Scientific Classification
Kingdom: Plantae
Subkingdom: Tracheobionta
Division/Phylum: Magnoliophyta/Tracheophyta
Class: Magnoliopsida/Angiospermae
Subclass: Asteridae
Category: Campanulids
Order: Asterales
Family: Asteraceae
Subfamily: Asteroideae
Tribe: Heliantheae
Subtribe: Ambrosiinae
Genus: Parthenium
Species: hysterophorus L.
Synonyms: Argyrochaeta bipinnatifida Cav.; Echetrosis pentasperma Phil; Parthenium lobatum Buckley; Parthenium pinnatifidum Stokes.
EPPO code: PTNHY
Name and Taxonomy
Parthenium weed (Parthenium hysterophorus L.) belongs to the Asteraceae family and Heliantheae tribe. It was initially classified under the Melampodiinae subtribe but later reassigned to the Ambrosiinae subtribe; the same subtribe under which other genera such as Ambrosia, Xanthium, Iva, and Parthenice belong (Stuessy Reference Stuessy1973). However, the exact phylogenetic relationships among these genera remain uncertain (Panero Reference Panero2005).
Parthenium hysterophorus is recognized by a multitude of names around the world, reflecting its widespread distribution and impact (Table 1).
Table 1. International and local names for Parthenium hysterophorus around the world.

Importance
Parthenium hysterophorus is a weed of global significance because of its widespread distribution, its aggressive invasive nature, and its detrimental impacts on agricultural production, animal and human health, and the environment (Chippendale and Panetta Reference Chippendale and Panetta1994; Jayachandra 1971; Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996). Initially, in its invaded range, P. hysterophorus was primarily seen as a weed of rangeland settings (Adkins and Shabbir Reference Adkins and Shabbir2014), but more recent reports indicate its proliferation in agricultural systems as well, with one study reporting up to 97% of sorghum [Sorghum bicolor (L.) Moench] yield losses attributed to P. hysterophorus infestation in Ethiopia (Tamado et al. Reference Tamado, Ohlander and Milberg2002). In India, one of the worst P. hysterophorus–affected countries in the world, yield losses have been reported to be as high as 40% in rice (Oryza sativa L.), 63% in tomato (Solanum lycopersicum L.), and 90% in sorghum (Kumar Reference Kumar2012; Oudhia Reference Oudhia2000). In Pakistan, P. hysterophorus infestation in maize (Zea mays L.) and wheat (Triticum aestivum L.) crops have caused regional food shortages (Khan et al. Reference Khan, Marwat, Hassan and Khan2013). Parthenium hysterophorus is known to outperform crops when competing for resources such as light, water, space, and nutrients (Zimdahl Reference Zimdahl2004); and under competition, crop growth, reproduction, and ultimately crop yields are all negatively affected. It also competes indirectly through its powerful allelopathic mechanisms (Bajwa et al. Reference Bajwa, Weston, Gurusinghe, Latif, Adkins and Weston2020; Shi and Adkins Reference Shi and Adkins2020) or by serving as an alternate host to pests and diseases (Shabbir Reference Shabbir2014b; Sharman et al. Reference Sharman, Persley and Thomas2009). Parthenium hysterophorus also causes indirect yield losses when its pollen grains fall onto the floral structures of various crops (Kanchan and Chandra Reference Kanchan and Chandra1980), which then causes poor seed set of the crop.
Apart from crop production losses, livestock production is the second major agricultural system affected by P. hysterophorus. Competition with fodder crops directly impacts the quality and yield of fodder, which in turn jeopardizes livestock health, milk and meat production, and meat quality. For example, in Australia, livestock that consume P. hysterophorus have been reported to produce a bitter-tasting milk, and the meat from sheep (Ovis aries Linn.) is said to emit a strong odor that spoils the taste and market value of the meat (Tudor et al. Reference Tudor, Ford, Armstrong and Bromage1982). Additionally, the invasion of P. hysterophorus into a given habitat is known to alter grazing community composition and diversity by reducing the richness and abundance of species, both in the above- and belowground communities (Belgeri et al. Reference Belgeri, Navie, Vivian-Smith and Adkins2014; Boja et al. Reference Boja, Girma and Dalle2022; Khatri-Chettri et al. Reference Khatri-Chettri, Rokaya and Shrestha2022; Mutua et al. Reference Mutua, Chiuri, Ngure and Kimani2022; Paneru et al. Reference Paneru, Maharjan, Devkota and Shrestha2023). Parthenium hysterophorus invasion also affects the physical and chemical properties of soil, such as its texture, pH, organic matter, nitrogen (N), phosphorus (P), and potassium (K) content (Karki Reference Karki2008; Osunkoya et al. Reference Osunkoya, Akinsanmi, Lim, Perret, Callander and Dhileepan2017; Timsina et al. Reference Timsina, Shrestha, Rokaya and Münzbergová2011), as well as soil invertebrates (Jeyalakshmi et al. Reference Jeyalakshmi, Doraisamy and Valluvaparidasan2011), faunal diversity (McClay et al. Reference McClay, Palmer, Bennett and Pullen1995), and water systems (Ashraf et al. Reference Ashraf, Ayub, Sajjad, Elahi, Ali and Ahmed2010; Patel et al. Reference Patel, Chitra, Prasanna and Krishnaraju2008).
Parthenium hysterophorus is also a serious threat to public health due to its ability to cause allergies and dermatitis as well as internal respiratory diseases like allergic asthma, bronchitis, and rhinitis (hay fever). Contact dermatitis is a common allergy caused by personal contact with P. hysterophorus (Allan et al. Reference Allan, BoYang, Adkins, Adkins, Shabbir and Dhileepan2019; Muddebihal et al. Reference Muddebihal, Sardana, Sinha and Kumar2023), and 40% of cases of plant dermatitis in India are related to P. hysterophorus (Agarwal et al. Reference Agarwal, Saini, Kumar and Vijay2021). Various allergies due to the pollen of P. hysterophorus have been recorded in almost all countries where the plant has been introduced (Goldsworthy Reference Goldsworthy2005; Gupta and Chanda Reference Gupta and Chanda1991; Lonkar et al. Reference Lonkar, Mitchell and Calnan1974; Nadeem et al. Reference Nadeem, Rani, Aman, Kazmi and Shabbir2005).
The socioeconomic losses caused by P. hysterophorus infestations are significant in some parts of the world. In India, Kumar and Varshney (Reference Kumar and Varshney2010) reported P. hysterophorus infestation of over 35 million ha of land and predicted that the cost of manual and chemical control would be around US$36.2 million yr−1. Additionally, the predicted costs for the treatment of P. hysterophorus–induced health problems were estimated to be US$1.06 million yr−1 (Kumar and Varshney Reference Kumar and Varshney2010). In Pakistan, the combined cost of the negative impacts of P. hysterophorus on crops, fodder, and animal and human health for a single rural household was estimated to be ca. US$3,244 yr−1 (Bajwa et al. Reference Bajwa, Farooq, Nawaz, Yadav, Chauhan and Adkins2019a). In Australia in the 1970s, the infestation of P. hysterophorus had reached ca. 600,000 km2 of rangelands and was predicted to be causing beef production losses of US$11.03 million (Chippendale and Panetta Reference Chippendale and Panetta1994). Adamson (Reference Adamson1996) estimated that if the rate of P. hysterophorus spread continues in Australia until the year 2050, the economic losses would reach US$72.2 million yr−1. This is one of the reasons that P. hysterophorus has been designated as a Weed of National Significance in Australia along with only 31 other invasive plant species.
Parthenium hysterophorus spread and impacts are predicted to be further aggravated by climate change, as its growth and reproduction traits are known to tolerate increasing temperature and drought conditions and to respond positively elevated atmospheric carbon dioxide (CO2) levels (Bajwa et al., Reference Bajwa, Wang, Chauhan and Adkins2019e; Belgeri Reference Belgeri2013; Navie Reference Navie2002; Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a). Because many grain crops are grown on marginal, less-fertile, and nonirrigated lands, the ability of P. hysterophorus to grow under water-stress conditions is a threat to dryland crop production. Under current and future climate scenarios with a temperature increase of +3 C, P. hysterophorus is likely to significantly expand into much larger areas that are under irrigation (Shabbir et al. Reference Shabbir, Zalucki, Dhileepan, Khan and Adkins2023).
Parthenium hysterophorus is known to cause significant negative impacts on the biodiversity of natural areas, such as natural reserves, national parks, forests, and other protected areas (Witt and Belgeri Reference Witt, Belgeri, Adkins, Shabbir and Dhileepan2019). Once it has invaded an area, P. hysterophorus reduces the native plant diversity and abundance and alters the soil nutrient dynamics (Osunkoya et al. Reference Osunkoya, Akinsanmi, Lim, Perret, Callander and Dhileepan2017; Shabbir and Bajwa Reference Shabbir and Bajwa2006; Etana et al., Reference Etana, Kelbessa and Soromessa2011; Timsina et al. Reference Timsina, Shrestha, Rokaya and Münzbergová2011). For instance, in Ethiopia, P. hysterophorus was reported to change the species composition and had significantly reduced the grass species diversity in a grassland (Nigatu et al. Reference Nigatu, Hassena, Sharma and Adkins2010). Another study in southeastern Ethiopia reported lower biomass and diversity of grasses across P. hysterophorus–invaded sites. The species diversity and evenness in aboveground vegetation were significantly lower in P. hysterophorus–invaded sites compared with uninvaded sites (Ayele et al. Reference Ayele, Nigatu, Tamado and Adkins2013).
Despite negative impacts of P. hysterophorus, it also has beneficial uses (Chandrasena and Rao Reference Chandrasena, Rao, Adkins, Shabbir and Dhileepan2019), including use in traditional medicine for alleviating skin inflammation, rheumatic pain, diarrhea, urinary tract infections, dysentery, and malaria (Patel Reference Patel2011); as a bioherbicide (Saha et al. Reference Saha, Marble, Stamps, Steed and Boyd2018); in making compost (Devi and Khwairakpam Reference Devi and Khwairakpam2021); in phytoremediation to clean contaminated soil; in biogas production due to its high biomass content; and as a natural dye for fabrics (Saini et al. Reference Saini, Aggarwal, Sharma, Kaur and Yadav2014).
Description
Parthenium hysterophorus is a rapidly growing annual or short-lived perennial herb that often grows in dense stands at an average height of 1.5 m, or up to 2.0 m in field conditions under adequate soil moisture conditions (Adkins and Shabbir Reference Adkins and Shabbir2014; Haseler Reference Haseler1976; Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996). It is a dicotyledonous plant and produces cotyledons measuring 3 by 6 mm in size that are hairless and exhibit a short petiole (Figure 1A). Following the cotyledonary stage, the first true leaves appear pale green in color and often form a basal rosette of leaves that measure ca. 15 cm in length and 2 to 4 cm in width. The leaves are alternately arranged onto the main stem and are deeply lobed with petioles of up to 2 cm in length. Parthenium hysterophorus forms a rosette growth habit under specific environmental conditions such as when the plant is under environmental stress or heavy competition. Upon stem elongation, leaves that are smaller and narrower are produced in the upper branches, while relatively larger leaves, measuring about 20-cm long and 4- to 8-cm wide are produced in the lower branches. Leaves are covered with small, stiff hairs called trichomes, which appear more frequently on the lower surface than on the upper surface, and trichomes also cover the stem. Stems are longitudinally grooved, and succulent in the early growth stages but become hard and inflexible as the plant matures. The plant produces a deep taproot with many small root hairs that help with nutrient and water extraction from greater depths within the soil profile (Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996). The plant can withstand mild winter frosts, normally while in the rosette stage, and can regrow following moderate chilling injury, but cannot survive severe frost (Shabbir Reference Shabbir2012). The chromosome number of P. hysterophorus is 2n = 34 (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019).

Figure 1. Growth stages of Parthenium hysterophorus: (A) cotyledon stage; (B) early seedling stage; (C) rosette stage; (D) flowering stage; (E) a close-up of a flower head; (F) cypselae, each containing a single achene; and (G) achenes (seed).
Flowering begins as early as 28 d after seedling emergence (Jayachandra 1971; Figure 1). The flower structure is arranged into flower heads, or capitula, which are produced in numerous clusters on the terminal panicles. Each capitulum is creamy-white in color, measures about 4 mm across, and consists of five ray florets or occasionally six to eight ray florets, to which two sterile disk florets and a bract or phyllary are attached to form a complex cypsela (Rollins Reference Rollins1950). Inside each cypsela is a single black achene (hereafter referred to as a seed), which is obovate, 2.0- to 2.5-mm long, light in weight, and crowned by persistent corolla appendages and a style, measuring about 2- to 3-mm long by 1- to 2-mm wide, without a pappus (EPPO 2014). The cypsela is light brown in the early stages of development but turns dark brown when mature (Figure 1).
Geographic Distribution
Parthenium hysterophorus is known to be present in at least 70 countries. The native range of this plant includes 30 countries from the United States to Mexico and throughout Central America and South America (Rollins Reference Rollins1950). The non-native global distribution, or introduced range of P. hysterophorus, spans 40 or more countries and islands that are part of Africa, Asia, Oceania, and Europe (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019; Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016; Mao et al. Reference Mao, Shabbir and Adkins2021b). The Global Biodiversity Information Facility (GBIF 2024) lists 26,297 occurrences. This includes a non-native distribution in Australia (3,696 occurrences), India (2,120 occurrences), Taiwan (1,351 occurrences), and Ethiopia (1,196 occurrences), and these rival the lists for its native range, which includes the southern region of the United States (3,988 occurrences), Mexico (4,529 occurrences), Central America (213 occurrences), and South America (2,468 occurrences) (Figure 2).
United States
Established populations of P. hysterophorus occur mainly south of 35°N and include the states of California, Oklahoma, Kansas, Arkansas, Louisiana, Mississippi, Alabama, Georgia, Texas, and Florida (Dale Reference Dale1981; GBIF 2024; Rollins Reference Rollins1950). Populations are most common in Texas, Oklahoma, Florida, Louisiana, and Mississippi (Boyd and Reuss Reference Boyd and Reuss2022; Dale Reference Dale1981; Fernandez et al. Reference Fernandez, Odero, MacDonald, Ferrell and Gettys2015; GBIF 2024; Reddy et al. Reference Reddy, Bryson and Burke2007) and have an allelopathic chemical composition, particularly sesquiterpene lactone (secondary metabolite), similar to those of populations found in Mexico and Central America, which suggests the plant’s native range encompasses the southern United States (Picman and Towers Reference Picman and Towers1982; Rollins Reference Rollins1950). Increased occurrences have been observed since the early 2000s in Alabama and Georgia, since the mid-2010s in California, and most recently in South Carolina in 2021 and 2022 (GBIF 2024; Reddy et al. Reference Reddy, Bryson and Burke2007).
Parthenium hysterophorus was introduced into Hawaii in the 1960s, most likely from the continental United States and has since been naturalized on the islands of Hawai’i, Kaua’i, Maui, Molokai, and O’ahu (PIER 2023; Wagner et al. Reference Wagner, Herbst and Sohmer1990). It has been reported northward of 35°N in the continental United States in New Mexico in 1904, Missouri in 1948, Illinois in 2007, Ohio in 1890, Michigan in 1917, Connecticut in 1908, Delaware in 2011, New York in 1921, and New Jersey in 1931 (GBIF 2024; Rollins Reference Rollins1950; USDA-NRCS 2023; Wiegand and Eames Reference Wiegand and Eames1925). There are no records beyond the initial observation of P. hysterophorus in any of these locations in the United States, and they therefore should be considered transient.
Mexico
The majority of the P. hysterophorus populations in Mexico are to be found in lower-elevation areas of the Tamaulipas and Yucatán provinces that border the Gulf of Mexico and the Tamaulipas and Sierra Madre Oriental provinces in the northeast (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019). Parthenium hysterophorus has a limited naturalized presence in the temperate parts of central Mexico but has become more concentrated in recent decades around the Federal District of Mexico and the surrounding Guanajuato, Morelos, and Querétaro provinces, as well as the Sonora and Chihuahua provinces in the northwest provinces (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019; GBIF 2024). Mexico and the region around the Gulf of Mexico are central to the distribution of the genus Parthenium and contain the highest diversity of its natural enemies (Bennett and McClay Reference Bennett and McClay1979; McClay et al. Reference McClay, Palmer, Bennett and Pullen1995; McFadyen Reference McFadyen1979).
Central America
Populations of P. hysterophorus occur throughout Belize, Guatemala, Honduras, Nicaragua, Costa Rica, and Panama (GBIF 2024). Several populations from these countries have been shown to have a composition of sesquiterpene lactone, an allelopathic chemical, similar to populations in Mexico and United States, suggesting the plant’s native range encompasses the region (Picman and Towers Reference Picman and Towers1982; Rollins Reference Rollins1950).
South America
Parthenium hysterophorus is recorded from virtually all countries in South America, but most occurrences are concentrated in two disjunct ranges: one throughout Paraguay, northern Argentina, southern Brazil, and southern Bolivia, and the second spanning the northern range of Venezuela, Columbia, Ecuador, and Peru (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019; Dale Reference Dale1981). Differences in the composition of sesquiterpene lactone, an allelopathic chemical, have been identified, with most plants in the South American range containing hymenin and having cream or yellow flowers (Picman and Towers Reference Picman and Towers1982), while parthenin is the dominant sesquiterpene lactone for the white-flowering biotypes in Central America, Mexico, the Bahamas, and the southern United States (Dale Reference Dale1981). Interestingly, the yellow-flowering biotype of P. hysterophorus is only found in southern Brazil, Bolivia, and northern Argentina (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018; Dale Reference Dale1981). The white-flowering form is also found in Brazil and is hypothesized to be a more recent introduction to the area (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018). The high diversity seen in populations in this region of South America is likely due to polyploidy or hybridization with the closely related species P. bipinnatifidum (Ortega) Rollins and P. confertum A. Gray (Picman and Towers Reference Picman and Towers1982; Rollins Reference Rollins1950).
In Uruguay, the distribution of P. hysterophorus is irregular, and it has not established widely based on survey data collected in 2010 (Belgeri Reference Belgeri2011) and from herbarium records made in the 1930s and 1960s (GBIF 2024). Three unverified records of P. hysterophorus have been made from near Saul, French Guiana, in 2021, with one further account represented by a herbarium record from 1883 (GBIF 2024). Like the records in French Guiana, the occurrence of P. hysterophorus in Guyana and Suriname should be considered transient based on the limited number and age of the herbarium records made in Guyana (1883) and Suriname (the mid- to late 1800s, 1936, and 1997) (GBIF 2024).
Bermuda to the Caribbean
Parthenium hysterophorus is widespread in all Commonwealth Caribbean countries and dependent territories, from the Bahamas to other countries in the region that include Cuba, Haiti, Antigua and Barbuda, Trinidad and Tobago, and the Federation of Saint Christopher and Nevis (Hammerton Reference Hammerton1981; Rollins Reference Rollins1950). The plant is predominantly found along roadsides, forests, and agricultural areas, with large and persistent infestations near the towns of San Juan, Puerto Rico, and Santo Domingo, Dominican Republic, and across the north coast of Jamaica (GBIF 2024; Wunderlin et al. Reference Wunderlin, Hansen, Franck and Essig2024). A recent survey indicated P. hysterophorus has spread to Dominica and Saint Kitts as recently as 2018, with observations in urban areas within both countries (A Witt, personal communication, 2023). Whether the West Indies are within the native range is difficult to determine, but P. hysterophorus is argued to have been introduced to this region from Mexico (Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996) due to there being no other species of the genus Parthenium there or the insects that exclusively target the weed (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018).
South Asia
The main P. hysterophorus infestation was introduced into India from a U.S.-based food grain shipment delivered in 1955 (Shabbir et al. Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c). This infestation spread aggressively across India and then to Pakistan, Nepal, Bangladesh, Bhutan, and Sri Lanka, in multiple introductions, as indicated by genetic and ecological studies (Bambaradeniya Reference Bambaradeniya2002; Jabeen et al. Reference Jabeen, Prentis, Anjum and Adkins2015; Shabbir and Bajwa Reference Shabbir and Bajwa2006; Shrestha et al. Reference Shrestha, Shabbir, Adkins and Bourdôt2015; Tomasello et al. Reference Tomasello, Stuessy, Oberprieler and Heubl2019). Parthenium hysterophorus is estimated to have invaded 35 million ha in India (Kohli et al. Reference Kohli, Batish, Singh and Dogra2006; Sushilkumar Reference Sushilkumar2012) and 1.3 million ha of vegetable production in a northern province of Sri Lanka (Kirshanthan et al. Reference Kirshanthan, Jeyaseelan and Nandakumar2016; Kishojini et al. Reference Kishojini, Pakeerathan and Mikunthan2018). The most severe infestations in Pakistan occur in the northeastern parts of the Punjab Province (Dhileepan and Senaratne Reference Dhileepan and Senaratne2009; Shabbir Reference Shabbir2013; Shabbir and Bajwa Reference Shabbir and Bajwa2007), but a southward range expansion is becoming more prevalent in agricultural land, especially in irrigated cropping systems (Shabbir et al. Reference Shabbir, Dhileepan and Adkins2012, Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c).
East and Southeast Asia
In southern and southeast China, P. hysterophorus is found in 9 of the 12 provinces and districts situated between 18°N to 25°N (Li and Gao Reference Li and Gao2012; Tang et al. Reference Tang, Wei, Zeng, Li, Tang, Zhong and Geng2009), with Chongqing being the most recent introduction (Chen et al. Reference Chen, Liu, Zhang, Jin, Han and Lin2016; Mao Reference Mao2018). In Zhejiang Province, a population was found in Ningbo, ca. 166 km northeast of the Wenzhou population (Mao Reference Mao2018; Wei et al. Reference Wei, Zhu, Pan, Wang, Hu, Zhou and Jin2021). In northern China, a population found in 2004 in Junan County of the Shandong Province (Wang and Hou Reference Wang and Hou2004) rapidly expanded to more than 400,000 ha by 2010 (Li and Gao Reference Li and Gao2012). This northern population in Shandong Province was shown to be a unique chloroplast haplotype, different from the southern population and considered to be a separate introduction into China (Tang et al. Reference Tang, Wei, Zeng, Li, Tang, Zhong and Geng2009). Apart from mainland China, populations of P. hysterophorus are also known in the island of Taiwan (Peng et al. Reference Peng, Hu and Kao1988), Vietnam (Nguyen Reference Nguyen2011), Japan (Tachikake and Nakamura Reference Tachikake and Nakamura2007; Tominaga Reference Tominaga2013), South Korea (Kim Reference Kim2013), Malaysia (Maszura et al. Reference Maszura, Karim, Norhafizah, Kayat and Arifullah2018; Shi et al. Reference Shi, Tang, Nguyen, Dhileepan, Adkins, Shabbir and Dhileepan2019; Sukumaran Reference Sukumaran2015), and Christmas Island (Dodd et al. Reference Dodd, Reeves and Eldershaw2012). In 2016, P. hysterophorus was reported in Lampoon Province, northern Thailand, within an agricultural area (Shabbir Reference Shabbir2017), and has since been reported in Chiangmai Province in northern Thailand, Nakhon Phanom and Nakhon Ratchasima provinces in the northeast, and in central Thailand near Kanchanaburi (S Zungsontiporn, personal communication, 2024).
Africa
In Southern Africa, P. hysterophorus is found in the countries of South Africa, Eswatini (formerly Swaziland), Mozambique, Zimbabwe, Botswana, and the western Indian Ocean islands such as Madagascar, Mauritius, Réunion, and the Seychelles (McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011; Nath Reference Nath1988; Strathie and McConnachie Reference Strathie, McConnachie, Adkins, Shabbir and Dhileepan2019). Parthenium hysterophorus thrives in the subtropical climate of South Africa and occupies about 4.4 million ha, with the most severe infestations in the KwaZulu-Natal Province (McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011; Terblanche Reference Terblanche2015). Populations have also been reported from Egypt in North Africa and the East African Countries of Ethiopia, Eritrea, Djibouti, Kenya, Rwanda, Somalia, Tanzania, and Uganda (Strathie and McConnachie Reference Strathie, McConnachie, Adkins, Shabbir and Dhileepan2019; Witt et al. Reference Witt, Kiambi, Beale and Van Wilgen2017). There is a widespread occurrence in Uganda from the eastern and southern to southwestern regions and in Ethiopia throughout in the south, central, and northern regions (Adem Reference Adem2010; McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011; Million et al. Reference Million, Nigatu, Bekeko and Legesse2021). The immediate origins of most of the P. hysterophorus populations in central and southern Africa are from the United States, but secondary transfer to South Africa has occurred from Mozambique (Picman and Towers Reference Picman and Towers1982; Towers andand Mitchell Reference Towers and Mitchell1983; Wise et al. Reference Wise, Van Wilgen, Hill, Schulthess, Tweddle, Chabi-Olay and Zimmermann2007) and from there into Botswana (Strathie and McConnachie Reference Strathie, McConnachie, Adkins, Shabbir and Dhileepan2019).
Eastern Mediterranean and Persian Gulf
Parthenium hysterophorus has a limited naturalized presence in the Arabian Peninsula, with populations found mainly along roadsides and on wastelands in Yemen (Kilian et al. Reference Kilian, Peter and Ali2002) and the United Arab Emirates (Mahmoud et al. Reference Mahmoud, Gairola and El-Keblawy2015) and, most recently, in agricultural areas and fallow fields in Oman (Shammas Reference Shammas2022) and Saudi Arabia (Thomas et al. Reference Thomas, Basahi, Al-Ansari, Sivadasan, El-Sheikh, Alfarhan and Al-Atar2015). Parthenium hysterophorus is thought to have been introduced into the Arabian Peninsula from populations in eastern Africa or South Asia (McConnachie and Witt Reference McConnachie, Witt, Adkins, Shabbir and Dhileepan2019) and from Yemen into Saudi Arabia, as populations are close to the border (A Witt, personal communication, 2023). In Israel, the first establishment of P. hysterophorus was found near the banks of several fishponds. It is thought that P. hysterophorus was introduced into Israel through contaminated low-grade grain that was subsequently used to make fish meal (Dafni and Heller Reference Dafni, Heller, di Castri, Hansen, Debussche and Invasions1990). Since then, the weed has rapidly expanded into agricultural areas in the Beit Shean Valley, Jezreel Valley, and Jordan Valley (Matzrafi et al. Reference Matzrafi, Raz, Rubin, Yaacoby and Eizenberg2021).
Oceania
Parthenium hysterophorus is widely naturalized in Queensland, Australia; occasional populations found in Western Australia, Northern Territory, and New South Wales (AVH 2023; Blackmore and Johnson Reference Blackmore, Johnson and Zydenbos2010; GBIF 2024; Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996) are immediately targeted for erdication. The weed was introduced into Queensland on two occasions, to Toogoolawah in the 1940s and into Clermont in the 1950s. The origin of both populations (biotypes) was the United States, as confirmed by genotypic studies (Graham and Lang Reference Graham and Lang1988; Hanif Reference Hanif2014) and characterized by plants containing parthenin as the major sesquiterpene lactone (Picman and Towers Reference Picman and Towers1982; Rodriguez et al. Reference Rodriguez, Yoshioka and Mabry1971). In Queensland, it was estimated that P. hysterophorus occupied 2.9 million ha by 1980, 17 million ha of rangelands by 1991, and 52 million ha by 2003, then effective control measures reduced the area of infestation to 36.9 million ha by 2013 (Chippendale and Panetta Reference Chippendale and Panetta1994; McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019). Vanuatu eradicated the first introduction of P. hysterophorus in 1971, but a new population has been reported in 2009 and has expanded into several islands, with the most severe infestations occurring on the Efate and Tanna islands (Day and Bule Reference Day and Bule2016; McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019). A recent population in Port Vila shares morphological characteristics with populations from both Vietnam and China (Mao et al. Reference Mao, Shabbir and Adkins2021b). Parthenium hysterophorus was introduced in 2001 in Papua New Guinea, near the city of Lea, but seems to be one of the few successful eradication efforts, with no plants found during annual surveys since 2006 (Kawi and Orapa Reference Kawi and Orapa2010; McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019). Populations are also known in New Caledonia and Tahiti (Florence et al. Reference Florence, Chevillotte, Ollier and Meyer2007; Gargominy et al. Reference Gargominy, Bouchet, Pascal, Jaffre and Tourneur1996).
Europe
Parthenium hysterophorus was found in Poland in 1938 and 2022 (GBIF 2024; Mirek et al. Reference Mirek, Piękoś-Mirkow, Zając and Maria2002), Belgium in 1999, 2003, 2013, 2017, and 2023 (GBIF 2024; Verloove Reference Verloove2006), Sweden in 1937 and 1967 (GBIF 2024), and the Netherlands in 1938 and 2014 (GBIF 2024), but this species has not persisted or established in these countries.
Habitat
According to a global Köppen-Geiger climate zone map, P. hysterophorus occurs across 16 global climate subtypes within a wide range of tropical (Group A), arid (Group B), temperate (Group C), and continental (Group D) climate groups. In the northern portion of the native distribution of P. hysterophorus, from the southern United States to Mexico, populations are predominately in temperate areas of humid subtropical (Cfa) and lowland (Cwa) climates, with additional occurrences in tropical savanna (Aw) regions along the Mexican Gulf Coast and the Yucatan Peninsula (Structured Appendix, Figure A1). Most populations in Mexico are found at elevations <1,500 m asl within regions with mean annual temperatures above 4 C or mean annual precipitation exceeding 600 mm (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019). Its northern native range is considered to extend into the arid regions of the southwestern United States to the Sonora and Chihuahua states of Mexico, where the BSh (steppe, hot) and BSk (steppe, cold) are dominant, with a few occurrences in hot (BWh) and cold (BWk) desert areas likely due to a long, dry winter season (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019). In Central America (native range) and Bermuda to the Caribbean (likely introduced range), P. hysterophorus occurs mainly in tropical climate subtypes Am, As, Aw (monsoon and savannah with dry summer or winter, respectively), apart from Honduras in the temperate climate subtype Cwa. In South America, the temperate areas of humid subtropical (Cfa) and lowland (Cwa) climates are dominant, followed by tropical savanna (Aw) regions, to some tropical rainforest (Af) regions in the north and warm-summer Mediterranean (Csb) regions in the southwest, and less commonly, in equatorial savanna (As) and steppe (BSh) climate subtypes in the east. Its distribution extends south of the 34th parallel in Argentina to the cold, steppe (BSk) regions, where it is commonly found around 1,500 m above sea level (asl) and as high as 3,580 m asl (Dale Reference Dale1981; GBIF 2024).

Figure 3. Parthenium hysterophorus infestations in pasturelands in Queensland, Australia (A) and Ethiopia (B), wheat fallow in Lahore, Pakistan (C), and maize crop in Arusha, Tanzania (D), along a water channel in Vietnam (E), and on campus of Tribhuvan in Nepal (F).
Globally, P. hysterophorus predominately occurs in one of five diverse climate types. It is most commonly present in areas of the world with a subtropical savanna biome with distinct wet and dry seasons (Aw; East Africa, central India, northern Australia) or humid subtropical climates (Cfa; central and southeastern China, Japan, eastern Australia). It thrives in these habitats with regular and abundant summer rains (Aw, Cfa), such as the major agricultural regions in Australia (McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019), east Africa (McConnachie and Witt Reference McConnachie, Witt, Adkins, Shabbir and Dhileepan2019), and in limited areas of China that receive at least 500 mm of annual rainfall (Shi et al. Reference Shi, Tang, Nguyen, Dhileepan, Adkins, Shabbir and Dhileepan2019; Structured Appendix, Table A1). The tropical savanna (Aw) and humid subtropical climates (Cfa) with average monthly temperatures exceeding 18 C in the tropical savanna (Aw) and humid subtropical climates (Cfa) promote optimal germination rates within temperature ranges of 15 to 25 C and 22 to 25 C for biotypes in Israel (Matzrafi et al. Reference Matzrafi, Raz, Rubin, Yaacoby and Eizenberg2021) and Australia (Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996), respectively, with flowering occurring more rapidly at warm temperatures (27/22 C; day/night) as compared with cooler temperature regimes (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019). The desert subtropical climate (Cwa) is the next most common climate type where P. hysterophorus is found, from south-central Africa to the majority of south Asia and eastern Australia.
Parthenium hysterophorus has a lesser, but significant presence in climate types such as the hot semiarid (Sh) regions of central and southern Africa, India, and parts of Australia (Structured Appendix, Figure A1). The hot (BSh) and cold (BSk) semiarid climates with dry winters or prolonged drought may limit the range expansion of P. hysterophorus (Doley Reference Doley1977; Royimani et al. Reference Royimani, Mutanga, Odindi, Zolo, Sibanda and Dube2019). Based on global populations that can germinate over a wide range of temperatures from 9 to 36 C in Australia (Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996), 10 to 30 C in Israel (Malka et al. Reference Malka, Eizenberg and Matzrafi2023), and 12 to 35 C in Ethiopia (Tamado et al. Reference Tamado, Ohlander and Milberg2002), seeds of P. hysterophorus are likely adapted to both the hot (BSh) and cold (BSk) semiarid climates. Populations are limited, but still often found, in areas of the world having a Mediterranean climate with cool, dry summers (Csb; parts of Kenya and Ethiopia) or hot, dry summers (Csa; Israel), and mainland coastal areas with hot, dry summers (Cfb; coastal South Africa and southeastern Australia). Parthenium hysterophorus tolerates subtropical highland climates (Cwb) but is likely restricted by dry winters in these regions of Mexico, South America, South Asia, and Africa. Nonetheless, this species is invading the high-altitude grasslands of Cwb climates in Bhutan, where it is mostly limited up to 1,700 m asl but reported as high as 2,320 m asl (Tshering and Adkins Reference Tshering and Adkins2012), from 1,500 to 2,400 m asl in the northwestern Indian Himalayas (Dogra et al. Reference Dogra, Sood and Sharma2011; Kohli et al. Reference Kohli, Dogra, Batish and Singh2004), up to 1,935 m asl in the northern highlands of Nepal, and up to 2,500 m asl in Lalibela, Ethiopia (Shrestha et al. Reference Shrestha, Shabbir, Adkins and Bourdôt2015; Tamado and Milberg Reference Tamado and Milberg2000). Known occurrences of P. hysterophorus are confirmed to tropical rainforest (Af) and monsoon (Am) climate types in parts of Madagascar and southern Thailand and solely tropical rainforest (Af) climates in parts of Hawaii, central Africa, Sri Lanka, and Papua New Guinea. Isolated populations in continental hot (Dsb) and warm (Dwa) summer climates are known to occur in northeastern Pakistan and South Korea, respectively.
Parthenium hysterophorus grows best on black, alkaline, cracking clay soils with high fertility (Adkins and Shabbir Reference Adkins and Shabbir2014). It is typically found in a wide range of soils textures, from sandy loams to clay loams and, in its native range, specifically, on eutric vertisols (base-rich cracking clays) or calcic leptosols (shallow, rocky, or gravelly soils) (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018; Adkins and Shabbir Reference Adkins and Shabbir2014; Dale Reference Dale1981). Parthenium hysterophorus can tolerate a wide range of soil types with various nutrient contents but is known to invade and thrive in nitrogen- and phosphorus-rich environments in its native range of Mexico and South America (Dale Reference Dale1981) and globally in areas such as Nepal (Timsina et al. Reference Timsina, Shrestha, Rokaya and Münzbergová2011), China (Shi et al. Reference Shi, Tang, Nguyen, Dhileepan, Adkins, Shabbir and Dhileepan2019), and Tanzania (Ojija and Manyanza Reference Ojija and Manyanza2021). Soil disturbance in fallow areas promotes its range expansion within its native range, regardless of soil type (Dale Reference Dale1981). A single soil disturbance in an Australian cropping system promoted the germination and persistence of a P. hysterophorus stand for a 4- to 6-yr period (White Reference White1994), and similarly, in Mexico, dense infestations have been observed to persist for ≥1 yr in frequently disturbed sites (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018).
Soil disturbance, anthropogenic activity, and waterways are important influences on seed distribution and establishment of this species. Parthenium hysterophorus inhabits waste areas and fallow lands to urban areas, roadsides and railway tracks, and field edges and agricultural land; it occurs along rivers and waterways and, less commonly, in open forest and shrub areas (Figure 3). In most invaded countries, P. hysterophorus spreads rapidly from small, isolated patches on roadsides, then into wastelands or fallow areas, and then into agricultural areas (Shi et al. Reference Shi, Tang, Nguyen, Dhileepan, Adkins, Shabbir and Dhileepan2019). The trans-border and long-distance movement of vehicles promotes seed dispersal of P. hysterophorus seed along road networks in southern Africa (Strathie and McConnachie Reference Strathie, McConnachie, Adkins, Shabbir and Dhileepan2019); East and North Africa (McConnachie and Witt Reference McConnachie, Witt, Adkins, Shabbir and Dhileepan2019; Mutua et al. Reference Mutua, Chiuri, Ngure and Kimani2022; Ojija and Manyanza Reference Ojija and Manyanza2021); and in the Persian Gulf, including Oman, Yemen, and the United Arab Emirates (Mahmoud et al. Reference Mahmoud, Gairola and El-Keblawy2015). Road maintenance practices of grading gravel-surfaced road networks and grass cutting along roadsides in a survey in southern Africa were found to have exacerbated the spread of P. hysterophorus seed along roadsides (Strathie and McConnachie Reference Strathie, McConnachie, Adkins, Shabbir and Dhileepan2019). A survey in Mexico attributed P. hysterophorus populations along roadways to increased moisture availability provided by water runoff from the road surface (Dale Reference Dale1981). For example, in the savanna climate of Kenya, P. hysterophorus was initially limited since its introduction in the early 1970s (McConnachie and Witt Reference McConnachie, Witt, Adkins, Shabbir and Dhileepan2019), but a 2022 survey in Nakuru County reported high densities alongside Kenyan roads following construction (Mutua et al. Reference Mutua, Chiuri, Ngure and Kimani2022). Unlike many plants, P. hysterophorus has strong mechanisms to tolerate environmental pollution in roadside communities (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019). Similarly, in wasteland areas, P. hysterophorus utilizes high bioaccumulation and translocation rates to tolerate soils contaminated with heavy metals of nickel, lead, copper, cobalt, chromium, and zinc (Hadi and Bano Reference Hadi and Bano2009; Irshad et al. Reference Irshad, Ahmad, Pervez and Inoue2015).

Figure 4. Road signs are erected in Queensland, Australia to assist in the prevention of further seed spread of Parthenium hysterophorus.
Waterways and irrigation channels are known to promote the transport of P. hysterophorus seeds; such movement has been reported from Pakistan, with seed being moved along the irrigation canal networks in the Punjab Province and the Indus River basin in south Pakistan (Anwar et al. Reference Anwar, Khan, Tahira and Suliman2012; Shabbir et al. Reference Shabbir, Dhileepan and Adkins2012), from the Mahaweli River body in Ethiopia by irrigation networks (Horo et al. Reference Horo, Gudisa, Worku and Tilahun2020), and in many parts of India along waterways (Adnan et al. Reference Adnan, Ali, Haider and Khan2015). Parthenium hysterophorus is frequently found along riverbanks and the edges of water channels in the otherwise inhospitable and seasonally dry (BWh/BSh) regions across the globe, such as central Pakistan (Shabbir et al. Reference Shabbir2012), and parts of Australia (Auld et al. Reference Auld, Hosking and McFadyen1982; McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019), Saudi Arabia (Thomas et al. Reference Thomas, Basahi, Al-Ansari, Sivadasan, El-Sheikh, Alfarhan and Al-Atar2015), Kenya (Njoroge Reference Njoroge1986), Ethiopia (Horo et al. Reference Horo, Gudisa, Worku and Tilahun2020; Seta et al. Reference Seta, Assefa, Mesfin and Balcha2013), and the Shandong and Guangxi provinces in China (Huang et al. Reference Huang, Liu, Tang and Zeng2012; Mao Reference Mao2018; Tang Reference Tang2012). For example, the invasion of P. hysterophorus in southern Pakistan is supported by moisture made available from irrigation canals and groundwater pumping when the climate conditions of hot summers and 250 mm of annual rainfall would otherwise be limiting (Shabbir et al. Reference Shabbir, Zalucki, Dhileepan, Khan and Adkins2023).
A comparison of the global distribution map of P. hysterophorus and the United Nations land-use assessment map (FAO 2013) shows its distribution is predominately associated with croplands and grasslands or sparse areas in both unmanaged systems and with livestock stocking rates from low to high densities. Shrub cover areas and forests having either agricultural activities or various stocking rates in areas in the southern United States, Mexico, southern and central Africa, and Australia are also correlated with known occurrences but likely limited to open shrub and forest areas with adequate light intensities that support the growth of P. hysterophorus (FAO 2013; Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996). Parthenium hysterophorus being found in low-rainfall climates that overlap with low- and high-intensity irrigation areas indicates increased water availably is promoting range expansion and that nearby non-invaded irrigated areas should be monitored.
Invasion History
The history of invasion, as far as is known, began with an accidental introduction to the island of Mauritius in the 18th century (Fusée-Aublet 1775). This and other early introductions were sporadic in nature but were usually associated with maritime trading (Binggeli Reference Binggeli, Goodman and Benstead2003). Thus, the global distribution of P. hysterophorus initially comprised island populations or locations close to large ports, including those of Kolkata, India (1810), Reunion in the Mascarenes (1878), Natal in South Africa (1880), the islands of New Caledonia (1881), the Seychelles (1908), and French Polynesia (1909) (Mao et al. Reference Mao, Shabbir and Adkins2021b). The pace of invasion never flagged and has increased dramatically in recent years. By the 1940s, P. hysterophorus was established in only about a dozen countries outside its native range, but since the 1950s, the speed of invasion has accelerated considerably (Mao et al. Reference Mao, Shabbir and Adkins2021b). A second and major introduction was made to India in 1955, which then spread over the entire country by the 1970s (Batish et al. Reference Batish, Kohli, Singh and Kaur2012), and into most neighboring countries by the early 2000s (Chhogyel et al. Reference Chhogyel, Kumar and Bajgai2021; Jayasuriya Reference Jayasuriya, Pullaiah and Ielmini2021; Shabbir et al. Reference Shabbir, Dhileepan and Adkins2012; Sushilkumar 2014). Similarly, a second major introduction in 1958 established the weed in Queensland, Australia. During the next 20 yr, only local spread occurred, followed by dramatic spread in the late 1970s, with invasions covering almost one-third of the state by 2000 (McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019), and repetitive incursions in New South Wales and other bordering states and territories (Blackmore and Johnson Reference Blackmore, Johnson and Zydenbos2010). The fastest spread in recent years has occurred in Asia and Africa, with new invasions taking place at a rate of ca. 10 countries per decade, a threefold rate of new introductions as compared with the 1950s (Mao et al. Reference Mao, Shabbir and Adkins2021b). Several countries of the Middle East (e.g., United Arab Emirates, Saudi Arabia) have recently been invaded by P. hysterophorus (McConnachie and Witt Reference McConnachie, Witt, Adkins, Shabbir and Dhileepan2019; Shabbir et al. Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c). Its spread is also increasing within already invaded countries, such as Oman (Shammas Reference Shammas2022).
Many attempts have been made to eradicate P. hysterophorus after invasion has occurred; however, this has proven to be extremely difficult. So far, eradication has only been successful in countries and regions where early detection of small populations was made. Eradication of such populations has been achieved in Papua New Guinea (Kawi and Orapa Reference Kawi and Orapa2010), the state of New South Wales, Australia (Blackmore and Johnson Reference Blackmore, Johnson and Zydenbos2010), and on Espiritu Santo Island, Vanuatu (McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019). In locations where the weed has become well established, containment, rather than eradication, is applied (Khan et al. Reference Khan, Navie, George, O’Donnell and Adkins2018; McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019; Shabbir et al. Reference Shabbir, Macdonald, Terblanche, Adkins, Shabbir and Dhileepan2019d; Figure 4). When used together with chemical and biological control and applied at the invasion front, containment can bring about local eradication, as has been achieved in regions of Australia (Blackmore and Johnson Reference Blackmore, Johnson and Zydenbos2010; Thorp Reference Thorp2001) and South Africa (Terblanche et al. Reference Terblanche, Nanni, Kaplan, Strathie, McConnachie, Goodall and van Wilgen2016). Sadly, eradication as well as containment of P. hysterophorus are still challenging to achieve on most occasions, especially in the countries of Asia and Africa, where the weed is spreading fast.

Figure 5. The life stages of Parthenium hysterophorus: seed containing cypsela, seedling, rosette, mature plant, and seed.
Life-Form and Life History
The life history of this annual forb commences from seed present in the soil seedbank on the soil surface (Figure 5). Fast germination, as well as rapid early seedling growth, enables this weed to enter the reproductive stage within 2 mo of germination under ideal conditions and then to live for up to 8 mo (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019) or, rarely, after regrowth in a subsequent season to more than 14 to 16 mo (Hassan Reference Hassan2011). When under stress, however, the weed can tolerate adverse effects by entering a rosette stage (Figure 5). Further rates of vegetative, then reproductive, growth vary under different environmental conditions (Kaur et al. Reference Kaur, Batish, Kaur, Singh and Kohli2017, Reference Kaur, Kaur, Singh, Batish and Kohli2019), thus phenotypic plasticity facilitates its survival under a wide range of conditions. Although plants do not survive in freezing temperatures, the soil seedbank serves as a reservoir for future generations, with seeds able to survive for more than a decade in the soil (Navie et al. Reference Navie, Panetta, McFadyen and Adkins1998; Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017b; Osunkoya et al. Reference Osunkoya, Ali, Nguyen, Perrett, Shabbir, Navie, Belgeri, Dhileepan and Adkins2014).

Figure 6. Global climatic suitability with occurrence records (green dots) of Parthenium hysterophorus using CLIMEX (Shabbir et al. Reference Shabbir, Zalucki, Dhileepan, Khan and Adkins2023).
The weed gains precedence in the long term by having a flexible life history: fast germination, excessive vegetative growth, prolonged reproduction, and tolerance of unfavorable conditions. When conditions allow, interspecific competition against other plants (Vehra and Khan Reference Vehra and Khan2011) and crops (Bajwa et al. Reference Bajwa, Shabbir, Adkins, Adkins, Shabbir and Dhileepan2019b, Reference Bajwa, Ullah, Farooq, Chauhan and Adkins2019c) is significant, especially when environmental disturbances are present (Cowie et al. Reference Cowie, Byrne and Witkowski2020a). With residues returned to the soil (Batish et al. Reference Batish, Singh, Pandher and Kohli2005), the weed also displays a moderate allelopathic effect (Shi and Adkins Reference Shi and Adkins2018), improving its chances of survival against other plants in the community.
Dispersal and Establishment
In an unmanaged population, P. hysterophorus is spatially dispersed by means of natural elements, such as wind and water. The structure of its cypsela, which contains a single mature achene, includes a winged assembly, consisting of two “air sacs” originating from two sterile florets, that assists its movement by wind or water (Auld et al. Reference Auld, Hosking and McFadyen1982; Nguyen Reference Nguyen2011). The wind-assisted dispersal of the cypsela is thought to be only locally important (Auld et al. Reference Auld, Hosking and McFadyen1982; Mao et al. Reference Mao, Shabbir and Adkins2021b), although the distance traveled could be considerable in the event of a whirlwind or storm (Haseler Reference Haseler1976; McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011). Water dispersal, on the other hand, could be over much longer distances and times. After 7 d of simulated water movement, 20% of the treated seeds remained floating, while still able to germinate (Mao et al. Reference Mao, Nguyen, Osunkoya and Adkins2019). These floating seeds provide an excellent opportunity for the weed to spread long distances downstream. Lawes and Grice (Reference Lawes and Grice2008) found P. hysterophorus growing along the entire Burdekin River catchment in Queensland, Australia, especially on the lower levels of the riverbanks. In addition, the Darling River catchment in New South Wales, Australia, was infested by P. hysterophorus along the edges of the watercourses and an eradication effort is underway (Shabbir et al. Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c). Thus, flooding in such areas could be suspected as the major opportunity for the dispersal of P. hysterophorus seeds.
Native animals, livestock, and feral animals are also believed to be involved in the dispersal of P. hysterophorus seeds over short distances (Parsons and Cuthbertson Reference Parsons and Cuthbertson1992). The spread of P. hysterophorus seeds by cattle from infested to un-infested land has been observed in southern Queensland and in dung in southeast Queensland (Khan Reference Khan2012). The human spread of seeds is mainly by vehicles as well as by agriculture machinery (Blackmore and Johnson Reference Blackmore, Johnson and Zydenbos2010). These pathways for seed spread can be over very long distances and are thought to be the most important pathways in most countries. Blackmore and Johnson (Reference Blackmore, Johnson and Zydenbos2010) reported that 73% of all the P. hysterophorus populations appearing in New South Wales arrived as seed carried on vehicles from Queensland. Parthenium hysterophorus seed can also be spread within fodder or within crop and feed seed lots (Gupta and Sharma Reference Gupta and Sharma1977). All these various means of seed dispersal play a role in the overall spread of the weed within invaded countries, making management more difficult. Other mechanisms of spread of P. hysterophorus are also known, including when the weed is used as an ornamental plant in floral bouquets, when its vegetative and reproductive parts are used as packaging for small items in crates, and when it is used as a green manure (Chandrasena and Rao Reference Chandrasena, Rao, Adkins, Shabbir and Dhileepan2019).
When seed arrives in a new location by means of dispersal, plant establishment requires additional environmental support. Once germination takes place, growth and reproduction are commonly completed in 2 to 6 mo when temperature, water and light conditions are adequate (Bajwa et al. Reference Bajwa, Chauhan and Adkins2017; Navie et al. Reference Navie, Panetta, McFadyen and Adkins1998; Pandey and Dubey Reference Pandey and Dubey1989). However, not all seeds germinate while support is in place; dormancy can ensure multiple germination events can occur over time, thus creating overlapping generations, which are commonly seen in field populations (Navie et al. Reference Navie, Panetta, McFadyen and Adkins1998). In tropical India, three cohorts of P. hysterophorus seedlings are observed in a single growing season (Pandey and Dubey Reference Pandey and Dubey1989), and consecutive seedling cohorts were observed in the Middle Mountain region in Nepal (Shrestha et al. Reference Shrestha, Dangol, Airi, Kharel, Thapa, Devkota and Shrestha2024), while in Australia, with lower annual rainfall, only two cohorts are generally found in one growing season (Mao 2020). In India, the highest survival rate of plants was found from the first cohort of the growing season, with mortality increasing in the later cohorts (Pandey and Dubey Reference Pandey and Dubey1989). Such mortality could be due to a water shortage later in the season, as well as greater competition from within the population (Pandey and Dubey Reference Pandey and Dubey1989). Successful sexual reproduction is vital to sustain a field population under unfavorable conditions, although new populations can be reestablished from the arrival of just a few seeds via biotic and abiotic vectors (Mao et al. Reference Mao, Shabbir and Adkins2021b).
Temporally, the new frontier of the weed’s invasion will be extended by seed dispersal. In a simulated spatial and temporal dispersal model (Mao 2020), a typical wind condition was predicted to disperse the population by 1.4 m yr−1 when the plant height attained was 1 m and when 40 seeds wk−1 were released during an 8-wk growing period. Under such an assumption, the population front may move by up to 14 m in a 20-yr period without other assistance (Mao 2020).
Invasion Risk
Predictive modeling can be used to assess P. hysterophorus’s potential global distribution, as well as the distribution of its biological control agents. Continental model predictions in Australia (Adkins et al. Reference Adkins, Navie and McFadyen1996; Thorp Reference Thorp2001), Asia (Ahmad et al. Reference Ahmad, Khuroo, Hamid, Charles and Rashid2019; Dhileepan and Senaratne Reference Dhileepan and Senaratne2009; Dorji et al. Reference Dorji, Lakey, Wangchen and Adkins2022; Masum et al. Reference Masum, Halim, Mandal, Asaduzzaman and Adkins2022), Africa (Dorji et al. Reference Dorji, Lakey, Wangchen and Adkins2022: McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011; Terblanche et al. Reference Terblanche, Nanni, Kaplan, Strathie, McConnachie, Goodall and van Wilgen2016), and Europe (Brunel et al. Reference Brunel, Panetta, Fried, Kriticos, Prasad, Lansink, Shabbir and Yaacoby2014; Kriticos et al. Reference Kriticos, Brunel, Ota, Fried, Oude Lansink, Panetta, Prasad, Shabbir and Yaacoby2015) have already been used to estimate the potential distribution of P. hysterophorus based on climatic data from the environments where the weed has shown sustained growth (Figure 6). These mapping activities show P. hysterophorus is already present in some of the projected high-risk areas that are dominated by humid subtropical and savanna climates and warm temperate areas (Mao et al. Reference Mao, Shabbir and Adkins2021b; Structured Appendix, Figure A1).

Figure 7. Some of the biological control agents released against Parthenium hysterophorus. An adult and late instar larvae of Zygogramma bicolorata (A), two stem galls produced by Epiblema strenuana (B), an adult pair of Smicronyx lutulentus, (C), a Listronotus setosipennis adult (D), and pustules produced by Puccinia abrupta (E).
Mapped areas, classified as medium- to low-risk in the current climate, are expected to be invaded over time based on the ability of P. hysterophorus to persist under wide temperature ranges and soil moisture conditions (Kriticos et al. Reference Kriticos, Brunel, Ota, Fried, Oude Lansink, Panetta, Prasad, Shabbir and Yaacoby2015). Of great concern in Africa is the continued spread into sub-Saharan Africa, such as from southwest Kenya to northern Tanzania, where some farmers in neighboring subsistence and pastoral agricultural areas have already been forced to abandon land heavily infested by P. hysterophorus (Mainali et al. Reference Mainali, Warren, Dhileepan, McConnachie, Strathie, Hassan, Karki, Shrestha and Parmesan2015; McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011). East Africa is at great risk for invasion, with climatically suitable areas spanning from Ethiopia to Mozambique, beyond which P. hysterophorus is already considered the most problematic weed in croplands and grazing areas (Tamado and Milberg Reference Tamado and Milberg2000), but the dry season may be a limiting factor for germination for part of the year (Tamado et al. Reference Tamado, Ohlander and Milberg2002).
Irrigation networks aiding seed distribution of P. hysterophorus and increasing soil moisture availability in cropping lands are expected to expand the invasion territory of P. hysterophorus into the large fertile areas of Australia, such as the Murray-Darling Basin, as well as throughout sub-Saharan Africa, and into southern Pakistan and other parts of the Middle East (Kriticos et al. Reference Kriticos, Brunel, Ota, Fried, Oude Lansink, Panetta, Prasad, Shabbir and Yaacoby2015; Shabbir et al. Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c, Reference Shabbir, Zalucki, Dhileepan, Khan and Adkins2023).
A change in climate can alter the habitat suitability boundaries for P. hysterophorus distribution (Guan et al. Reference Guan, Guo, Chen, Li, Liu, Gong and Ge2020; Mushtaq et al. Reference Mushtaq, Reshi, Shah and Charles2021). Using the maximum entropy (MaxEnt) modeling approach, Adhikari et al. (Reference Adhikari, Lee, Poudel, Lee, Hong and Park2023) suggested the current distribution of P. hysterophorus between 35°N and 35°S of the Equator would be retained in a future climate scenario, while its habitat suitability range would also extend as far as 65°N of the Equator. Currently uninvaded areas such as those in central China would face an increasing risk of invasion (Mainali et al. Reference Mainali, Warren, Dhileepan, McConnachie, Strathie, Hassan, Karki, Shrestha and Parmesan2015; Wang et al. Reference Wang, Liu, Zhong, Luo, Lin, Mao, Wang and Deng2023). Land-use systems of crop production, livestock production, and forestry were predicted by Kriticos et al. (Reference Kriticos, Brunel, Ota, Fried, Oude Lansink, Panetta, Prasad, Shabbir and Yaacoby2015) to have the greatest potential for colonization by P. hysterophorus. The northern range expansion of P. hysterophorus into the southern and central-western parts of Europe and most Mediterranean countries under a climate change scenario may occur due to an increased habitat suitability in riparian zones, agricultural landscapes, and actively managed forests of this region (Brunel et al. Reference Brunel, Panetta, Fried, Kriticos, Prasad, Lansink, Shabbir and Yaacoby2014; Kriticos et al. Reference Kriticos, Brunel, Ota, Fried, Oude Lansink, Panetta, Prasad, Shabbir and Yaacoby2015). Within its native range in the United States, isolated weed populations in the semiarid West and Southwest are at risk of expanding along the West Coast and into parts of the Midwest, with these important agronomic systems commonly under irrigation being particularly at risk due to increased soil moisture availability (Kriticos et al. Reference Kriticos, Brunel, Ota, Fried, Oude Lansink, Panetta, Prasad, Shabbir and Yaacoby2015; Shabbir et al. Reference Shabbir, Zalucki, Dhileepan, Khan and Adkins2023). Conversely, P. hysterophorus is expected to spread to the arid and hot southern parts of Pakistan through irrigated areas where the excess moisture will allow the weed to tolerate the predicted climate change conditions (Shabbir et al. Reference Shabbir, Dhileepan and Adkins2012). The effect of the irrigation network, allowing incursions of P. hysterophorus beyond its normal range, would be a threat to the cotton (Gossypium hirsutum L.) industry of Pakistan (Shabbir et al. Reference Shabbir, Dhileepan and Adkins2012).
Many climatic models indicate a trend of a northerly shift of P. hysterophorus populations alongside a decrease in the area of habitat suitability due to temperature increases and more variable precipitation patterns in some regions of India (Ahmad et al. Reference Ahmad, Khuroo, Hamid, Charles and Rashid2019), Bhutan (Chhogyel et al. Reference Chhogyel, Kumar and Bajgai2021; Thiney et al. Reference Thiney, Banterng, Gonkhamdee and Katawatin2019), Sri Lanka (Kariyawasam et al. Reference Kariyawasam, Kumar and Ratnayake2019), Oman (Al-Ruheili et al. Reference Al-Ruheili, Al Sariri and Al Subhi2022), and South Korea (Adhikari et al. Reference Adhikari, Lee, Poudel, Lee, Hong and Park2023). Rising temperatures may drive the distribution limit into higher latitudes and altitudes in parts of the world (Ziska and McConnell 2016), as is predicted to occur in Asia (Dhileepan and Senaratne Reference Dhileepan and Senaratne2009; Masum et al. Reference Masum, Halim, Mandal, Asaduzzaman and Adkins2022). For example, population densities are projected to increase in Nepal and northeast India to the western Himalayas due to more suitable climatic conditions of cooler temperatures and moisture availability compared with the surrounding regions (Ahmad et al. Reference Ahmad, Khuroo, Hamid, Charles and Rashid2019; Mainali et al. Reference Mainali, Warren, Dhileepan, McConnachie, Strathie, Hassan, Karki, Shrestha and Parmesan2015; Shrestha et al. Reference Shrestha, Pokhrel, Paudel, Poudel, Shabbir and Adkins2019). Parthenium hysterophorus may move ca. 240 km northward in China (Guan et al. Reference Guan, Guo, Chen, Li, Liu, Gong and Ge2020) and may gain greater altitude in mountainous areas such as Nepal (Maharjan et al. Reference Maharjan, Shrestha, Joshi, Devkota, Muniappan, Adiga and Jha2019; Shabbir et al. Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c) and Bhutan (Thiney et al. Reference Thiney, Banterng, Gonkhamdee and Katawatin2019), shifting ca. 753 upward to 2,931 m asl (Dorji et al. Reference Dorji, Lakey, Wangchen and Adkins2022). McConnachie et al. (Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011) determined that among the indicators used for CLIMEX on climatic suitability, altitude acted poorly when modeling range limits of P. hysterophorus. The plant’s range limits being minimally influenced by altitude agrees with survey data in Ethiopia by McConnachie et al. (Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011), who reported most populations to occur between 1,500 to 2,000 m asl, with some infestations reaching up to 2,627 m asl. Such shifts to higher altitudes may also be accelerated by the elevation of atmospheric CO2 concentration, as the weed becomes more competitive under such conditions (Cowie et al. Reference Cowie, Venter, Witkowski and Byrne2020b; Mao et al. Reference Mao, Bajwa and Adkins2021a; Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a; Rice et al. Reference Rice, Wolf, Fleisher, Acosta, Adkins, Bajwa and Ziska2021).
Based on CLIMEX models, it appears that P. hysterophorus has the capacity to spread into the midwestern and coastal regions in the United States and further into the central region of South America (Kriticos et al. Reference Kriticos, Brunel, Ota, Fried, Oude Lansink, Panetta, Prasad, Shabbir and Yaacoby2015; Figure 6), but there are no specific studies in its native range to understand the extent of natural competition and enemies keeping populations in check. Cold winter temperatures in the midwestern United States may limit the establishment of P. hysterophorus in this area. Large areas of southern, eastern, and western Australia are indicated by climate models to be suitable for P. hysterophorus (Mainali et al. Reference Mainali, Warren, Dhileepan, McConnachie, Strathie, Hassan, Karki, Shrestha and Parmesan2015; McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011), but on-ground reports indicate P. hysterophorus growth and establishment are limited in environments with precipitation mostly occurring in the winter months followed by a hot and dry summer (McFayden et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019). Although there are gaps in the known occurrences of P. hysterophorus in regions in Asia and Africa with climatic suitability, it is probable that known populations have established in neighboring countries such as Afghanistan (Khan et al. Reference Khan, Marwat, Hassan, Khan and Hashim2014a), Myanmar and Brunei (Shi et al. Reference Shi, Tang, Nguyen, Dhileepan, Adkins, Shabbir and Dhileepan2019), and several countries in sub-Saharan Africa (Mainali et al. Reference Mainali, Warren, Dhileepan, McConnachie, Strathie, Hassan, Karki, Shrestha and Parmesan2015; McConnachie and Witt Reference McConnachie, Witt, Adkins, Shabbir and Dhileepan2019; McConnachie et al. Reference McConnachie, Strathie, Mersie, Gebrehiwot, Zewdie, Abdurehim, Abrha, Araya, Asaregew, Assefa, Gebre-Tsadik, Nigatu, Tadesse and Tana2011) but such occurrences have not yet been adequately reported (Shabbir Reference Shabbir2012).
Invasion Pathways
Parthenium hysterophorus spreads through various pathways. The small-sized, lightweight seed can disperse seeds unnoticed through many local activities, while new populations can establish easily from the arrival of just a few seeds. Regionally, road networks were responsible for most population spread (36%), while direct cross-border travel contributed to more than half (51%) of the incoming sources of new populations into a country (Mao et al. Reference Mao, Shabbir and Adkins2021b). Other pathways of spread at the local or regional scale include by wind, water, contaminated machinery, and agricultural produce (Khan et al. Reference Khan, Navie, George, O’Donnell and Adkins2018; Mao et al. Reference Mao, Nguyen, Osunkoya and Adkins2019; Shabbir et al. Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c). These pathways can provide multiple conduits for spread to allow local establishment. For example, in Australia, ca. 6,000 P. hysterophorus viable seeds were found in every ton of material washed off vehicles at a roadside cleaning facility near a heavily infested region of central Queensland (Nguyen Reference Nguyen2011); an infestation found along Elsey Creek, in the Northern Territory of Australia, is thought to have originated from the Roper Highway 8 km upstream, where the weed was first observed (DENRNT 2017); a report suggested that a major flooding event in 2010 to 2011 brought new populations of the weed from Queensland in to New South Wales, Australia, and this event caused local outbreaks of the weed in that state (Blackmore and Charlton Reference Blackmore and Charlton2011). These events are believed to occur in all invaded regions, although many of them go undocumented.
Conversely, the pathway through international trade is thought to have contributed the most to the spread of the weed on a larger scale (Mao et al. Reference Mao, Shabbir and Adkins2021b; Shabbir et al. Reference Shabbir, McConnachie, Adkins, Adkins, Shabbir and Dhileepan2019c). A third of all new introductions have been traced back to the importation of contaminated seed lots and other plant commodities (Mao et al. Reference Mao, Shabbir and Adkins2021b). For example, seed imports from the United States, especially as part of USAID programs, were the source of P. hysterophorus establishments in Israel (Dafni and Heller Reference Dafni and Heller1982), Mozambique (Wise et al. Reference Wise, Van Wilgen, Hill, Schulthess, Tweddle, Chabi-Olay and Zimmermann2007), and Ethiopia and India (Adkins and Shabbir Reference Adkins and Shabbir2014). Used vehicles carried weed seeds from Queensland, Australia, into Papua New Guinea (McFadyen et al. Reference McFadyen, Dhileepan, Day, Adkins, Shabbir and Dhileepan2019), while the weed itself was first brought into Australia from United States through contamination of a shipment of aviation parts (Adkins and Shabbir Reference Adkins and Shabbir2014). In addition, trading ports have been sites of unintentional introductions, both in the early 20th century and in recent years. The Republic of Korea reported P. hysterophorus populations present in the export zone of Masan City (Kim Reference Kim2013), while China experienced a second introduction into the northern province of Shandong, following the discovery of a plant in the busy port of Lianyungang a year before (Mao Reference Mao2018). However, seeds may still manage to slip into new countries without invasive populations being formed. Such events have occurred in Belgium and Poland, where the local climate was not suitable for establishment (Mirek et al. Reference Mirek, Piękoś-Mirkow, Zając and Maria2002; Verloove Reference Verloove2006). Fewer introductions are now being observed due to improved border control and quarantine procedures in many countries.
Growth and Development
Morphology
Parthenium hysterophorus displays an upright growth pattern and a robust taproot that firmly anchors it in the soil (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018). Its invasiveness can be attributed to a distinct set of morphological features (Table 2).
Table 2. Key morphological features of Parthenium hysterophorus and their role in its resilient growth habita.

a The information presented here is adopted and modified from Bajwa (Reference Bajwa2019) and Bajwa et al. (Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016).
These features contribute to competitive ability, invasiveness, and adaptability (Adkins and Shabbir Reference Adkins and Shabbir2014; Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016). The rosette stage in the growth cycle of P. hysterophorus can play a critical role, influencing the timing of subsequent stages based on the prevalent environmental conditions. This adaptive flexibility in growth stages aids in the plant’s successful invasion (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018).
Eco-physiological Responses to Climate Change
Parthenium hysterophorus has an extraordinary ability to emerge, compete, and reproduce under different climatic conditions (Mao et al. Reference Mao, Bajwa and Adkins2021a). It has evolved morphological and physiological adaptations to sustain its growth while completing its life cycle under climate change conditions. Parthenium hysterophorus’s response and adaptive biology under major climate change elements are discussed in the following sections.
High Temperature
Parthenium hysterophorus has demonstrated an impressive ability not only to germinate but also to grow vigorously and reproduce across a wide range of temperatures. The weed has evolved phenological flexibility, allowing it to adapt to variations in temperature. This adaptability is a key factor in its successful establishment in regions with vastly different average temperatures, ensuring its presence throughout the year in certain countries. In India, Kaur et al. (Reference Kaur, Batish, Kaur, Singh and Kohli2017) reported changes in ambient temperature and relative humidity to have a significant impact on the phenology of P. hysterophorus. However, crucially, these changes do not inhibit its ability to germinate or flower. In a controlled-environment study, Nguyen et al. (Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a) found that P. hysterophorus plants grown at warmer day/night temperatures (35/20 C) grew faster and taller and produced more seed with a high seed-fill rate and predicted longevity than plants grown at cooler day/night temperatures (30/15 C). However, the life span of plants grown under warmer temperatures was shorter compared with those exposed to the lower-temperature regime. These adaptive responses underline the resilience and invasiveness of P. hysterophorus, which enable it to thrive in diverse climatic conditions and to successfully reproduce, even in challenging and fluctuating climatic conditions.
In addition to phenological adaptations, P. hysterophorus displays impressive physiological adaptations to high temperatures. Plants grown under high, summer temperatures (ranging from 35 to 45 C) exhibited enhanced growth rates, increased chlorophyll content, higher levels of soluble sugars, and increased protein quantities compared with plants grown at cool temperatures (22 to 30 C) within a greenhouse (Kapoor Reference Kapoor2014). Conversely, these attributes are not expressed when plants are exposed to low temperatures (7 to 15 C) during winter. Another study conducted by Sharma et al. (Reference Sharma, Bhullar, Rakhra and Mamik2014) reported a substantial upregulation of antioxidant and heat shock–related stress-tolerant biochemicals in response to high temperatures. These heightened levels of heat-induced proteins are associated with the effective capturing of reactive oxygen species, leading to improved regulation of plant physiological functions (Sharma et al. Reference Sharma, Bhullar, Rakhra and Mamik2014). Such adaptive physiological changes play a vital role in the success of P. hysterophorus in harsh tropical and subtropical environments.
Drought
Parthenium hysterophorus demonstrates remarkable adaptability to low soil moisture conditions by modifying its morphology, growth phases, and life cycle. For instance, the cuticle (14.0 μm) and palisade layers (145.6 μm) of P. hysterophorus leaves growing in Yunnan Province, China, were the thickest among nine weed species, and presumably are helpful to reduce water loss (Pu et al. Reference Pu, Song, Wang, Yang, Wu, Yu and Wang2023). Studies have shown that when grown at 50% of soil water-holding capacity (WHC), P. hysterophorus plants adjust their life cycle, flowering ca. 17 d earlier and producing seeds about 18 d earlier than those grown under normal moisture conditions. This adaptation results in a shortened life span by ca. 32 d under drought conditions (Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a). Interestingly, while drought negatively affects seed fill, it does not significantly reduce total seed production or predicted seed longevity (Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a).
Moreover, under drought conditions, P. hysterophorus plants produced significantly higher amounts of antioxidants such as superoxide dismutase, glutathione reductase, catalase, glutathione S-transferase, ascorbate, and proline compared with normal moisture conditions (Ahmad et al. Reference Ahmad, Bashir, Bagheri, Baig, Al-Huqail, Ibrahim and Qureshi2017). The plants also upregulate various defense proteins in response to oxidative stress, enhancing their ability to tolerate the adverse effects of drought stress (Ahmad et al. Reference Ahmad, Bashir, Bagheri, Baig, Al-Huqail, Ibrahim and Qureshi2017). Similar findings have been reported for Australian biotypes of P. hysterophorus under moisture-stress conditions (Bajwa et al. Reference Bajwa, Chauhan and Adkins2017). These physiological adaptations underscore the resilience and invasive potential of P. hysterophorus, which allow it to thrive and spread even in arid and drought-prone environments.
Soil Constraints
Parthenium hysterophorus exhibits a notable tolerance to elevated salinity levels, enabling its invasion of salt-affected and coastal areas. Research by Khurshid et al. (Reference Khurshid, Nasim, Bajwa and Adkins2012) demonstrated that P. hysterophorus can withstand high salinity levels both in controlled conditions (up to 30 mM NaCl) and in field conditions (10 dS m−1). However, it is important to note that, comparatively, P. hysterophorus was found to be more sensitive to salinity stress than to drought (Ahmad et al. Reference Ahmad, Bashir, Bagheri, Baig, Al-Huqail, Ibrahim and Qureshi2017).
Moreover, P. hysterophorus has the capacity to uptake and accumulate a variety of heavy metals, including cadmium, copper, cobalt, lead, nickel, chromium, and zinc, from soils contaminated with industrial waste (Ajmal et al. Reference Ajmal, Rao, Ahmad and Khan2006; Lata et al. Reference Lata, Garg and Gupta2008; Malik et al. Reference Malik, Husain and Nazir2010). This ability to cope with phytotoxic stress induced by heavy metals makes it feasible for P. hysterophorus to establish itself in disturbed environments. The weed thrives in roadside communities that are heavily polluted with vehicle emissions, dust, and dirt, indicating a strong tolerance mechanism toward air, soil, and water pollution (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018; Strathie and McConnachie Reference Strathie, McConnachie, Adkins, Shabbir and Dhileepan2019).
Elevated CO2
The rise in atmospheric CO2 concentration profoundly influences the morphology and physiology of plants, a phenomenon extensively investigated in P. hysterophorus (Mao et al. Reference Mao, Bajwa and Adkins2021a). As a C3/C4 intermediate plant species, in its C3 adult form P. hysterophorus enhances its photosynthetic activity in response to elevated atmospheric CO2 levels. This results in a significant improvement in its growth, biomass accumulation, competitive ability, allelopathic ability, and seed production (Adkins et al. Reference Adkins, McClay, Bajwa, Adkins, Shabbir and Dhileepan2018; Khan et al. Reference Khan, Navie, George, O’Donnell and Adkins2018; Moore et al. Reference Moore, Franceschi, Cheng, Wu and Ku1987; Shi and Adkins Reference Shi and Adkins2020). Rice et al. (Reference Rice, Wolf, Fleisher, Acosta, Adkins, Bajwa and Ziska2021) showed that P. hysterophorus has already reaped benefits from the historical increase in atmospheric CO2 concentrations over the past century. An invasive biotype of P. hysterophorus from Australia grown under modern-day CO2 concentrations (400 ppm) exhibited close to a 50% increase in size and produced 50% more parthenin (a major allelochemical/allergen) compared with when it was grown under preindustrial-era CO2 concentrations (300 ppm).
Various studies have consistently reported substantial increases in growth, competitive ability, and reproductive output of P. hysterophorus plants when exposed to elevated atmospheric CO2 concentrations, like those predicted to prevail in the coming decades (Bajwa et al. Reference Bajwa, Wang, Chauhan and Adkins2019e; Khan et al. Reference Khan, George, Shabbir, Hanif and Adkins2015; Navie et al. Reference Navie, McFadyen, Panetta and Adkins2005). For instance, Shabbir et al. (Reference Shabbir, Dhileepan, Khan and Adkins2014) observed a significant increase in height (52%), biomass (55%), branching (62%), leaf area (120%), photosynthesis (94%), and water-use efficiency (400%) of P. hysterophorus plants at an elevated atmospheric CO2 concentration (550 ppm) compared with plants at an ambient CO2 concentration (380 ppm).
Interactive Effects
The interactive effects of different climate change elements are more important given the fact that all these factors coexist under natural conditions. Nguyen et al. (Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a) studied the growth and fecundity of P. hysterophorus plants at normal (100% WHC) and low (50% WHC) soil moisture in combination with high (35/20 C) and low (30/15 C) day/night temperatures. The plants had significantly higher growth and seed production under a combination of normal moisture and low temperature compared with those grown at low moisture in combination with high temperature (Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a). Bajwa (Reference Bajwa2019) reported that P. hysterophorus plants grown under elevated CO2 better tolerated moisture stress condition than their counterparts grown under ambient CO2. Similarly, P. hysterophorus plants better tolerated soil salinity when grown in a CO2-enriched environment (Saravanane et al. Reference Saravanane, Bajwa, Djanaguiraman and Adkins2023). These compensatory improvements were attributed to enhanced metabolic activity and better growth regulation. This demonstrates that the interaction of different climate change elements could have a significant impact on the morphology, physiology, phenology, and reproductive capacity of P. hysterophorus.
Reproduction
Floral Biology
Flower initiation can start as early as 28 d after seedling emergence from the soil (Jayachandra 1971), while others report that flower initiation will take up to 42 to 63 d (Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996). The flower head or capitulum consists of a conical receptacle surrounded by an outer involucre of five persistent bracts, five (sometimes six to eight) peripheral fertile ray florets, and 12 to 20 central cylindrical staminate disk florets, each bearing four connate anthers (Navie et al. Reference Navie, Panetta, McFadyen and Adkins1998). The appearance of reddish-brown spots on the stigmas of the ray florets indicates successful pollination has taken place. Pollen grains are mostly spheroidal, 12 to 20 µm in size, and have short to medium-length spines often permeated with micropores (Lewis et al. Reference Lewis, Dixit and Wedner1991). On average, 150,000 to 350,000 pollen grains are produced in each of thousands of capitula, so pollen production by an average plant is extremely large, ca. 850 million pollen grains plant−1 (Kanchan and Jayachandra Reference Kanchan1979; Lewis et al. Reference Lewis, Dixit and Wedner1988) or more than 10 billion pollen grains m−2 in a typical stand of the weed. Large amounts of airborne pollen from P. hysterophorus plants have been detected both in the United States and India at various altitudes (2 to 915 m asl) and at considerable distances from P. hysterophorus populations (Lewis et al. Reference Lewis, Dixit and Wedner1991).
There are conflicting reports as to whether P. hysterophorus is self- or cross-pollinated, and what the actual mechanism of pollination is. Esau (Reference Esau1946) reported that apomixis did not occur in P. hysterophorus and that the species was only known to produce seed after pollination. Lewis et al. (Reference Lewis, Dixit and Wedner1988), working on plants from the native range, considered the species to exhibit a high degree of self-pollination (95% of seeds produced in this way were viable) with little or no insect pollination. They concluded that wind or self-pollination accounted for most seed produced. In later studies, Lewis et al. (Reference Lewis, Dixit and Wedner1991) advanced the notion that the mechanism of wind pollination in P. hysterophorus was less developed than that seen in many other wind-pollinated species, indicating that self-pollination was probably the most common form of pollination in this species. However, Gupta and Chanda (Reference Gupta and Chanda1991) noted that P. hysterophorus plants in the invaded range of India appeared to be insect pollinated or, at least, pollen was dispersed mainly by insects. The main pollinating agents were thought to be honey bees (Apis Spp.), ants (Lasius niger Linn.), house flies (Musca domestica Linn.), and other dipterans that frequently visited its flowers. They concluded that P. hysterophorus is not normally self-pollinating, but ants may occasionally induce the process of self-pollination after visiting flowers from the same plant. More recently, Hanif (Reference Hanif2014) has raised the very interesting idea that in certain parts of the invaded range, where the plants are extremely invasive, they are mainly cross-pollinating plants, while less invasive plants are self-pollinated.
Seed Production
Parthenium hysterophorus is a very prolific seed producer and will continue to flower and fruit profusely until senescence (Haseler Reference Haseler1976). Seed (retained within the cypsela) is shed gradually throughout the latter stages of growth, while other seed is retained in the flowers until after senescence (Parsons and Cuthbertson Reference Parsons and Cuthbertson1992). Each flower head produces up to five blackish achenes, occasionally six to eight, of uniform size and weigh (Auld et al. Reference Auld, Hosking and McFadyen1982; Lewis et al. Reference Lewis, Dixit and Wedner1988), which are individually enclosed in the straw-colored cypsela along with two lateral attached sterile florets (Navie et al. Reference Navie, McFadyen, Panetta and Adkins1996). While there have been a range of estimations of P. hysterophorus seed production per plant in the field (ca. 15,000 [Haseler Reference Haseler1976] to ca. 156,000 [Dhileepan Reference Dhileepan2012]), the most recent and accurate counts for glasshouse-grown plants is between ca. 18,000 to 26,000 filled seeds per plant (Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017b). In India, Kanchan and Jayachandra (Reference Kanchan1979) found that there was an average of 15 plants m−2 in a typical stand of P. hysterophorus. Such data indicate that more than 300,000 seeds m−2 could be produced in many field situations. These figures for seed production are only applicable when sufficient moisture and warm temperatures are available to produce vigorously growing stands of plants, as Nguyen et al. (Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a) have shown that filled seed production will be reduced (from ca. 20,000 to ca. 9,000) when plants are grown under cool/dry conditions compared with warm/wet environmental conditions. Pandey and Dubey (Reference Pandey and Dubey1988) reported P. hysterophorus to produce polymorphic seeds that vary in size and weight. They suggested that the variation in seed morphology may be due to differences in the maturation time of the capitula produced at different positions on the parent plant. They also found that small seeds were more commonly produced at lower latitudes (i.e., in southern India) compared with the larger seeds produced at higher latitudes (i.e., in northern India). Therefore, it seems that the climatic conditions have a bearing on both seed production and seed size (Dubey and Pandey Reference Pandey and Dubey1988).
Seedbanks, Seed Viability, and Germination
Little is known concerning the longevity of P. hysterophorus seeds, either on the soil surface or in the soil seedbank. Navie et al. (Reference Navie, Panetta, McFadyen and Adkins1998) reported more than 70% of seed buried 5 cm below the soil surface could live for at least 2 yr, with a half-life of 7 yr. Tamado et al. (Reference Tamado, Ohlander and Milberg2002) reported more than 50% of soil-buried seed remained viable for up to 2.5 yr, with an anticipated life span of 3 to 4 yr. One additional study (Nguyen Reference Nguyen2011) using a controlled ageing test suggested that the quality of the seed at the time of burial may also affect longevity, with higher-quality seed predicted to live longer in the soil seedbank than poor-quality seed. The longevity of surface-lying seeds seems to be quite short, with most dying within 6 mo (Navie et al. Reference Navie, Panetta, McFadyen and Adkins1998). In Australia, Navie et al. (Reference Navie, Panetta, McFadyen and Adkins2004) determined the size of the germinable P. hysterophorus soil seedbank to range from 3,200 to 5,100 seed m−2 in a black clay soil to 20,500 to 44,700 seed m−2 in a sandy loam soil. At these two sites, the P. hysterophorus seedbank accounted for 47% to 73% and 65% to 87%, respectively, of the total seedbank present. In a riparian habitat invaded by P. hysterophorus near Lahore, Pakistan, a soil seedbank of 4,400 m−2 has been reported (Shabbir Reference Shabbir2015). In other locations, much larger seedbanks have been recorded; for example, Joshi (Reference Joshi1991) estimated an Indian P. hysterophorus soil seedbank in an abandoned field to be 200,000 m−2.
Parthenium hysterophorus seeds have a very high rate of fill, and those seeds have a high viability of 85% or greater when collected directly from the adult plant (Pandey and Dubey Reference Pandey and Dubey1988; Williams and Groves Reference Williams and Groves1980). Research has demonstrated that water-soluble germination inhibitors (i.e., parthenin and certain phenolic acids) are present in the layers surrounding the seed and that these inhibitors need to be leached out before maximum germination can be attained (Picman and Picman Reference Picman and Picman1984). Parthenium hysterophorus seed may also be induced into a state of quiescence by certain ambient environmental conditions, such as deep burial (White Reference White1994). Recently, Nguyen et al. (Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a) have shown that the proportion of dormant seeds produced by a P. hysterophorus plant is determined by the maternal environment in which those seeds matured, with a warm/wet environment producing up to ca. 2,000 dormant seed plant−1, while a cool/dry environment producing only ca. 100 dormant seeds plant−1. This result is supported by Shrestha et al. (Reference Shrestha, Dangol, Airi, Kharel, Thapa, Devkota and Shrestha2024), who found the germination of summer-produced seed to be significantly lower than that of winter-produced seed.
Navie et al. (Reference Navie, McFadyen, Panetta and Adkins1996) found that the optimal temperature for germination of Australian seeds was between 22 and 25 C, but with a much wider range of temperatures over which some seed would germinate (9 to 36 C). Bajwa et al. (Reference Bajwa, Chauhan and Adkins2018a) reported superior germination ability of the highly invasive Australian biotype of P. hysterophorus compared with its non-invasive counterpart, Toogoolawah biotype, across a range of temperature, pH, salinity, and osmotic stress levels under laboratory conditions. According to Tamado et al. (Reference Tamado, Ohlander and Milberg2002), fluctuating temperature regimes of 12/20 C to 35/20 C (day/night) were all suitable for the germination of Ethiopian seed. Williams and Groves (Reference Williams and Groves1980), working with Australian seed, reported that the maximum germination (88%) could be achieved in the dark, while Pandey and Dubey (Reference Pandey and Dubey1988), using seed of Indian origin, found the highest germination to occur in both continuous light and continuous dark, suggesting that this species does not have a strict light requirement for germination. Soil germination decreased from 91% when the soil was at field capacity, to just 50% when the soil moisture was reduced to −0.07 MPa, and to 0% when the soil moisture was reduced to −0.90 MPa. This demonstrates that P. hysterophorus seed germination depends on high moisture availability (Williams and Groves Reference Williams and Groves1980). According to Tamado et al. (Reference Tamado, Ohlander and Milberg2002), the depth of seed burial can have a significant impact upon germination and/or seedling emergence. A depth of 0.5 cm seems to be ideal for this weed’s germination, while 5.0 cm or more will result in slow or no germination. In one germination study conducted in India, the percentage of seeds germinating gradually increased as their seed size increased (Pandey and Dubey Reference Pandey and Dubey1988).
Vegetative Reproduction
Parthenium hysterophorus does not reproduce vegetatively from plant parts or by apomixis. However, it has been observed that P. hysterophorus can regrow from an overwintering stem base created by either grazing or physical weed management (G Hassan, personal communication, 2010).
Population Dynamics
In some climates where P. hysterophorus occurs, it can grow at any given time of year due to its ability to germinate, grow, and flower over a wide range of photoperiod and temperature conditions (Haseler Reference Haseler1976). Its main season of growth, however, is the rainy season during the summer months in regions such as North and Central America, Mexico, and Australia, or during the wet season in India, Pakistan, and Nepal (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019). A field season with sufficient moisture and warm temperatures typically supports P. hysterophorus stands of 15 to 25 plants m−2 in India (Kanchan and Jayachandra Reference Kanchan1979; Joshi Reference Joshi1991), 315 plants m−2 in eastern Ethiopia (Tamado and Milberg Reference Tamado and Milberg2004), to field reports of 800 plants m−2 in central Queensland (Dhileepan Reference Dhileepan2003, Reference Dhileepan2012). It has been estimated that P. hysterophorus may produce more than 300,000 seeds m−2 within a season under these conditions (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019). A season with frequent and abundant rainfall may allow the emergence of three or more successive cohorts (Pandey and Dubey Reference Pandey and Dubey1989), with each cohort having variable life spans depending on seasonal conditions and the prevailing competition for light, water, or other resources (see “Life-Form and Life History”). For example, field observations made in India show three successive cohorts of P. hysterophorus to be recruited after the first rains of the new season, and the average recruitment over the 2-yr study was 110 plants m−2, of which 14 plants m−2 (13%) achieved maturity (Pandey and Dubey Reference Pandey and Dubey1989). The study found seedling density and survivorship declined over successive cohorts until the third cohort had very few plants surviving past the seedling stage, which indicated the first, well-established cohort adversely affected the growth, and probably the survivorship, of the latter cohorts through resource competition (Pandey and Dubey Reference Pandey and Dubey1989) and possibly allelopathy (Belz et al. Reference Belz, van der Laan, Reinhardt and Hurle2009; Shabbir and Javaid Reference Shabbir and Javaid2010a, Reference Shabbir and Javaid2010b). Late-emerging cohorts in the autumn months in northeastern Mexico are known to produce large rosettes and overwinter in this form from October to March (Adkins et al. Reference Adkins, Shabbir and Dhileepan2019), likely as a response to cooling temperature, shortening photoperiods, or water stress (McClay Reference McClay1983).
As P. hysterophorus often grows in pure stands, only a few studies have been conducted on its population dynamics in relation to competition with other species in the field. A field report from Nepal has shown P. hysterophorus to outcompete native vegetation as quickly as 1 to 2 yr after agricultural land has been abandoned (Shrestha et al. Reference Shrestha, Shabbir, Adkins and Bourdôt2015). Also, P. hysterophorus populations in Australia and Texas have been shown to remain dominant over native populations for more than 15 yr following soil disturbance (Dale Reference Dale1981). These interactions may, in part, be attributed to direct or indirect soil-based allelopathic activity through phytotoxic effects that suppress root growth of neighboring plants (Belz et al. Reference Belz, van der Laan, Reinhardt and Hurle2009). Parthenin-containing leaf leachates are also known to stimulate chlorophyll content (Kumari and Kohli Reference Kumari and Kohli1987) and may be a self-stimulatory allelopathic mechanism that helps form pure, dense stands of P. hysterophorus (Belz et al. Reference Belz, van der Laan, Reinhardt and Hurle2009). Parthenium hysterophorus is also known to indirectly manipulate plant communities by inhibiting the growth of beneficial microorganisms (Belz et al. Reference Belz, van der Laan, Reinhardt and Hurle2009; Osunkoya et al. Reference Osunkoya, Akinsanmi, Lim, Perret, Callander and Dhileepan2017). A field study undertaken in the Yucatán, Mexico, reported the microorganism population was much different in non-invaded soils compared with invaded soils. The study showed 5.2% of P. hysterophorus plants to be colonized by arbuscular mycorrhizal fungi, suggesting the microbial community could aid its invasion and establishment in certain edaphic regimes (Jeyalakshmi et al. Reference Jeyalakshmi, Doraisamy and Valluvaparidasan2011).
The density and fecundity of P. hysterophorus stands are based on competition dynamics and habitat factors as well as climatic conditions. Frequent and abundant rainfall events have been reported to help P. hysterophorus quickly colonize fallow areas and bare soils (Royimani et al. Reference Royimani, Mutanga, Odindi, Zolo, Sibanda and Dube2019). For example, a field survey in the province of Kwazulu-Natal, South Africa, indicated a correlation between low rainfall events from 2006 to 2012 with a decline in P. hysterophorus population size, followed by a high rainfall event in 2016 that rapidly expanded the area occupied by P. hysterophorus (Royimani et al. Reference Royimani, Mutanga, Odindi, Zolo, Sibanda and Dube2019). Flooding events exacerbate the spread of P. hysterophorus seed into new areas, as indicated by heavily infested areas in the wetlands of Sri Lanka (Jayasuriya Reference Jayasuriya2005) and Mozambique (Bandeira et al. Reference Bandeira, Massingue Manjate and Filipe2006) and the establishment of large colonies over floodplains and waterways in central Queensland, Australia (Parsons and Cuthbertson Reference Parsons and Cuthbertson1992), Tanzania (Wabuyele et al. Reference Wabuyele, Lusweti, Bisikwa, Kyenune, Clark, Lotter, McConnachie and Mersie2014), and Shandong Province, China (Shi et al. Reference Shi, Tang, Nguyen, Dhileepan, Adkins, Shabbir and Dhileepan2019). Severe weather events are also known to aid P. hysterophorus seed dispersal and have subsequently increased population densities in Ethiopia (Ayele Reference Ayele2007) and Oman (Ghazanfar Reference Ghazanfar2017).
Detection and Recognition
Using a field spectroscopy approach, Iqbal et al. (Reference Iqbal, Balzter and Firdaus-e-Bareen2021) discriminated P. hysterophorus from co-occurring native and introduced species in a protected reserve forest in Pakistan. Of various spectral indices, the Normalized Difference Vegetation Index (670- and 830-nm wavelengths) best discriminated P. hysterophorus from the rest of seven plant species. In contrast, Kganyago et al. (Reference Kganyago, Odindi, Adjorlolo and Mhangara2017) found that the subsect of two red-edge (685 and 707 nm), one near-infrared (1,115 nm) and seven short-wave infrared bands (1,971, 1,982, 1,990, 1,966, 2,003, 2,005, and 2,013 nm) showed the greatest discrimination ability of P. hysterophorus from its co-occurring species in South Africa. In another study, different growth stages (seedling, rosette, and flowering) of P. hysterophorus were detected and discerned from two grasses, kangaroo grass (Themeda triandra Forssk.) and desert bluegrass [Bothriochloa ewartiana (Domin) C.E. Hubb.], using images collected on glasshouse-grown plants with RGB and hyperspectral cameras (Costello et al. Reference Costello, Osunkoya, Sandino, Marinic, Trotter, Shi, Gonzalez and Dhileepan2022). Similarly, Ullah et al. (Reference Ullah, Shakir, Iqbal, Iqbal, Ali, Shafique, Rehman and Godwin2021) performed spectral discrimination of P. hysterophorus from four co-occurring plant species using hyperspectral data, using a combination of spectral angle mapper and genetic algorithms to successfully classify training and testing datasets, both with an accuracy of more than 90%. In summary, P. hysterophorus can be detected and classified using remote sensing and machine learning approaches. Further investigation is needed to scale up the data from distinct spectral bands to airborne and satellite sensors. This will facilitate the comprehensive documentation of P. hysterophorus distribution at the landscape level.
Management Options
Legislative Measures
Effective legislation addressing prevention and containment of weed is for weed management. In Australia, P. hysterophorus is classified as a declared pest plant in all states and territories. Each state and territory authority has its own legislation to prevent the entry and spread of P. hysterophorus based on the perceived level of risk (Campbell et al. Reference Campbell, Vogler, Tana, Adkins, Shabbir and Dhileepan2019). For instance, in New South Wales, specific legislative requirements outline the requirement of interstate movement of agricultural machinery such as harvesters and other equipment. The regulation mandates that equipment needs to be thoroughly cleaned to remove any weed seeds and to be checked by the state border inspector (Campbell et al. Reference Campbell, Vogler, Tana, Adkins, Shabbir and Dhileepan2019). Similarly other countries such as South Africa, Kenya, and Sri Lanka have implemented legislation to contain the spread of P. hysterophorus into un-infested areas (Adkins and Shabbir Reference Adkins and Shabbir2014). However, in many other countries where P. hysterophorus has invaded, effective legislation does not exist; thus the weed continues to spread within invaded areas and into new regions (Dorjee et al. Reference Dorjee, Buckmaster and Downey2021).
Phytosanitary Measures
Phytosanitary measures play a crucial role in the prevention, introduction, and further spread of weeds into new areas. In New South Wales, Australia, 59% of P. hysterophorus outbreaks on new properties were attributed to contaminated harvesters coming from the state of Queensland, where this weed is a significant problem (Blackmore and Johnson Reference Blackmore, Johnson and Zydenbos2010). Establishment of roadside vehicle wash-down facilities in weed-infested areas and along the state border crossings has proven effective in removing P. hysterophorus seeds and minimizing its further spread (Bajwa et al. Reference Bajwa, Nguyen, Navie, O’Donnell and Adkins2018b).
Phytosanitary measures are essential to minimize the spread of P. hysterophorus to new regions and countries. Within the Euro-Mediterranean region, P. hysterophorus is only established in Israel. To prevent the potential risk of P. hysterophorus spreading to other countries in the region, the European and Mediterranean Plant Protection Organization performed a pest risk analysis on this weed (Brunel et al. Reference Brunel, Panetta, Fried, Kriticos, Prasad, Lansink, Shabbir and Yaacoby2014) and recommended it be regulated as a quarantine pest for the member countries (EPPO 2014). Similar measures are required to prevent seed spread at the local level, for instance, the state of Queensland has developed a weed hygiene declaration form that require providers to include the weed seed status in any grain, feed, or animals being purchased (Chamberlain and Gittens Reference Chamberlain and Gittens2004).
Chemical Control
Chemical control of P. hysterophorus, as for most weeds, is the most effective, quickest, and most economical weed control method, especially in agroecosystems (Bajwa et al. Reference Bajwa, Ullah, Farooq, Chauhan and Adkins2019d). A range of pre- and postemergence herbicides have been used to control P. hysterophorus in non-cropped and cropped areas (Table 2). In non-cropped areas, glyphosate and 2,4-D have been the major herbicides used to suppress P. hysterophorus growth and biomass production (Tanveer et al. Reference Tanveer, Khaliq, Ali, Mahajan and Chauhan2015). Several other chemicals, including isoproturon, metribuzin, flumioxazin, dicamba, diuron, and picloram, have also been reported to provide effective control of this weed in non-cropped situations (Fernandez et al. Reference Fernandez, Odero, MacDonald and Ferrell2014; Odero Reference Odero2012; Reddy et al. Reference Reddy, Bryson and Burke2007; Shabbir Reference Shabbir2014a; Singh et al. Reference Singh, Yadav, Balyan, Malik and Singh2004). Most of the postemergence herbicides provided effective control of P. hysterophorus when applied at the seedling or rosette stages, but their efficacy was reduced when applied to mature plants (Reddy et al. Reference Reddy, Bryson and Burke2007). However, a relatively smaller number of studies have reported successful chemical control of P. hysterophorus in crops (Table 3).
Table 3. Chemical options that provided an effective control (>90%) of Parthenium hysterophorus in different crops when applied either preemergence (PRE) or postemergence (POST) to the crop.

Some studies have also highlighted the challenge of effectively controlling P. hysterophorus with certain commonly used herbicides. For example, research by Tamado and Milberg (Reference Tamado and Milberg2004) indicated that 2,4-D, a widely used herbicide, showed inconsistent control of P. hysterophorus in sorghum crops in Ethiopia. In Sri Lanka, Nishanthan et al. (Reference Nishanthan, Sivachandiran and Marambe2018) found that a preemergence application of oxyfluorfen was ineffective in controlling P. hysterophorus in capsicum (Capsicum annuum L.) crop. Bajwa et al. (Reference Bajwa, Ullah, Farooq, Chauhan and Adkins2019d) reported that the application of pendimethalin (preemergence) or bispyribac-sodium + bensulfuron-methyl (postemergence) failed to provide good control in a direct-seeded rice crop in Pakistan. Given the widespread infestation of P. hysterophorus in various crops globally, this information underscores the need for comprehensive research to identify effective chemical control options for managing this weed across a range of crops.
Cultural
In some countries, the manual removal of P. hysterophorus is not considered to be a cost-effective approach due to the size of the weed infestations present and the cost of the labor in that country. However, this method of management may affect the health of the workers who are employed to do this job, as P. hysterophorus is known to cause contact dermatitis and asthma (Allan et al. Reference Allan, BoYang, Adkins, Adkins, Shabbir and Dhileepan2019). Parthenium hysterophorus populations also readily regenerate from the seedbank after manual removal has been applied and will regrow from cut or partly pulled plants that still have a root system. Despite such drawbacks, the hand-pulling strategy is commonly used in developing countries such as India, Pakistan, Bangladesh, and Nepal where labor is cheap, and people are not aware of the associated health risks of pulling up the plant (Khan Reference Khan2011). Slashing and plowing are only effective for the short term, as these strategies stimulate regrowth and seed germination, respectively (Bhowmik and Sarkar Reference Bhowmik and Sarkar2005; Haseler Reference Haseler1976).
Physical Control
Physical removal of P. hysterophorus is effective but labor-intensive and poses health risks due to the allergenic nature of the weed. Parthenium hysterophorus can quickly regrow from slashed or mowed plants, so uprooting plants before they seed set is important (Campbell et al. Reference Campbell, Vogler, Tana, Adkins, Shabbir and Dhileepan2019). The burning of P. hysterophorus plants in a rangeland setting can result in the reduction in its numbers but fire can also create an ideal germination bed for surviving P. hysterophorus seeds (Vogler et al., Reference Vogler, Navie, Adkins and Setter2006). In a field study in northern Australia, Vogler et al. (Reference Vogler, Navie, Adkins and Setter2006) found that the size of the P. hysterophorus seedbank in soil was not affected by the fire. In addition, smoke and heat did not impact P. hysterophorus seed germination. However, different responses to fire have been observed (Shabbir Reference Shabbir2007). Within a wheat-cropping system in which the wheat residues were burnt after crop harvest, P. hysterophorus seedlings emerged rapidly. This emergence was, however, within the residue patches that had escaped the fire and not in the burnt areas.
Biological Control
Biological control involving natural enemies (insect herbivores, plant pathogens, and other organisms) is a widely used approach to manage P. hysterophorus in several countries. To date, nine countries have released one or more biological control agents against this weed, with another four countries having some agents arrive fortuitously or originally released for control of other weed species (Dhileepan Reference Dhileepan2009; Strathie et al. Reference Strathie, Cowie, McConnachie, Chidawanyika, Musedeli, Sambo, Magoso and Gareeb2021; Table 4; Figure 7).
Table 4. Biological control agents released or present in different countries around the world to aid in the management of Parthenium hysterophorus.a

a Adapted and modified from Dhileepan and McFadyen (Reference Dhileepan, McFadyen, Julien, McFadyen and Cullen2012).
b Originally released against annual ragweed (Ambrosia artemisiifolia).
Australia is leading the way in using biological control for P. hysterophorus management with 11 agents (9 insects and 2 pathogens) so far released since the initiation of the program in 1976 (Table 3). Most of these released agents have become established, with some that are now widespread and abundant and having a significant impact upon weed populations (Dhileepan and MacFadyen Reference Dhileepan, McFadyen, Julien, McFadyen and Cullen2012; Dhileepan et al. Reference Dhileepan, Callander, Shi and Osunkoya2018). Zygogramma bicolorata (a leaf-feeding beetle), Listronotus setosipennis (a stem-boring weevil), Smicronyx lutulentus (a seed-feeding weevil), and Epiblema strenuana (a stem-galling moth) are some of the widespread and effective insect biocontrol agents (Dhileepan et al. Reference Dhileepan, Callander, Shi and Osunkoya2018; McFadyen Reference McFadyen1992). The most effective agents are now being redistributed to south and southeast Queensland, where P. hysterophorus is now spreading from its core infestations in central Queensland (Dhileepan et al. Reference Dhileepan, Callander, Shi and Osunkoya2018).
Zygogramma bicolorata is the most widely distributed agent, released or arrived fortuitously, in 10 countries (Table 3). Both the larvae and adults of Z. bicolorata feed on the leaves of P. hysterophorus (Figure 7), and just a few beetles can completely defoliate a plant, especially when present on the early growth stages of the weed (Shabbir et al. Reference Shabbir, Sheema and Mujahid2016). Field occurrences of Z. bicolorata are now common in central Queensland, Australia, resulting in a significant reduction in populations of P. hysterophorus (Dhileepan et al. Reference Dhileepan, Callander, Shi and Osunkoya2018).
After its first release in Australia in 1981, the stem-boring weevil Listronotus setosipennis has now been released to four other countries, including South Africa, Ethiopia, Uganda, and Pakistan. The larvae of L. setosipennis bore through the stem, feed on the pith or epidermis, and can subsequently kill the plant (Wild et al. Reference Wild, McFadyen, Tomley and Willson1992). Smicronyx lutulentus is a seed-feeding weevil, first released in Australia in 1981; since then, it has been released in South Africa and Ethiopia. The adult weevils feed on developing leaf buds and emerged leaves, but they inflict minimal damage. The female lays eggs on the flower buds, and the emerging larvae feed on developing seeds, which causes the seeds to swell greatly, inflicting damage not only to the infested seeds but also to the other seeds in the capitulum that often do not develop (Dhileepan et al. Reference Dhileepan, Callander, Shi and Osunkoya2018).
Epiblema strenuana is a stem-galling moth first released in Australia in 1982. This agent is also present in China, where it was originally released for the biological control of annual ragweed (Ambrosia artemisiifolia L.) (Liu et al. Reference Liu, He, Li, Tan, Li and Zeng2013). After its first release in Australia, E. strenuana readily established in most areas where P. hysterophorus occurred at that time. The female lays eggs singly on leaves, and the emerging larvae feed on leaf tissue for short periods of time before boring into the stem. The feeding larvae produce stem galls in which developing larvae pupate and emerge out through a hole 4 to 6 wk later (Dhileepan et al. Reference Dhileepan, Callander, Shi and Osunkoya2018; Figure 7). The stem-galling moth is a widespread and effective agent that can reduce the growth and seed production of P. hysterophorus (Dhileepan Reference Dhileepan2003; Dhileepan and McFadyen Reference Dhileepan and McFadyen2001). This agent was found to retain its effectiveness to suppress the growth of P. hysterophorus under climate change conditions involving an atmosphere of elevated CO2 (Shabbir et al. Reference Shabbir, Dhileepan, Zalucki and Adkins2019b).
Two rust species, Puccinia abrupta var. partheniicola (winter rust) and Puccinia xanthii var. parthenium hysterophorae (summer rust) were released in Australia and established in the field, but their prevalence and impact are highly variable and sporadic depending upon the local climatic conditions (Dhileepan et al. Reference Dhileepan, Madigan, Vitelli, McFadyen, Webster and Trevino1996). The winter rust is now present in six other countries outside Australia, where its source is unknown (Table 3), with Pakistan being the most recent country where winter rust is reported (Iqbal et al. Reference Iqbal, Ali, Evans, Rehman, Seier, Shabbir and Weyl2020; Figure 7). South Africa imported the summer rust from Australia in 2004, and it was subsequently approved for field release after host specificity testing and has now established (Retief et al. Reference Retief, Ntushelo and Wood2013; Strathie et al. Reference Strathie, Cowie, McConnachie, Chidawanyika, Musedeli, Sambo, Magoso and Gareeb2021).
Suppressive plants, also known as competitive plants, can suppress the growth of nearby plants, including invasive plants, through direct physical competition for resources (light, water, space, nutrients) or by releasing allelopathic chemicals. Sowing beneficial suppressive plants in a P. hysterophorus–infested areas is an effective strategy to manage this weed (Belgeri et al. Reference Belgeri, Bajwa, Shabbir, Navie, Vivian-Smith and Adkins2020; Khan et al. Reference Khan, George, Shabbir, Hanif and Adkins2015; Shabbir et al. Reference Shabbir, Ali, Khan, Belgeri, Khan and Adkins2019a; van der Laan et al. Reference Van der Laan, Reinhardt, Belz, Truter, Foxcroft and Hurle2008). Several suppressive pasture plants, both grass and leguminous species, have been reported to suppress the growth of P. hysterophorus (Khan et al. Reference Khan, Shabbir, George, Hassan and Adkins2014b, Reference Khan, George, Shabbir, Hanif and Adkins2015). This approach has been used to manage P. hysterophorus in different parts of the world such as Australia, India, and South Africa (Belgeri et al. Reference Belgeri, Bajwa, Shabbir, Navie, Vivian-Smith and Adkins2020; Khan et al. Reference Khan, George, Shabbir and Adkins2019; Knox et al. Reference Knox, Jaggi and Paul2011; Shabbir et al. Reference Shabbir, Ali, Khan, Belgeri, Khan and Adkins2019a; van der Laan et al. Reference Van der Laan, Reinhardt, Belz, Truter, Foxcroft and Hurle2008). In Australia, more than 30 pasture species (both grasses and legumes) were systematically selected based on their superior growth traits (fast growth rate, higher biomass, tall stature, etc.) and good palatability to suppress the growth of P. hysterophorus and at the same time provide fodder for livestock (Bowen et al. Reference Bowen, Ji and Adkins2007; Khan et al. Reference Khan, Shabbir, George, Hassan and Adkins2014b, Reference Khan, George, Shabbir, Hanif and Adkins2015; O’Donnell and Adkins Reference O’Donnell and Adkins2005). Most of these species suppressed P. hysterophorus growth under glasshouse conditions (Khan et al. Reference Khan, Marwat, Hassan and Khan2013), while some of them [e.g., Purple pigeon grass, Setaria incrassata (Hochst.) Hack.; buffel grass, Cenchrus ciliaris L.; T. triandra; creeping bluegrass, Bothriochloa insculpta (Hochst. ex A. Rich.) A. Camus; Indian bluegrass, Bothriochloa pertusa (L.) A. Camus; butterfly pea, Clitoria ternatea L.; bull Mitchel grass, Astrebla squarrosa C.E. Hubb.] have also proven to be suppressive at different field locations (Khan et al. Reference Khan, Shabbir, George, Hassan and Adkins2014b, Reference Khan, George, Shabbir, Hanif and Adkins2015). In a field study in central Queensland, Australia, Shabbir et al. (Reference Shabbir, Ali, Khan, Belgeri, Khan and Adkins2019a) tested additional species and found that the introduced pasture grasses [digit grass, Digitaria milanjiana (Rendle) Stapf.; C. ciliaris (L.) Link] and legumes [round-leaf cassia, Chamaecrista rotundifolia (Pers.) Greene; C. ternatea], suppressed the biomass of P. hysterophorus by 60% to 80%, with C. ciliaris and C. ternatea producing large amounts of dry fodder biomass (i.e., 0.41 and 0.36 kg m−2, respectively). Belgeri et al. (Reference Belgeri, Bajwa, Shabbir, Navie, Vivian-Smith and Adkins2020) studied the suppressive effect of plant mixtures on the growth of P. hysterophorus in Australian grasslands. This study found that the mixture composed predominantly of native species [forest bluegrass, Bothriochloa bladhii (Retz.) S.T. Blake; Queensland bluegrass, Dichanthium sericeum (R. Br.) A. Camus] exhibited greater suppression of P. hysterophorus biomass compared with those rich in introduced species. It should be noted that the suppressive legume yellow lucerne (Medicago falcata L.) can decrease the root to shoot ratio of P. hysterophorus, while alfalfa (Medicago sativa L.) increased this ratio, possibly through beneficial nitrogen fixation (Ran et al. Reference Ran, Zhang, Liu and Zhang2024).
Integrating suppressive plants with other management strategies, including biological control, can provide enhanced control of P. hysterophorus (Shabbir et al. Reference Shabbir, Dhileepan, O’Donnell and Adkins2013, Reference Shabbir, Dhileepan, O’Donnell, Khan, Hanif and Adkins2015, Reference Shabbir, Bajwa, Dhileepan, Zalucki, Khan and Adkins2018, Reference Shabbir, Dhileepan, Zalucki and Adkins2020a, Reference Shabbir, Dhileepan, Zalucki, Khan and Adkins2020b). Some of the biological control agents released in Australia (E. strenuana, Z. colorata, and P. abrupta) have been shown to work synergistically or additively with selected suppressive plants (e.g., C. ternatea and A. squarrosa) to provide good control of P. hysterophorus (Shabbir et al. Reference Shabbir, Dhileepan, O’Donnell and Adkins2013, Reference Shabbir, Bajwa, Dhileepan, Zalucki, Khan and Adkins2018). Further, the complementary effects of suppressive plants with biological control could be retained under an atmosphere of elevated CO2 in various changing climate scenarios (Shabbir et al. Reference Shabbir, Dhileepan, Zalucki and Adkins2019b, Reference Shabbir, Dhileepan, Zalucki, Khan and Adkins2020b). In summary, sowing suppressive plants in P. hysterophorus–infested areas has shown good potential to aid the management of P. hysterophorus, and it can be combined with other strategies such as biological control to provide resilient management across diverse landscapes. Exploring new suppressive plants suited to local climates would provide effective management of P. hysterophorus and promote environmental sustainability.
General Outlook
Parthenium hysterophorus is a highly invasive plant species of global concern. It impacts agriculture, natural ecosystems, human and animal health, and livelihoods around the world. Already, the weed has invaded more than 50 countries across its introduced range and is expanding even in parts of its native range, particularly in the southwestern United States. Global climatic suitability modeling indicates that this weed has not yet reached its full spread potential, and there are many more areas (e.g., sub-Saharan and western Africa, eastern Europe, southeast Asia) that are highly susceptible to future invasion. The rapid spread of P. hysterophorus in densely populated countries like China, India, and Pakistan poses a significant threat to the environment, human health, and the sustainability of resources. The ability of this plant to produce many highly viable seeds and disperse them through various vectors, coupled with intensified global trade and travel, pose a serious threat to all those suitable uninvaded areas. Climate change is also in favor of P. hysterophorus, promoting its growth and reproduction under increasing temperature and atmospheric CO2 concentrations. Current control options are partially effective in most circumstances. Effective biosecurity legislation and investment in border control of this weed are the most cost-effective ways to protect new areas from invasion; however, these would be expensive to implement in most developing and low-income countries. A suite of biological control agents has been released against P. hysterophorus in Australia and South Africa with partial success. However, a combination of approaches built into a sustainable integrated weed management plan is advised. Other countries with a prevailing P. hysterophorus problem could learn from these experiences and benefit from this strategy, and at a much lower cost and with rapid delivery. Collaboration among researchers, policy makers, and local communities is key to addressing the P. hysterophorus issue, on both local and global scales.
Acknowledgments
We thank Stephen Johnson and Bernie Dominiak (Department of Primary Industries and Regional Development, NSW) for internal review of this article and providing numerous suggestions that improved it. We also appreciate the valuable suggestions from two anonymous reviewers.
Funding statement
This research did not receive any specific grant from funding agencies.
Competing interests
The authors declare no conflicts of interest.
Parthenium hysterophorus at a glance…
Impacts
-
Outcompetes native vegetation and reduces biodiversity
-
Reduces agricultural production and impacts socioeconomic health in rural communities
-
Causes allergies and other health problems in humans and animals
Dispersal
-
Seed dispersed by wind and water, via movement of livestock and native and feral animals, and attached to agricultural machinery and vehicles
Management
-
Several herbicides provide effective control
-
Suite of biocontrol agents available
-
Suppressive plants also effective alone and or in combination with biocontrol
-
True integrated management lacking
Biosecurity
-
One of the world’s worst invasive species
-
Recognized as a major biosecurity threat and a Weed of National Significance in Australia
-
Spread through contaminated seed, feed, and machinery
-
Most of sub-Saharan Africa, eastern Europe, and southeast Asia at risk of invasion
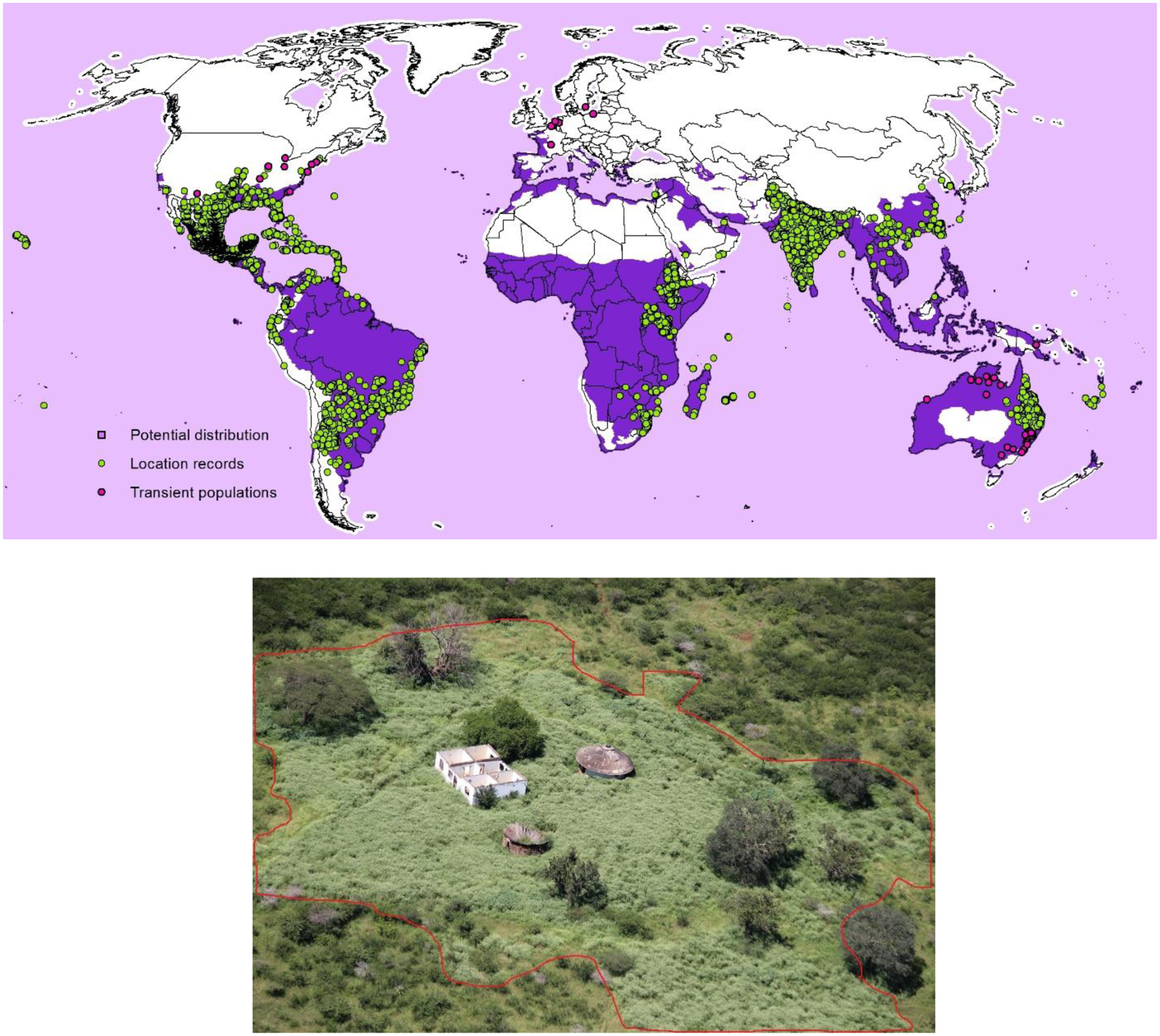
Structured Appendix
Methods for Distribution Data and Köppen-Geiger Climates
Plant localities were taken from GBIF (2024), REMIB database (2024) (Ghazanfar Reference Ghazanfar2017; Kawi and Orapa Reference Kawi and Orapa2010; Shabbir Reference Shabbir2017; Sushilkumar Reference Sushilkumar2012; Tominaga Reference Tominaga2013), published surveys (Jayasuriya Reference Jayasuriya2005; Kirshanthan et al. Reference Kirshanthan, Jeyaseelan and Nandakumar2016; Shabbir et al. Reference Shabbir, Dhileepan and Adkins2012), and recent, but unpublished, surveys in Thailand (S Zungsontiporn, personal communication, 2024), Saudi Arabia, Eritrea, and Bermuda to the Caribbean region (A Witt, personal communication, 2024). Locality data were refined by (1) correcting coordinate errors, where possible; (2) removing duplicate records; (3) removing instances with a locational error or an artificial cultivation of Parthenium hysterophorus (e.g., university campuses, botanical gardens); and (4) reevaluating coordinates older than 50 yr and/or with no additional locations near them, deeming them transient, and removing them in Belgium, France, Poland, Sweden, the Netherlands, and parts of the United States.
Climatic data were downloaded from World Maps of Köppen-Geiger Climate Classification (http://koeppen-geiger.vu-wien.ac.at). Distribution maps with Köppen-Geiger climates as a background were generated using ArcMap (ESRI, Redlands, CA). We used the CLIMEX model developed by Shabbir et al. (Reference Shabbir, Zalucki, Dhileepan, Khan and Adkins2023) to show the potential distribution of P. hysterophorus around the world.
Table A1. Köppen-Geiger climate zone classifications globally that indicate habitat suitability for the survival and growth of Parthenium hysterophorus


Figure A1. The known occurrence of Parthenium hysterophorus populations overlaid on climatic zones based on the Köppen-Geiger classification system. Parthenium hysterophorus is mainly found in subtropical and tropical regions of the world. Köppen-Geiger legends are explained in Table A1.

















