Management Implications
This study demonstrates that pretreatment genetic monitoring combined with herbicide characterization of a prioritized set of clones could aid herbicide management decisions for the invasive aquatic plants Myriophyllum spicatum (Eurasian watermilfoil) and Myriophyllum spicatum × Myriophyllum sibiricum (hybrid watermilfoil). For example, our study confirmed resistance to the operational rate of fluridone used in Michigan to control Myriophyllum (6 µg L−1) in accessions from five different lakes containing the same microsatellite multilocus genotype (MG-237) and from a second lake with a different genotype (MG-377). Therefore, we suggest that fluridone would not be an effective treatment for invasive Myriophyllum control in lakes where MG-237 or MG-377 occurs at a high frequency. Conversely, our study identified several multilocus microsatellite genotypes that appeared susceptible to 6 µg L−1 fluridone, and some of these genotypes have been identified in multiple Michigan lakes. Therefore, we suggest that fluridone would likely be an effective treatment in lakes that consist mostly of one or more of the susceptible genotypes identified in our study. More generally, our study suggests that building a “catalog” of prioritized genotypes (e.g., those that occur most commonly across the landscape) and their response to commonly used herbicides could help predict herbicide outcomes without an immediate need for identifying the genetic basis of herbicide resistance. Further, clones are frequently shared across nearby waterbodies, so managers may be able to pool resources to identify herbicide responses to common clones in their region.
Introduction
When used effectively, herbicides are estimated to do the work of approximately 70 million workers (Gianessi and Reigner Reference Gianessi and Reigner2007). However, the effectiveness of herbicides is threatened by the evolution of herbicide resistance (Baucom Reference Baucom2019; Oreke Reference Oreke2005). Currently there are 262 species of weeds resistant to at least one mode of herbicide action (Heap Reference Heap2020). One potential tool to help maintain the efficacy of herbicides is pretreatment screening for molecular markers that identify resistant versus susceptible individuals. Identification of individuals in a population slated for herbicide treatment that are resistant to certain herbicides can reduce the evolution and spread of resistance. There are currently genetic marker assays in some species of managed weeds for genes that confer herbicide resistance. For example, alleles of acetyl-CoA carboxylase (ACCase; Menchari et al. Reference Menchari, Camilleri, Michel, Brunel, Dessaint, Le Corre and Delye2006) and phytoene desaturase (Benoit and Les Reference Benoit and Les2013) are known to confer resistance to ACCase-inhibiting herbicides and fluridone (1-methyl-3-phenyl-5-[3-(trifluromethyl) phenyl]-4(1H)-pyridinone), respectively. Copy number of 5-Enolypyruvyl-shikamate-3-phosphate synthase can also be used to determine resistance to glyphosate (Chatham et al. 2015). Pretreatment identification of resistant individuals using these types of genetic markers can then inform herbicide decisions to maintain resistant individuals at a low frequency in managed weed populations.
For clonal organisms, characterizing herbicide response of widespread clones would potentially inform herbicide response of the same clones in all locations where they are found. Therefore, genetic markers that can identify ramets of the same genet and distinguish unique genets may also accurately predict herbicide response. Because all genes of a clone are essentially linked, clone identification may be as good a predictor of herbicide response as causal herbicide resistance alleles in the short term.
Eurasian watermilfoil (Myriophyllum spicatum L.) including its hybrid with native northern watermilfoil (Myriophyllum spicatum × Myriophyllum sibiricum Kom.) are heavily managed with herbicides in the United States (Bartodziej and Ludlow Reference Bartodziej and Ludlow1998). Experimental studies of Myriophyllum clearly indicate that distinct genotypes can differ in vegetative growth and herbicide response properties (Netherland and Willey Reference Netherland and Willey2017; Taylor et al. Reference Taylor, McNair, Guastello, Pashnick and Thum2017; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012). Although Myriophyllum is capable of sexual reproduction, clonal reproduction is common (Hartleb et al. Reference Hartleb, Madsen and Boylen1993), and the same clones of Myriophyllum have been distinguished using multilocus microsatellite genotyping (MG) within and among regions (Taylor et al. Reference Taylor, McNair, Guastello, Pashnick and Thum2017; Thum et al. Reference Thum, Chorak, Newman, Eltawely, Latimor, Elgin, Parks and McNair2020). Therefore, if herbicide response were characterized for widespread Myriophyllum genotypes, managers might be able to make informed herbicide treatment decisions based on the genotypes that are present in a lake.
The herbicide fluridone (WSSA Group 12) is commonly used in aquatic plant management and is typically an effective tool to reduce Myriophyllum populations (Berger et al. Reference Berger, Netherland and MacDonald2012; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012). However, a failed treatment of M. spicatum × M. sibiricum using fluridone in Townline Lake, MI, raised concerns for the possibility of herbicide resistance. Laboratory testing found that M. spicatum × M. sibiricum collected from Townline Lake exhibited resistance to the operational rate of fluridone used in Michigan (Berger et al. Reference Berger, Netherland and MacDonald2012, Reference Berger, Netherland and MacDonald2015; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012).
A recent genetic survey of Myriophyllum across the state of Michigan identified the same genotype found in Townline Lake (hereafter referred to as MG-237) in at least seven other lakes in Michigan (Thum et al. Reference Thum, Chorak, Newman, Eltawely, Latimor, Elgin, Parks and McNair2020; RAT, additional unpublished data). Given that it is unlikely that the same genotypes arose independently through sexual reproduction, it follows that all MG-237 individuals are clones of the same lineage. We therefore expect that the same genotypes will exhibit the same resistance phenotype in response to fluridone, because they are the same clonal lineage that has spread across the landscape. However, genotypes that share ancestry through clonal reproduction can still differ in their fluridone response because of somatic mutations (e.g., Michel et al. Reference Michel, Arias, Scheffler, Duke, Netherland and Dayan2004). For example, if mutation(s) conferring fluridone resistance arose before MG-237 spread to other lakes, then we would expect all lakes where MG-237 clones occur to exhibit fluridone resistance. Alternatively, it is possible that an ancestral, fluridone-sensitive MG-237 spread across the landscape and that subsequent somatic mutation(s) conferred fluridone resistance in one or a subset of lakes where it occurs.
In this study, we used a 6 µg L−1 fluridone response assay to determine the susceptibility of the same and different Myriophyllum genotypes to the operational rate of fluridone used in Michigan. Specifically, we tested whether MG-237 clones sampled from different lakes in Michigan exhibited resistance to the Michigan operational rate of fluridone, as would be expected if the genotype spread across the landscape after fluridone resistance evolved. While studies of Townline Lake Myriophyllum have shown resistance to fluridone (Berger et al. Reference Berger, Netherland and MacDonald2012; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012), it is unknown how common fluridone resistance is in Myriophyllum. For this reason, we tested the response of several other genotypes to begin building a “catalog” of fluridone responses for different Myriophyllum genotypes. This catalog may also be used to inform fluridone treatment decisions in the future when managers see a characterized genotype in their lakes.
Materials and Methods
We tested the response to fluridone of 13 Myriophyllum accessions. Here, we define an accession by the combination of its taxon (M. spicatum vs. M. spicatum × M. sibiricum), multilocus microsatellite genotype, and where/when it was collected, as the same multilocus microsatellite genotype can be found in different lakes (Table 1). A culture of each accession was initiated from a single meristem collected from the field and vegetatively propagated at Montana State University’s Plant Growth Center (Bozeman, MT). The multilocus microsatellite genotypes for each accession were determined in a previous study (Thum et al. Reference Thum, Chorak, Newman, Eltawely, Latimor, Elgin, Parks and McNair2020).
Table 1. Description of the 13 Myriophyllum accessions in this study, including multilocus microsatellite genotype, taxon (EWM, Myriophyllum spicatum; hybrid, Myriophyllum spicatum × Myriophyllum sibiricum), and the lake, U.S. state, and year collected from the field.
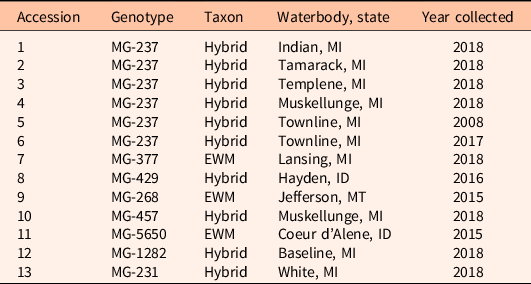
To determine the extent of fluridone resistance in clones of MG-237 found in multiple waterbodies, seven accessions of MG-237 were included in our fluridone assays. Accessions 1 through 5 were all MG-237 but were collected in different lakes (Table 1). Accessions 5 and 6 were MG-237 collected in the originally identified fluridone-resistant lake (Townline Lake, MI), but in different years; the accession collected in 2008 preceded the documentation of fluridone resistance (Berger et al. Reference Berger, Netherland and MacDonald2012; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012), whereas the accession collected in 2017 represents a recolonization of that same genotype after several years of management with alternative herbicides (Table 1). To begin building a catalog of fluridone responses, seven additional accessions with unique multilocus microsatellite genotypes were also included (accessions 7 to 13) in our fluridone assays (13 total accessions) (Table 1).
To measure the response of each accession to fluridone, we used a treatment versus control assay. While the initial identification of herbicide resistance in a species should use a dose–response assay with several herbicide concentrations above and below the operational field rate (Burgos Reference Burgos2015; Burgos et al. Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013), fluridone resistance has previously been confirmed using a dose–response assay in Townline Lake, MI Myriophyllum (MG-237; Berger et al. Reference Berger, Netherland and MacDonald2012; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012). As is common in herbicide-resistance screens (Burgos Reference Burgos2015), we were logistically constrained by space for housing large tanks suitable for growing and testing multiple accessions at multiple treatment levels. We therefore decided to prioritize the number of accessions to assay over the number of treatment levels. Because regulation in Michigan, where MG-237 is widespread, restricts the use of fluridone above 6 µg L−1, we chose this as the discriminating dose for our fluridone-resistance assays. Our assays directly followed the recommendations of Burgos (Reference Burgos2015) and Burgos et al. (Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013) for confirming herbicide resistance and included three replicate tanks of both the discriminating dose (6 µg L−1) and untreated control.
We replicated the whole fluridone assay twice on all 13 accessions of Myriophyllum (hereafter referred to as Trials 1 and 2). For each trial, we vegetatively propagated each accession in separate tanks to generate enough meristems for the assay. We planted 8-cm meristems of each accession into Cone-tainers™ (Stuewe and Sons., Inc. Tangent, OR) with soil (1:1:1 peat:topsoil:sand) capped with pure sand. For each of the two trials, we used six 378.5-L steel cattle tanks with approximately 10 cm of soil capped with sand on the bottom and filled with approximately 296.5 L of Smart and Barko (Reference Smart and Barko1985) buffered water. To account for tank variance, three of each Cone-tainer™–planted accession was randomly arranged into each of the six tanks by inserting the Cone-tainer™ approximately 7.5-cm deep into the soil at the bottom of each tank. Only one meristem of accessions 6 and 8 was included per tank in Trial 1, because there were not enough meristems in culture to draw from. Both trials were conducted under greenhouse conditions with ambient and supplemental incandescent lighting to maintain a 16-h light:8-h dark period. Adequate aeration of the water in each tank was maintained with an air bubbler for both trials.
Our target concentration exposure time (CET) for both trials was 6 µg L−1 fluridone for 50 d. After a 12-d establishment period, we randomly selected three of the six tanks in each trial to receive a treatment of fluridone (Sonar Genesis®, SePRO, Carmel, IN). Approximately 14 d after treatment, we measured fluridone concentration in each tank (SePRO FasTEST®) to determine whether fluridone concentration needed to be adjusted in the treated tanks. Based on the fluridone concentration measurements, we discovered a clerical error in the first trial regarding the stock concentration of the fluridone used, which resulted in an initial concentration of only 3 µg L−1. Therefore, at 20 d after the initial fluridone treatment, the concentration in treated tanks was increased to 6 µg L−1 fluridone. Because our target CET was 6 µg L−1 fluridone for 50 d, we extended the first trial for 10 d (60-d total treatment time) to equal the same total fluridone exposure for the two trials (3 µg L−1 * 20 d + 6 µg L−1 * 40 d = 300 µg L−1d = 6 µg L−1 * 50 d). In Trial 2, the target 6 µg L−1 fluridone concentration was achieved and maintained for 50 d in treated tanks. While the two trials differed as noted, we saw an overall fluridone treatment effect (Table 2). Over the treatment period in both trials, water temperatures ranged from 18 to 22 C, and pure water was replaced weekly as it evaporated. At the end of the trial, plants were harvested and dried to a constant mass. We then measured the dry biomass of each plant.
Table 2. ANOVA (type II Satterthwaite’s method) table for the linear mixed-effects regression model (dry biomass ~ treatment*accession + (1 | trial : tank) + (1 | tank)) determining the effects of the accession of Myriophyllum exposed to control and 6 µg L−1 fluridone treatments on dry biomass over a 50-d exposure period.

We used a linear mixed-effects model (lme4 package in R) to test for fixed effects of treatment and accession while removing random effects of tank and trial. Trials also included three replicates of each accession planted within a tank, but these tank accession replicates were not included as a random effect in the model, because the model fit (using the Akaike information criterion) was higher without them. We then used the emmeans package in R to calculate estimated marginal means (emmeans) and pairwise contrasts between the control and 6 µg L−1 fluridone treatments within each accession (Supplementary Appendix S1).
Results and Discussion
Overall tank dry biomass was reduced in the 6 µg L−1 fluridone–treated tanks compared with the control tanks (effect of treatment in Table 2; see also accession responses in Figure 1), indicating that 6 µg L−1 fluridone was effective at decreasing overall Myriophyllum biomass. However, an ANOVA on the linear mixed-effects model of the data also showed a significant accession by treatment interaction (Table 2), indicating that different accessions responded differently to the 6 µg L−1 fluridone treatment. This trend can also be seen in the qualitatively differing slopes of the interaction plot of control and 6 µg L−1 treatment estimated marginal means in each accession (Figure 1).
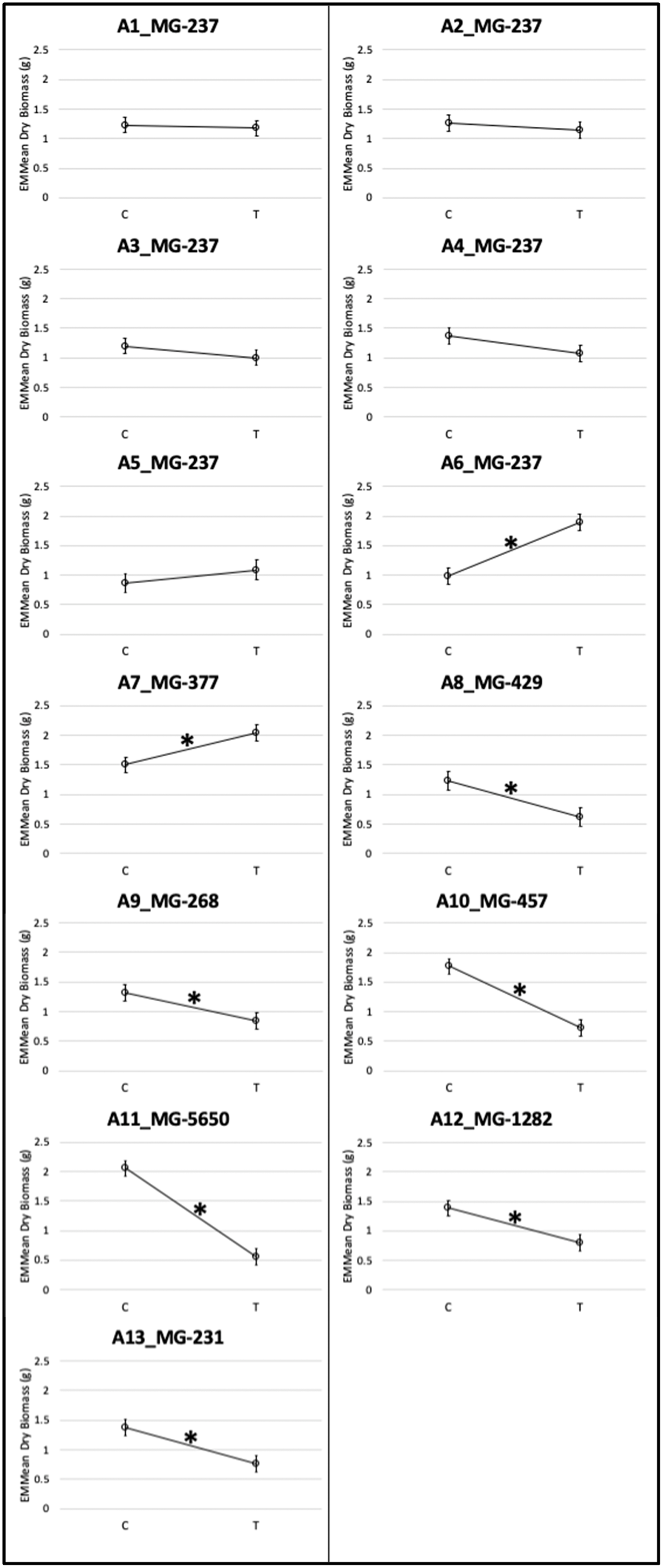
Figure 1. Interaction plot of the estimated marginal means (emmeans) of dry biomass for each accession (A) in both control (C) and 6 µg L−1 fluridone (T) treatment environments. Error bars around the emmeans represent the standard error and asterisks (*) represent significant (P < 0.05) differences between control and 6 µg L−1 fluridone treatment emmeans within that accession. The slopes of the lines between the control and 6 µg L−1 treatment emmeans indicate how much an accession was affected by 6 µg L−1 fluridone treatment. The title of each plot also includes the multilocus genotype (MG) of the accession plotted.
None of the MG-237 accessions were significantly negatively affected by 6 µg L−1 fluridone treatments (Figure 1). Accessions 1 through 5 showed a nonsignificant difference in dry biomass emmeans between control and 6 µg L−1 fluridone treatments (P = 0.1323 to 0.7831; Figure 1). Accession 6 showed a significant difference in emmean biomass between control and 6 µg L−1 fluridone treatments (P < 0.0001), but mean dry biomass was greater in 6 µg L−1 fluridone than in control treatments for that accession (Figure 1). These results align with previous dose–response assays that showed MG-237 is resistant to 6 µg L−1 fluridone (Berger et al. Reference Berger, Netherland and MacDonald2012; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012) and a failed 6 µg L−1 fluridone treatment in Townline Lake, Michigan (Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012). Therefore, we conclude that 6 µg L−1 fluridone treatments would not be effective at reducing MG-237 biomass, because MG-237 accessions tested here also showed resistance to the Michigan field rate of 6 µg L−1 fluridone. These results also align with the expected pattern of a clonal lineage that evolved resistance before its spread across the landscape. We therefore recommend that any lakes found to be dominated by MG-237 individuals should not be treated with fluridone, as they will likely exhibit resistance to the typical 6 µg L−1 fluridone treatment for M. spicatum.
Accession 7 also showed resistance to 6 µg L−1 fluridone treatments, because emmean dry biomass in 6 µg L−1 fluridone treatments was significantly larger than in control treatments for that accession (P = 0.0069; Figure 1). Accession 7 is a unique genotype (MG-377) from Lake Lansing, MI. We therefore conclude that the Michigan field rate of 6 µg L−1 fluridone treatment is likely to be ineffective in the long term at controlling Myriophyllum biomass in Lake Lansing. Further genetic monitoring is needed to determine whether MG-377 is present in other lakes; if so, fluridone is likely to be an ineffective treatment method in those lakes as well.
As predicted, several accessions were significantly reduced with 6 µg L−1 fluridone (Figure 1). Accessions 8 through 13 all showed a significant decrease in emmean dry biomass in 6 µg L−1 fluridone–treated versus control treatments (P = <0.0001 to 0.0167; Figure 1). This significant decrease in emmean dry biomass in 6 µg L−1–treated versus control indicates that these accessions are more likely to be effectively controlled by 6 µg L−1 fluridone treatments than MG-237 and MG-377. However, the slopes of the lines between control and treatment emmeans in Figure 1 suggest that significantly reduced accessions identified here likely exhibit variation in the amount of dry biomass reduction with 6 µg L−1 fluridone. Our data suggest that there is variation in the response to fluridone of significantly reduced accessions tested here. Dose–response assays on these accessions are therefore warranted to determine variation in sensitivity to fluridone treatments.
One interesting trend that came out in this study is that accessions 6 and 7 showed an increase in growth in the fluridone treatment compared with the untreated control (Figure 1). Previous dose–response studies of Townline Lake Myriophyllum also found more growth in the 6 µg L−1 fluridone treatment compared with the untreated control. (Berger et al. Reference Berger, Netherland and MacDonald2012; Thum et al. Reference Thum, Wcisel, Zuellig, Heilman, Hausler, Tyning, Huberty and Netherland2012). This increased growth in the 6 µg L−1 fluridone treatment may indicate some density regulation of growth in resistant accessions. Further investigation into the mechanism(s) of fluridone resistance in these accessions of Myriophyllum may elucidate the cause of this and whether it is a potential resistance trade-off.
This study also demonstrates that the characterization of herbicide response for common and/or putatively problematic Myriophyllum genotypes is likely to predict efficacy outcomes for managers who perform pretreatment genetic monitoring. For instance, if managers detect MG-237 at a high frequency in a lake, then according to the results here, fluridone would not be an effective control tactic for reducing biomass of Myriophyllum in that lake. Conversely, if lakes are mostly dominated by one of the significantly affected genotypes identified here, then fluridone may be an effective control tactic to reduce Myriophyllum biomass. Preliminary data suggest that Myriophyllum clones are frequently shared across nearby lakes (Thum et al. Reference Thum, Chorak, Newman, Eltawely, Latimor, Elgin, Parks and McNair2020). Therefore, the continuation of assays like these and dose–response assays to build a catalog of herbicide responses for genotypes of Myriophyllum found across the managed landscape may be an effective short-term management tool for Myriophyllum with a region-wide benefit for managers.
While the above catalog will be invaluable for lakes harboring characterized genotypes, there may be too many genotypes to exhaustively characterize them all. Thus, in the long-term, the genetic basis of fluridone resistance in Myriophyllum should be explored. Distinguishing the (or closely linked) gene(s) resulting in fluridone resistance would ultimately be a more effective marker to scan for in populations. Similar to the mutation that indicates fluridone resistance in the aquatic plant hydrilla [Hydrilla verticillata (L. f.) Royle], this type of marker could predict resistance regardless of genetic background (Benoit and Les Reference Benoit and Les2013; Michel et al. Reference Michel, Arias, Scheffler, Duke, Netherland and Dayan2004). However, in the short term, identifying and prioritizing specific genotypes for herbicide characterization could help preserve the effectiveness of currently used herbicides for Myriophyllum control.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2020.34
Acknowledgments
No conflicts of interest have been declared. This project was supported by the Michigan Invasive Species Grant Program (www.michigan.gov/invasives), with additional support from the Montana Agricultural Experiment Station (Project MONB00249). We thank Jennifer Lachowiec for guidance on the statistical analyses in this study. We thank SePRO for providing the fluridone used in this assay. We thank Bre Grabill, Doug Pullman, Jake Britton, Paul Hausler, Rick Buteyn, Ben Scofield, Jens Beets, Jeff Pashnick, and Tom Woolf for collection of the accessions used in this project. We thank Hannah Hoff, Emma Rice, and Jeff Pashnick for assisting in experimental setup and take down. Finally, we thank Hannah Hoff, Emma Rice, Jeff Pashnick, Katheryn Gannon, and Zach Kuzniar for providing friendly review of this article.





