziflam application.
In the western United States, approximately 23 million ha of rangelands are infested with nonnative annual species (DiTomaso et al. Reference DiTomaso, Monaco, James and Firn2017), presenting a considerable challenge to restore and preserve these working lands. Areas with extensive monocultures of nonnative plants require novel and innovative approaches, such as seedbank depletion, to achieve adequate long-term control; however, these same methods may not be appropriate at all levels of infestation or with all nonnative annual species. In areas where the seedbank is composed mostly of nonnative species that have formed persistent soil seedbanks, indaziflam is a valuable tool to deplete the seedbank of target species before active revegetation efforts (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2020). However, in areas with minimal infestations, an existing diverse plant community, and therefore likely a diverse seedbank, the use of indaziflam and its impacts on the seedbank of nontarget species should be carefully considered before broadscale use. Additionally, to determine whether active management is warranted, the actual impacts of an invader should be considered (Rew et al. Reference Rew, Lehnhoff and Maxwell2007). Our results suggest Alyssum spp. is a weak invader, and using indaziflam to control it may do more harm than the target species itself in high-elevation sagebrush steppe.
Indaziflam significantly reduced species richness and diversity, particularly of nontarget annual forb species, resulting in a community more dominated by perennial species. Native annual forb species are often overlooked, though they occupy an important early successional niche and contribute to the biodiversity of rangelands. Maintaining biodiversity should be a priority for land managers, as communities that are higher in diversity are typically more resistant to ecosystem alteration and more resilient after disturbances (Chambers et al. Reference Chambers, Bradley, Brown, D’Antonio, Germino, Grace, Hardegree, Miller and Pyke2014; Hobbs and Huenneke Reference Hobbs and Huenneke1992; Standish et al. Reference Standish, Hobbs, Mayfield, Bestelmeyer, Suding, Battaglia, Eviner, Hawkes, Temperton, Cramer, Harris, Funk and Thomas2014). Understanding and minimizing the non-target effects of invasive plant control on existing intact vegetation is critical when developing management strategies (Rew et al. Reference Rew, Lehnhoff and Maxwell2007), especially in arid rangelands. Future studies should address the long-term impacts of indaziflam to plant community composition and explore the tolerances and seedbank longevities of native species for restoration seed mixes.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2021.31
Acknowledgments
Thanks to Stacey Robbins and Emily Daniels who helped with field sampling. JM-M was funded by Yellowstone National Park through Rocky Mountain CESU. ML, JM, CZ, and LJR are supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture Hatch: MONB00359, MONB00243, MONB00397, MONB00363, respectively. No conflicts of interest have been declared.












