Scientific Classification
Domain: Eukaryota
Kingdom: Plantae
Phylum: Magnoliophyta
Class: Angiospermae
Order: Asparagales
Family: Iridaceae
Subfamily: Iridoideae
Genus: Iris
Species: Iris pseudacorus L. (Linnaeus Reference Linnaeus1753)
Synonyms: Acorus adulterinus Ludw., Iris acoriformis Boreau, Iris acoroides Spach, Iris bastardii Boreau, Iris curtopetala Redouté, Iris flava Tornab., Iris lutea Ludw., Iris pallidior Hill, Iris paludosa Pers., Iris palustris Gaterau, Iris pseudacorus var. longifolia DC., Iris pseudacorus subsp. acoriformis (Boreau) K. Richt., Iris pseudacorus ssp. bastardii (Boreau) K. Richt., Iris sativa Mill., Limnirion pseudacorus (L.) Opiz, Limniris pseudacorus (L.) Fuss, Moraea candolleana Spreng., Pseudo-iris palustris Medik., Vieusseuxia iridioides Redouté, Xiphion acoroides (Spach) Alef., Xiphion pseudacorus (L.) Schrank, Xyridion acoroideum (Spach) Klatt, Xyridion pseudacorus (L.) Klatt.
EPPO Code: IRIPS
Names and Taxonomy
Among the plethora of common names used to identify Iris pseudacorus, the most common ones are yellow iris, yellow flag, yellow flag iris, pale-yellow iris, water iris, and water flag. In other languages, the species is referred to as iris de marais, iris faux-acore, and iris jaune (France); giaggiolo acquatico and iris palustre (Italy); lirio amarillio and falso acoro (Spain); gele lis (Netherlands); and sumpf-schwertlilie (Germany).
Iris pseudacorus belongs to the genus Iris, within the family Iridaceae (Wilson Reference Wilson2006). This is among the largest families of the order Asparagales, including more than 2,000 species divided among 65 to 75 genera (Goldblatt et al. Reference Goldblatt, Rodriguez, Powell, Davies, Manning, Van der Bank and Savolainen2008). Virtually worldwide in distribution, the family has a marked diversity (ca. 63% of species) in sub-Saharan Africa, in contrast to Eurasia and North Africa (ca. 19%), the Americas (ca. 16%), and Australasia (ca. 2%) (Goldblatt Reference Goldblatt2000).
The genus Iris is taxonomically difficult. Conflicting classifications based on anatomical and morphological characters do not reflect the evolutionary relationships illuminated in recent molecular studies, highlighting the need for further consideration (Boltenkov et al. Reference Boltenkov, Artyukova, Kozyrenko, Erst and Trias-Blasi2020; Wheeler and Wilson Reference Wheeler and Wilson2014).
The genus name Iris (from classical Greek Iρις, rainbow) refers to the wide variety of flower colors found among its species (Manning and Goldblatt Reference Manning and Goldblatt2008). The specific epithet pseudacorus (from classical Greek ψϵυδής, false) refers to the similarity of its leaves to those of Acorus calamus (Acoraceae), another common wetland plant species.
Iris includes approximately 260 species widely distributed in temperate regions across the Northern Hemisphere (Wilson Reference Wilson2011). Although some species are found in mesic and even wetland environments, most occur in arid, semiarid, or dry habitats. The genus has long been subdivided into six subgenera based on morphological characters such as underground organs and sepal beards (Wilson Reference Wilson2006). However, recent phylogenetic analyses based on chloroplast data showed the subgeneric classification to be more complex (Wilson Reference Wilson2011). Iris pseudacorus is currently positioned within the subgenus Limniris (Wilson Reference Wilson2011), which includes a clade of species with an affinity for wetland habitats. Also positioned within the subgenus Limniris is the stinking iris [Iris foetidissima L.], a close relative of I. pseudacorus, which is reported to be invasive in New Zealand (Howell Reference Howell2008).
Importance
Negative Impacts
Iris pseudacorus becomes readily established and colonizes new habitats due to its very prolific nature (Alpert et al. Reference Alpert, Bone and Holzapfel2000; Silvertown Reference Silvertown2008) and the presumed absence of specialized natural enemies in its introduced range (Gervazoni et al. Reference Gervazoni, Fuentes-Rodriguez, Sosa, Del Río, Sabater and Franceschini2021; Sandenbergh Reference Sandenbergh2021). After establishment, I. pseudacorus becomes aggressively invasive in natural, urban, and agricultural wetland ecosystems (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020), where its fast-growing and fast-spreading nature allows it to cause substantial impacts on biodiversity and ecosystem functioning at local and landscape scales (Global Invasive Species Database 2022; Stone Reference Stone2009; Thomas Reference Thomas1980; USDA-APHIS 2013).
In natural areas, I. pseudacorus can invade and dominate a variety of vegetation types, reducing native plant and animal diversity and altering successional trajectories (Tu Reference Tu2003). For instance, in the United States, I. pseudacorus has completely excluded native marsh vegetation such as bullrushes (Typha spp.) (Poaceae) (Raven and Thomas Reference Raven and Thomas1970) and other marsh plants such as sedges (Carex spp.), common tule (Schoenoplectus acutus (Muhl. ex Bigelow) Á. Löve & D. Löve) (both Cyperaceae), and water horsetail [Equisetum fluviatile L.] (Equisetaceae) (Stone Reference Stone2009). This impact also includes threatened native irises, such as zigzag iris [Iris brevicaulis Raf.], dwarf iris [Iris verna L.], and dixie iris [Iris hexagona Walter] (Mopper et al. Reference Mopper, Wiens and Goranova2016; USDA-APHIS 2013; Weatherbee et al. Reference Weatherbee, Somers and Simmons1998), as well as arrow arum [Peltandra virginica (L.) Schott] (Araceae), whose fruits are an important food source for wood ducks (Aix sponsa) during the nesting season (Cox Reference Cox1999). Furthermore, the resulting transformation in riparian vegetation structure has been linked to habitat reduction for several important salmon species (King County Noxious Weed Control Program 2009).
In a recent study, I. pseudacorus invasion was shown to disrupt the composition and function of native plant communities across brackish estuarine gradients in North America (Gallego-Tévar et al. Reference Gallego-Tévar, Grewell, Whitcraft, Futrell, Bárcenas-Moreno and Castillo2022). At the local scale, this species forms tall, dense, monospecific stands that overshadow smaller native plants. Iris pseudacorus was found to greatly reduce plant richness and diversity in California, USA, at local and watershed scales, while native populations in Andalusia, Spain, were associated with high plant species richness, evenness, and diversity in similar tidal wetlands (Gallego-Tévar et al. Reference Gallego-Tévar, Grewell, Whitcraft, Futrell, Bárcenas-Moreno and Castillo2022). In Japan, the density of I. pseudacorus infestations was linked to a significant decrease in the number of native plant species and a concomitant increase in the number of invasives (Hayasaka et al. Reference Hayasaka, Fujiwara and Uchida2018). Similarly, invasive I. pseudacorus populations in China have been associated with the displacement of indigenous plant assemblages and a decline in native wetland biodiversity (Xiong et al. Reference Xiong, Wang, Wu, Xiao, Li and Bowler2023). Furthermore, the attractiveness of I. pseudacorus flowers has been hypothesized as a cause for reduction in pollination frequency of native flowering species, such as the North American orchid [Galearis spectabilis (L.) Raf.] (Dieringer Reference Dieringer1982).
Although I. pseudacorus invasions reduce the diversity of native vegetation and the associated biota, including invertebrates, fish, and waterfowl (Global Invasive Species Database 2022; Hayasaka et al. Reference Hayasaka, Fujiwara and Uchida2018; Jacobs et al. Reference Jacobs, Pokorny, Mangold and Graves-Medley2011; King County Noxious Weed Control Program 2009; Preece Reference Preece1964; Stone Reference Stone2009; Thomas Reference Thomas1980; USDA-APHIS 2013; Xiong et al. Reference Xiong, Wang, Wu, Xiao, Li and Bowler2023), knowledge of the effect of this weed on invertebrate assemblages and biotic interactions remains largely unknown.
From a landscape perspective, once a founding population has been established on a shoreline, plant rhizomes retain sediment and organic matter, affecting the hydrology, functioning, and structure of large wetland ecosystems. In this sense, I. pseudacorus can be considered an ecosystem engineer, like North American beavers (Castor canadensis, Castoridae) in Southern Patagonian wetlands (Henn et al. Reference Henn, Anderson and Pastur2016; Huertas Herrera et al. Reference Huertas Herrera, Lencinas, Toro Manríquez, Miller and Martínez Pastur2020; Sonntag Reference Sonntag2021).
The intricate rhizome mat compacts soil and elevates topography, creating a drier habitat type with increased rates of siltation and sedimentation (Tu Reference Tu2003). This creates a positive feedback loop, preventing the germination and seedling growth of other native plant species while improving habitat suitability for I. pseudacorus (Morgan et al. Reference Morgan, Berent and Fusaro2020; Sutherland Reference Sutherland1990; Thomas Reference Thomas1980; Tu Reference Tu2003). In Montana, USA, I. pseudacorus was shown to reduce stream width by up to 25 cm annually by trapping sediment and creating new stream banks dominated by I. pseudacorus seedlings (King County Noxious Weed Control Program 2009). Other observations show that, by preventing the germination and seedling recruitment of characteristic plant species such as willows (Salix spp., Salicaeae), and providing a raised substrate for the seedbed, I. pseudacorus contributes to the conversion of riparian marshes into swamps and mesic forests dominated by ashes (Fraxinus spp.) (Oleaceae) (Thomas Reference Thomas1980; Tu Reference Tu2003).
Dense I. pseudacorus infestations are known to clog small streams, irrigation systems, and flood control structures, often leading to increased flooding (King County Noxious Weed Control Program 2009; Preece Reference Preece1964; Stone Reference Stone2009; USDA-APHIS 2013; Van Slooten Reference van Slooten2016). Ecosystem processes and services provided by native aquatic and riparian vegetation can also be detrimentally altered by invasion (USDA-APHIS 2013). By decreasing stream width, promoting sedimentation, and preventing access to water, I. pseudacorus infestations can restrict agricultural, recreational, and fishing activities, having adverse effects on the tourism industry (Wildland Consultants 2011).
Iris pseudacorus has been considered poisonous due to glycoside concentrations found within its tissues (Forsyth Reference Forsyth1976) and has been reported as unpalatable or even poisonous to livestock (Bossuyt et al. Reference Bossuyt, de Fre and Hoffmann2005; Stone Reference Stone2009). The glycoside concentrations found within I. pseudacorus tissue can act as a skin irritant, causing severe dermatitis (Crocker Reference Crocker1906; Fuller and McClintock Reference Fuller and McClintock1986; Williams and Champion Reference Williams and Champion2008), with effects varying between plant populations. In the United Kingdom, gastroenteritis occurred after livestock consumed I. pseudacorus leaves, and acute diarrhea occurred in domestic cattle (Bos taurus) after rhizome consumption (Sutherland Reference Sutherland1990 and references therein). Conversely, extensive grazing of I. pseudacorus by wild horses (Equus ferus), cattle, sheep (Ovis aries), and goats (Capra hircus) has been documented during field research in Spain and France (Grewell et al. Reference Grewell, Gallego-Tévar, Bárcenas-Moreno, Whitcraft, Thorne, Buffington and Castillo2023), and deer herbivory has recently been observed in California. However, careful consideration should be given before using cattle grazing as a control method due to the plant’s toxicity.
In natural wetlands of the introduced range, protected areas are of major concern, as they contribute to biodiversity conservation, especially in Afrotropical and Neotropical ecozones, which support substantial areas of macrophyte diversity and endemism around the world (Chambers et al. Reference Chambers, Lacoul, Murphy and Thomaz2008; Murphy et al. Reference Murphy, Efremov, Davidson, Molina-Navarro, Fidanza, Betiol, Chambers, Grimaldo, Martins, Springuel, Kennedy, Mormul, Dibble, Hofstra and Lukács2019, Reference Murphy, Carvalho, Efremov, Grimaldo, Molina-Navarro, Davidson and Thomaz2020). A recent study in Argentinian wetlands shows that 15 protected areas are invaded by I. pseudacorus, of which 4 have international conservation status (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020). The invasion of this species in Argentina also represents a threat to the artificial rice (Oryza sativa L.) wetlands that cover large areas of land in the northeastern region of the country. This is especially relevant, considering that I. pseudacorus has previously been reported as a weed of this crop inGen other countries (Rahimi et al. Reference Rahimi, Grouh, Solymani, Bahermand and Meftahizade2011). Despite the impact of I. pseudacorus invasion being evident at both local and landscape scales, studies that investigate the socioeconomic costs of I. pseudacorus invasions in natural and human-modified wetland ecosystems are scarce (Zilio et al. Reference Zilio, Restrepo, Gervazoni, Minuti, Muzón, Djeddour and Sosa2025).
Positive Attributes
Iris pseudacorus has showy flowers and is easy to grow, making it a popular ornamental plant for ponds and water bodies in its native and introduced ranges (Hayasaka et al. Reference Hayasaka, Fujiwara and Uchida2018). Aside from its primary horticultural value, I. pseudacorus is also considered a potential candidate for phytoremediation in constructed wetlands, eutrophic water systems, and urban wastewaters (Ansola and De Luis Reference Ansola and De Luis1994; Larue et al. Reference Larue, Korboulewsky, Wang and Mevy2010; Wu et al. Reference Wu, Cui and Cheng2013; Yousefi and Mohseni-Bandpei Reference Yousefi and Mohseni-Bandpei2010; Zhang et al. Reference Zhang, Liu, Yang and Chen2007; Zhao et al. Reference Zhao, Wang and Ji2015; Zhou et al. Reference Zhou, Huang, Yu, Gu, Zhao, Han and Fu2010), although risk of escape from treatment wetlands is a concern. This species is reported to reduce the concentrations of heavy metals (Branković et al. Reference Branković, Glišić, Topuzović and Marin2015), organic chemicals (Larue et al. Reference Larue, Korboulewsky, Wang and Mevy2010), insecticides (Wang et al. Reference Wang, Yang, Li, Xiao and Que2013), and bacterial loads (Jacobs et al. Reference Jacobs, Pokorny, Mangold and Graves-Medley2011; Sutherland Reference Sutherland1990) in these systems. It is also a suitable plant for use in erosion control, and for phyto-stabilization of contaminated soils in its native range (Pérez-Sirvent et al. Reference Pérez-Sirvent, Hernández-Pérez, Martínez-Sánchez, García-Lorenzo and Bech2017; Tu Reference Tu2003). In the introduced range, the use of I. pseudacorus for phytoremediation should be avoided, as other weeds take advantage of nutrient-rich conditions, making them difficult to control biologically, as has been the case with water hyacinth [Pontederia crassipes Mart.] (Coetzee and Hill Reference Coetzee and Hill2012).
Several aquatic plants are used in attempts to prevent recurrence of diseases, with extraction of natural products used as alternative medicine and/or drug precursors for the pharmaceutical industry (Bharthi et al. Reference Bharthi, Kavya, Shantha, Prathapa Reddy, Kavya, Rama Rao, Ishnawa and Venkateshwarlu2015; Mandal and Mondal Reference Mandal and Mondal2011; Tulika and Mala Reference Tulika and Mala2015). Iris pseudacorus is not an exception, and its rhizomes are used in India as a part of Ayurveda, a system of traditional medicine, due to its diuretic properties and its effect of preventing the recurrence of urinary calculi (Ahmed et al. Reference Ahmed, Ahsan Maliick and Hasan2017; Sharma et al. Reference Sharma, Raval, Koneri, Sharma and Chandrul2022). In the past, there have been several recreational and/or medicinal uses for I. pseudacorus. Sutherland (Reference Sutherland1990) reports the plant being smoked during World War II. In Turkey, rhizomes are used as a diuretic, to prevent gas, and to treat eczema, while roasted seeds are used as a substitute for coffee (Coffea spp.) (Stone Reference Stone2009), and minced rhizomes are mixed with couscous in a popular dish in northern Africa (IUCN 2012). However, adverse effects on human health have also been reported, including gastric distress after ingestion and irritation when sap comes in contact with the skin (King County Noxious Weed Control Program 2009).
Description, Identification, and Diagnostic Characteristics
Iris pseudacorus is an emergent aquatic macrophyte, ∼0.5 to 2.17 m in height (Chambers et al. Reference Chambers, Lacoul, Murphy and Thomaz2008). Although I. pseudacorus is a perennial species, under unfavorable growing conditions, plants may retain leaves in addition to retaining their roots and rhizome material (Campbell et al. Reference Campbell, Higman, Slaughter and Schools2010; Lui et al. Reference Lui, Butler, Allen, Snyder, da Silva, Brownson and Ecclestone2010). When in bloom, I. pseudacorus is easy to distinguish by the shape and color of its flowers (Goodridge et al. Reference Goodridge, Bixby, Winter-Gorsline, Tu, Anderson, Harber, Douglas, McMahan and Chan2011). The flowers flutter like flags in the breeze, explaining the common name. Flowers are yellow, radially symmetrical, with a perianth with two different-looking whorls, the external broadly ovate tepals and internal spatulate ones. The style has three yellow petaloid stylar blades arched over the external tepals (Figure 1A–D).

Figure 1. Morphology of Iris pseudacorus (Iridaceae) and its reproductive structures. (A) Iris pseudacorus general structure, (B–E) details of the flowers, (F) detail of the fruit, and (G) plant size.
It can be difficult to distinguish I. pseudacorus from other similar iris species or cultivars when it is not in bloom (Lui et al. Reference Lui, Butler, Allen, Snyder, da Silva, Brownson and Ecclestone2010; Sarver et al. Reference Sarver, Treher, Wilson, Naczi and Kuehn2008). Iris pseudacorus has a rhizome rather than a bulb or root tuber characteristic of some irises; it lacks beard or crest ornamentation on its sepals like many irises; it does not have arial outgrowths covering its seeds; and it has a thick, pronounced midrib. The fruits and the numerous thick, fleshy pink rhizomes are also important for differentiation and identification (Campbell et al. Reference Campbell, Higman, Slaughter and Schools2010; Kalesnik and Malvárez Reference Kalesnik and Malvárez2004; King County Noxious Weed Control Program 2009).
Distribution
The native range of I. pseudacorus extends from northern Africa throughout Europe, western Asia, and parts of the Middle East (Sutherland Reference Sutherland1990; Figure 2). While the threat status of native European I. pseudacorus is currently Least Concern on the International Union for Conservation of Nature (IUCN) Red List of threatened species (Flora Europaea 2022), the species is a protected endangered species in Finland’s Oulu and Lapland provinces (Nature Conservation Decree 1997).

Figure 2. Worldwide distribution of Iris pseudacorus. Top: political map of regions where I. pseudacorus is reported to occur (invasion status inferred from the literature; see text for details). Bottom: introduced (A–E, red) and native (F, green) range records for I. pseudacorus downloaded from GBIF (2022).
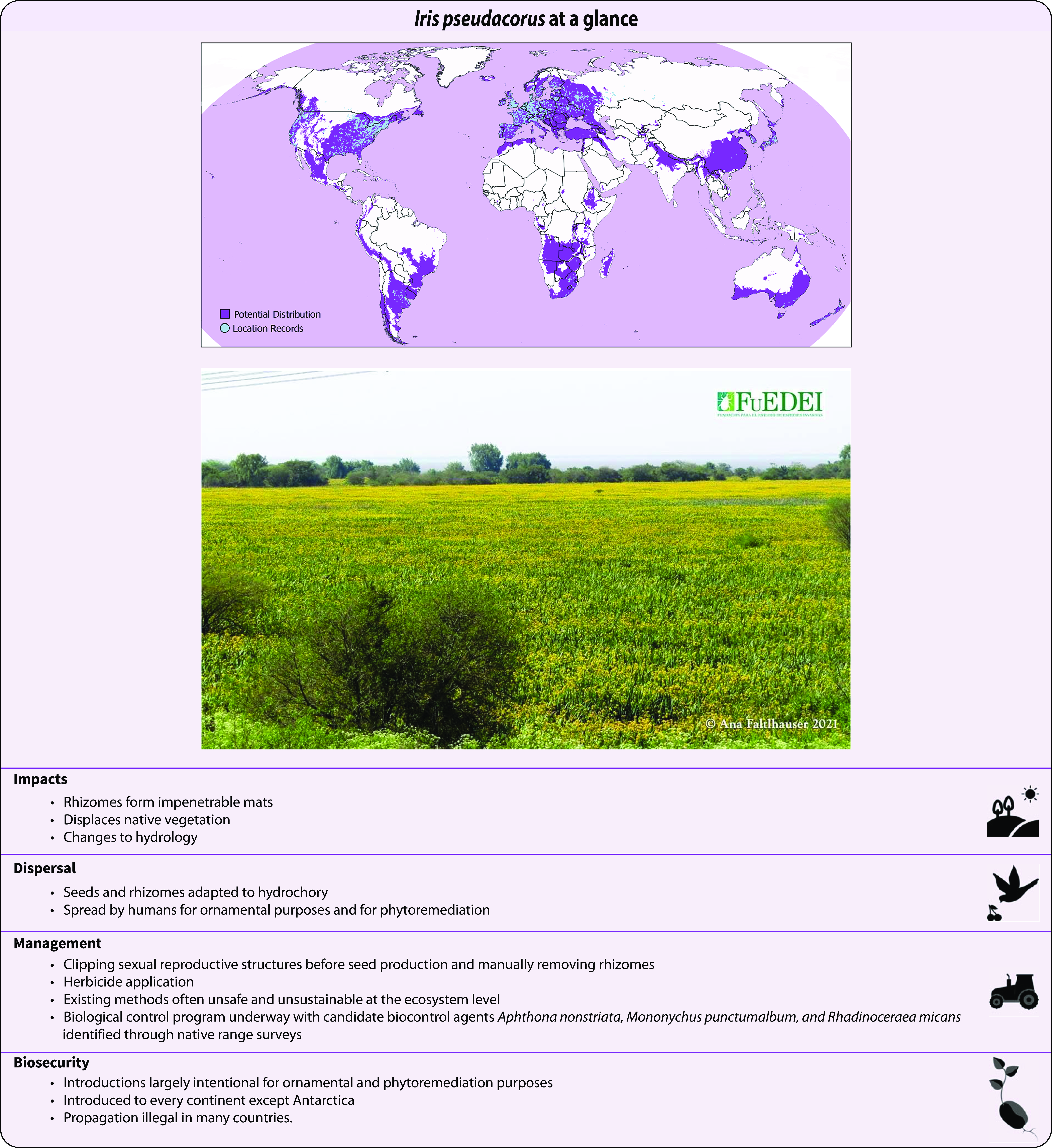
Due to its ornamental attributes, the plant has been introduced to every continent except Antarctica and is now considered naturalized or invasive in parts of Canada, the United States, Argentina, Chile, Uruguay, South Africa, Australia, New Zealand, China, Japan, and the Korean Peninsula (POWO 2022; USDA-APHIS 2013), and it is also present in Mexico and Zimbabwe (Hyde et al. Reference Hyde, Wursten, Ballings and Coates Palgrave2022; Naturalista 2022). While the recorded distribution of I. pseudacorus is expansive, due to limited data and mapping capacities, the true distribution of the species is likely far greater.
Iris pseudacorus is reported as present across much of the United States (Stoneburner et al. Reference Stoneburner, Meiman, Ocheltree, Nissen and Bradfield2021), although invasions are most prevalent in East and West Coast states and the Great Lakes region (GBIF 2022). The plant is also recorded in eight Canadian provinces, noticeably along the U.S. border (USDA-APHIS 2013; Figure 2A), and a single verified record of I. pseudacorus was found in Mexico (Naturalista 2022). The species has been observed in eight provinces in Argentina, with the majority of records concentrated in the Buenos Aires and Córdoba provinces (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020; Figure 2B). Iris pseudacorus is also present in the coastal region of Uruguay (Masciadri et al. Reference Masciadri, Brugnoli and Muniz2010) and is listed among the alien flora of Chile (Ugarte et al. Reference Ugarte, Lira, Fuentes and Klotz2011; Figure 2B).
Iris pseudacorus is listed as a cultivated plant in Zimbabwe, with records of infestations in public parks (Hyde et al. Reference Hyde, Wursten, Ballings and Coates Palgrave2022). In South Africa, I. pseudacorus is present in eight of the country’s nine provinces, with the majority of infestation reports coming from Johannesburg and Cape Town, two of the country’s major cities (Jaca Reference Jaca2013; Jaca and Mkhize Reference Jaca and Mkhize2015; NEMBA 2014; Sandenbergh et al. Reference Sandenbergh, Petruzzella and Coetzee2024; Figure 2C). Australian I. pseudacorus infestations are confined to Tasmania and the southeast region of the mainland (AVH 2022; Figure 2D). In New Zealand, I. pseudacorus has been recorded as an environmental weed in numerous wetlands across the country (Howell Reference Howell2008; Figure 2D). The species has been recorded in the Korean Peninsula (Chang et al. Reference Chang, Kim and Chang2014), in Japan (Kadono Reference Kadono2004), and in China, where it has successfully established across 26 provinces, autonomous regions, and municipalities (Xiong et al. Reference Xiong, Wang, Wu, Xiao, Li and Bowler2023; Figure 2E).
Invasion Risk
A potential distribution model of I. pseudacorus was developed in order to identify areas with climatic suitability for this species and to prioritize areas at risk of invasion. The model was developed with the software Maxent v. 3.4.1 (Phillips et al. Reference Phillips, Anderson, Dudík, Schapire and Blair2017; see methods detailed in Appendix), which has been shown to be efficient in handling presence-only data (Elith et al. Reference Elith, Kearney and Phillips2010, Reference Elith, Phillips, Hastie, Dudík, Chee and Yates2011).
The model showed areas at high risk of invasion by I. pseudacorus across different continents, in both the Northern and Southern Hemispheres (Figure 3). In the Northern Hemisphere, in addition to the climatic suitability across Europe (native range), the eastern regions of China and Japan also show a high probability of the plant thriving in Asia. In North America, areas in the northeastern United States, mainly around the Great Lakes and parts of the southeast such as Georgia and South Carolina, show high suitability as well. On the other hand, areas with moderate suitability are predicted on the West Coast of the United States, particularly in California. Regarding the Southern Hemisphere, in South America, some areas in southern Brazil and northeastern Argentina have moderate to highly suitable conditions for the establishment of the species. Moderate to highly suitable regions are also observed in South Africa, New Zealand, and southeastern Australia (Figure 3).

Figure 3. Global climatic suitability for Iris pseudacorus computed in Maxent (see methods in the Appendix).
The invasion risk of I. pseudacorus has been studied previously not only by determining its current climatic suitability and potential distribution (Minuti et al. Reference Minuti, Stiers and Coetzee2022), but also by studying its future distribution (2040 to 2060), taking into account several climate change scenarios (Minuti et al. Reference Minuti, Coetzee and Stiers2023). According to that study, in North America and eastern Asia, the potential distribution of the plant is expected to increase and shift northward, but in the Southern Hemisphere (South America, southern Africa, and Australasia), the future distribution is predicted to be reduced in response to climate change (Minuti et al. Reference Minuti, Coetzee and Stiers2023).
Invasion Pathways
Yellow-flag iris is an aesthetically pleasing plant, given its beautiful yellow flowers, and has been widely planted as a garden plant. This high ornamental value of I. pseudacorus is, unfortunately, one of the primary invasion pathways allowing this plant to spread anthropically across continents and large regions (Cody Reference Cody1961; Jaca and Mkhize Reference Jaca and Mkhize2015; USDA-APHIS 2013). Likewise, the use of this plant for its phytoremedial properties constitutes another anthropic factor that induces the introduction of this plant to new sites (Ediviani et al. Reference Ediviani, Priadi and Moersidik2018; Mohsin et al. Reference Mohsin, Nawrot, Wojciechowska, Kuittinen, Szczepańska, Dembska and Pappinen2023).
The exchange of specimens for gardens together with its use for water purification enabled extensive human-mediated distribution of I. pseudacorus. For instance, in Argentina, a citizen science study demonstrated its association with urban centers, where the trade and sale of specimens and seeds in nurseries, and even on online platforms, significantly increase their populations (Gervazoni et al. Reference Gervazoni, Fuentes-Rodriguez, Sosa, Del Río, Sabater and Franceschini2021).
Natural means of dispersal of this species include the production and release of propagules (seeds and rhizomes fragments) to water currents. The buoyant seeds of I. pseudacorus can remain viable over a long period (even up to 2 yr), floating in the water and consequently arriving at new, distant sites, promoting new invasions (Coops and Van Der Velde Reference Coops and Van Der Velde1995; Gaskin et al. Reference Gaskin, Pokorny and Mangold2016), allowing the species to spread over long distances, particularly when associated with flowing lotic water bodies (Ramey and Peichel Reference Ramey and Peichel2001). Additionally, human modifications of freshwater ecosystems through hydraulic structures (such as embankments, dams, dikes, and causeways) can facilitate the spread of aquatic invasive species like I. pseudacorus (Thomson et al. Reference Thomson, Davies, Lawn, Kushneryk, Brouard-John, Nelson and Gerwing2021).
Habitat
Climate
Iris pseudacorus occurs across a wide variety of climatic and environmental conditions (Figure 4). In its native range, according to the Köppen-Geiger climate types (Beck et al. Reference Beck, Zimmermann, McVicar, Vergopolan, Berg and Wood2018), it occupies mostly humid temperate (Cfa), oceanic (Cfb), and continental (Dfa, Dfb) climates, but it is also present, albeit less common, in semiarid (BSk) and Mediterranean (Csa, Csb) areas. The species is absent from the Alps and the Pyrenees, but in its northernmost distribution, it is observed within boreal climates (Dfc). The climates occupied by the species outside its native range vary depending on the region of introduction. In North America, I. pseudacorus is most abundant across the continental (Dfa, Dfb) and humid temperate (Cfa) climates of the East Coast, but it is also observed within the semiarid (BSk) and Mediterranean (Csa, Csb) climates of the western United States (Figure 4A). In South America, the most invaded regions are the humid subtropical (Cwa, Cwb, Cfa) and oceanic (Cfb) climates of the Argentinian pampa and coastal Uruguay (Figure 4B). A similar scenario is observed in South Africa, where a high representation of humid subtropical and subtropical highland (Cfa, Cwa, Cwb) and Mediterranean (Csb) climates occur. Additionally, the temperate oceanic (Cfb) climate zone is well represented in the invaded South African range (Figure 4C). In Australasia, I. pseudacorus has invaded the oceanic climates (Cfb) of New Zealand and southeastern Australia, with a slight expansion toward semiarid (BSk) and Mediterranean (Csa, Csb) regions (Figure 4D). Finally, in eastern Asia, the plant is found mostly across the humid temperate (Cfa) climates of eastern China and southern Japan and the continental climates (Dfa, Dfb, Dwa) of northern Japan and the Korean Peninsula (Figure 4E). This species is most common from sea level up to 300 m above sea level (m asl), but has been recorded at elevations over 1,000 m asl (Sutherland Reference Sutherland1990). In its invaded South African range, it occurs in the elevated interior, well above 1,200 m asl.

Figure 4. Köppen-Geiger climate zones occupied by Iris pseudacorus worldwide. The map was created based on I. pseudacorus distribution and the climate classification provided by Beck et al. (Reference Beck, Zimmermann, McVicar, Vergopolan, Berg and Wood2018). Pie charts represent the relative density of occurrence points within each range: (A) North America, (B) South America, (C) South Africa, (D) Australasia, (E) eastern Asia, and (F) Europe.
Land Use Associations
Iris pseudacorus occurs in habitats associated with water. It is found on the banks of lakes and rivers, in wetlands like ponds, streams, swamps, and marshes, but also in woodlands, open woods, and forest edges where the soil is moist or regularly flooded (Stone Reference Stone2009; Sutherland Reference Sutherland1990). Being disturbance adapted and commonly planted as an ornamental, it often occurs in human-modified habitats such as meadows, wet pastures, roadside ditches, irrigation channels, artificial wetlands, and gardens (Stone Reference Stone2009).
Soil Types
Iris pseudacorus usually grows in sites with high soil-water content, although it does not require constant submersion and can tolerate extended periods of drought (Jacobs et al. Reference Jacobs, Graves and Mangold2010a; Sutherland Reference Sutherland1990). This species is commonly found on water-deposited substrates such as silt, sand, gravel, and cobbles and is associated with calcareous, sandy loams, clay loams, and other soils derived from sandstone and schist (Mulqueen and Gleeson Reference Mulqueen and Gleeson1988; Stone Reference Stone2009; Sutherland Reference Sutherland1990). Iris pseudacorus occurs in fens and fen woodland, but is less frequent in areas of chalk (Sutherland Reference Sutherland1990). It can colonize a variety of soil types ranging from shingle, peat soils, permanently submerged organic and inorganic matter on gravel or sand, to orthodox gleys and shell hash (Gerwing et al. Reference Gerwing, Thomson, Brouard-John, Kushneryk, Davies, Lawn and Nelson2021; Sutherland Reference Sutherland1990). It persists in the higher zones of salt marshes and can tolerate soil acidity (at 0- to 30-mm depth) from pH 3.6 to 7.7 (Sutherland Reference Sutherland1990). Being a nitrophile, it prefers high-nutrient soils and grows well in eutrophic conditions (Stone Reference Stone2009; Sutherland Reference Sutherland1990; Tu Reference Tu2003).
Invasion History
Early occurrence records (17th to 19th century) of I. pseudacorus are scant and are mainly limited to herbarium records from the United Kingdom and France. The species has been introduced from the Palearctic ecozone into many areas worldwide, including the Neotropics, Afrotropics, Nearctic, Indomalaya, and Australasia. Its distribution area in the introduced range has been increasing over time and now comprises at least 13 countries (Howell Reference Howell2008; Masciadri et al. Reference Masciadri, Brugnoli and Muniz2010; USDA-APHIS 2013; Figure 5).

Figure 5. Records of occurrence of Iris pseudacorus in the Global Biodiversity Information Facility (GBIF) platform. Although this open database does not provide all existing records of Iris pseudacorus, it allows for the visualization of the increase over time in its geographic distribution (GBIF 2022). (A) 1600 to 1800, (B) 1900, (C) 2000, and (D) 2022. The shading of the dots represents the number of occurrence records, with darker (red) shading indicating many records and lighter (yellow) shading indicating fewer records.
Northern Hemisphere
Available records indicate that I. pseudacorus was documented outside its native range for the first time in the Nearctic ecozone. These introductions before 1800 were intentional, as I. pseudacorus was used as an ornamental pond plant to the United States (Champion et al. Reference Champion, Wong and Pagad2022; Natural History Museum 2014; Wells and Brown Reference Wells and Brown2000). While gardens are believed to be the most frequent source of introductions, I. pseudacorus was included on a list of ballast-water plants documented in New York and Philadelphia harbors (Torrey Botanical Club 1888), suggesting ship ballast water is a likely introduction source elsewhere.
The oldest report in this region corresponds to 1771, with I. pseudacorus being cultivated in Virginia (Wells and Brown Reference Wells and Brown2000). By 1800 it was noted in records of vascular plants introduced in low forest habitat along the upper Potomac tidal river at Mount Vernon, Virginia (Wells and Brown Reference Wells and Brown2000). By the 1860s, I. pseudacorus had escaped cultivation and had established along the Potomac River in Delaware and the Hudson River valley in New York. It was naturalized at Lake Ontario in the Great Lakes region in 1886, and by 1900, herbarium records place it in the Chesapeake Bay estuary. A voucher specimen collected in 1911 was reported as having escaped from household gardens in Newfoundland, Canada, and subsequently spread rapidly to swamps and other moist habitats, forming extensive stands (Cody Reference Cody1961; Fernald Reference Fernald1950). The species was well naturalized in southern Nova Scotia by at least 1915 (Fernald Reference Fernald1921; Roland Reference Roland1945).
Written records and herbarium specimens suggest the I. pseudacorus invasion in North American was a result of multiple independent introductions. It was established in far western Canadian wetlands in British Columbia in 1931 before the earliest documented occurrences in eastern and central Canada at Prince Edward Island (1939), Ontario (1940), Quebec (1943), and Manitoba (1953), but invasive spread was most rapid in Ontario (Cody Reference Cody1961). Invasions were also underway in the Pacific Northwest and California by 1948, and it was well established in the Merced River watershed and the San Francisco Bay region (California Academy of Sciences (https://www.calflora.org/) and California Department of Food and Agriculture (https://doi.org/10.15468/phc373) herbaria databases), but the first naturalized records in Montana were from the late 1950s (Preece Reference Preece1964). By the 1960s, I. pseudacorus was abundant in Canadian wetlands and in many regions of the United States (Hitchcock et al. Reference Hitchcock, Cronquist, Ownbey and Thompson1969; Raven and Thomas Reference Raven and Thomas1970), and it has now invaded 8 Canadian provinces and 48 of 50 states in the United States (Stoneburner et al. Reference Stoneburner, Meiman, Ocheltree, Nissen and Bradfield2021). In 1890, I. pseudacorus was first reported as introduced in a new Palearctic area, Japan, where it was introduced and cultivated intentionally for ornamental purposes (Kadono Reference Kadono2004).
Southern Hemisphere
In the Australasian ecozone, I. pseudacorus was reported growing wild in New Zealand for the first time in Lower Hutt in 1938 (Te Waihora Co-Governance 2019), and in Australia by 1945, according to herbarium specimens (AVH 2022). In New Zealand, it has since spread to many other parts of the country with dense, severe infestations occurring on the lower Avon River in Christchurch and in particular the lower Waikato River catchment (Maw Reference Maw2010; Wildland Consultants 2011). Iris pseudacorus was first recorded in the Waikato region in January 1990 at Lake Hakanoa, Huntly, and this is believed to be the source population of the current infestation along the lower Waikato River catchment (Champion et al. Reference Champion, de Winton and de Lange1993). Aerial surveys conducted along the Waikato River showed that up to 50 km of riverbank and river island shoreline contained I. pseudacorus (Wildland Consultants 2011). High seed production levels and nitrogen runoff from pastures into the Waikato River are suggested to have exacerbated the spread of I. pseudacorus within the Waikato region (McGrannachan and Barton Reference McGrannachan and Barton2019; Wildland Consultants 2011). Because of its threat to native biodiversity and natural ecosystems, I. pseudacorus has been classified as an unwanted organism in New Zealand under the Biosecurity Act (1993). It is also prohibited from being sold and distributed in New Zealand due to its listing as a National Plant Pest Accord Species (McGrannachan and Barton Reference McGrannachan and Barton2019).
The first record of I. pseudacorus in the Neotropics was made in 1931, when it was documented in Buenos Aires, Argentina (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020; Global Invasive Species Database 2022). Distribution studies have shown that after its introduction, the number of invaded localities increased significantly over the years (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020). However, the general status of the invasion in the country was unknown until recent years (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020). After the first report in Buenos Aires, I. pseudacorus was reported in 1964 in the northwest of Argentina, in Jujuy Province. Currently, I. pseudacorus is present across at least eight provinces, throughout a diversity of habitats, ecoregions, water conditions, and latitudes (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020). In Argentina, it is catalogued as an alien invasive species (Kalesnik and Malvárez Reference Kalesnik and Malvárez2004) and is currently a Restricted Species with mandatory control under the Conservation of Biodiversity Program (Ministry of Environment and Sustainable Development of Argentina 2022). The species is also reported on the coastal region of Uruguay, in the cities of Montevideo, Maldonado, Rocha, and San José, where it is considered invasive (InBUy 2011).
The first naturalized population of this plant in the Afrotropics was reported in 2004 in South Africa, where it was growing along the Vaal River in Gauteng Province (Jaca and Mkhize Reference Jaca and Mkhize2015; Sandenbergh et al. Reference Sandenbergh, Petruzzella and Coetzee2024). While the weed’s invasion in South Africa is still in the “lag” phase (Blackburn et al. Reference Blackburn, Pyšek, Bacher, Carlton, Duncan, Jarošik, Wilson and Richardson2011), the number of I. pseudacorus infestation records has increased substantially since it was first recorded. About a decade after the first report, Jaca and Mkhize (Reference Jaca and Mkhize2015) reported 23 new infestations in South Africa. The number of records of I. pseudacorus in South Africa continues to increase rapidly, with a recent study reporting more than 110 confirmed localities in all provinces except the arid Northern Cape (Sandenbergh et al. Reference Sandenbergh, Petruzzella and Coetzee2024). Iris pseudacorus has been categorized as a 1A invader under the National Environmental Management: Biodiversity Act and is listed as an eradication target (Jaca and Mkhize Reference Jaca and Mkhize2015).
The wide dispersion of I. pseudacorus over the introduced range shows a great adaptability in this species to invade under a wide range of environmental conditions, which is alarming due to the profound modification it produces in invaded ecosystems and the economic damage it causes. Due to the scarcity of ecological studies of I. pseudacorus in the Southern Hemisphere, a Global South collaboration alliance was initiated to study its distribution and ecology and to develop appropriate management strategies (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020).
Life-Form and Life History
Iris pseudacorus is categorized as a telmatophyte or helophyte according to the Raunkiaer system (Raunkiaer Reference Raunkiaer1934), as it is a perennial plant that almost always has its rhizomes and resting buds under the waterline. Nonetheless, the plant can remain in dry soil for long periods (Jacobs et al. Reference Jacobs, Graves and Mangold2010a; Sutherland Reference Sutherland1990). Leaves have aerenchyma, and they are always above the waterline. Iris pseudacorus typically occurs on high ground on the shore and wetlands, because seeds and seedlings require exposed soil conditions (Coops and Van der Velde Reference Coops and Van Der Velde1995).
Iris pseudacorus individuals take 2 (unpublished data) to 3 yr (Tyron Reference Tyron2006) to mature before flowering, but this can vary as a result of different growing conditions. Flowers typically bloom from April to July in the Northern Hemisphere (Good Reference Good1986; Lui et al. Reference Lui, Butler, Allen, Snyder, da Silva, Brownson and Ecclestone2010; Sutherland Reference Sutherland1990), although the timing varies among climate zones and hydrological settings. Bloom time is from September to December in the Southern Hemisphere (Gervazoni Reference Gervazoni2024; Sandenbergh Reference Sandenbergh2021).
After the reproductive period, depending on climatic and environmental conditions, the plant may remain green over winter (King County Noxious Weed Control Program 2009). Above- and belowground biomass increases seasonally, with the highest values of aboveground biomass in summer and negligible in winter (Larue et al. Reference Larue, Korboulewsky, Wang and Mevy2010). The seasonal accumulation of storage materials in the belowground organs of the plant result in the rhizomes forming a series of annual segments or “bulges,” providing a record of the plant’s growth history (Rakhimov et al. Reference Rakhimov, Kholmuradova and Shoyakubov2006; Sutherland Reference Sutherland1990). The plant biomass of I. pseudacorus in the native range was estimated at 0.7 to 0.8 kg m− 2 (Falińska Reference Falińska1986; Sutherland Reference Sutherland1990), which is lower than the biomass produced by other coexisting emergent aquatic macrophytes (Neiff Reference Neiff1990).
Dispersal and Establishment
Propagules of I. pseudacorus are produced both sexually and asexually, through the fragmentation of rhizomes and the production of seeds (Gaskin et al. Reference Gaskin, Pokorny and Mangold2016; Lamote et al. Reference Lamote, Roldán-Ruiz, Coart, De Loose and Van Bockstaele2002; Sutherland Reference Sutherland1990). Clonal reproduction by rhizome was initially considered the primary mode of spread for I. pseudacorus (Barrett Reference Barrett2015). Rhizome fragments are adapted for hydrochory and are often spread downstream after flooding events (Sutherland Reference Sutherland1990), and it was observed that rhizome fragments of 2 cm can develop into a new plant (Jaca Reference Jaca2013). However, when reproducing sexually, I. pseudacorus populations produce a vast number of highly buoyant seeds, adapted to dispersal by water (Coops and Van der Velde Reference Coops and Van Der Velde1995; van den Broek et al. Reference van den Broek, van Diggelen and Bobbink2005), and studies carried out in both the native and introduced ranges compared populations of I. pseudacorus and found genetic divergence between them, indicating that the propagation and spread of this species are predominantly a result of sexual reproduction (Gallego-Tévar et al. Reference Gallego-Tévar, Grewell, Gaskin and Castillo2024; Gaskin et al. Reference Gaskin, Pokorny and Mangold2016; Lamote et al. Reference Lamote, Roldán-Ruiz, Coart, De Loose and Van Bockstaele2002).
The relative employment of each reproductive strategy appears to be context specific, and the data so far suggest that introduced I. pseudacorus populations employ sexual reproductive strategies to a greater degree than native I. pseudacorus populations (Gaskin et al. Reference Gaskin, Pokorny and Mangold2016; Lamote et al. Reference Lamote, Roldán-Ruiz, Coart, De Loose and Van Bockstaele2002). However, Gallego-Tévar et al. (Reference Gallego-Tévar, Grewell, Gaskin and Castillo2024) found higher genetic diversity in the native range (Spain) than was the case for the invaded range (California).
In tidal wetlands, floating wrack mats composed of senescent plant debris and live plant propagules are significant vectors of macrophyte seed dispersal into wetlands (Huiskes et al. Reference Huiskes, Koutstaal, Herman, Beeftink, Markusse and de Munck1995). Tide-transported wrack mats are often deposited at high-elevation tide strandlines where I. pseudacorus regularly occurs, and seed burial by wrack mats can limit seedling recruitment of macrophytes. Castillo et al. (Reference Castillo, Gallego-Tévar and Grewell2023) found I. pseudacorus seedling recruitment can be limited by up to 8-cm depth of seed burial by wrack, but quiescent seeds persist in the seedbank and can germinate and emerge in disturbance-generated gaps or as wrack decomposes.
Anthropogenic dispersal also plays an important role in facilitating the spread of I. pseudacorus due to its high ornamental value. Propagules are often exchanged by horticulturalists and sold in nurseries and online, allowing for long-distance dispersal of propagules into novel environments, aggravating the risk of new establishments and subsequent invasions (Mercado Libre 2022; Raghu et al. Reference Raghu, Morin and Pratt2017). In South Africa, for example, the species was promoted for use in the trout (Oncorhynchus mykiss) farm industry, as well as for phytoremediation and the prevention of soil erosion, further promoting its dispersal and establishment (Sandenbergh et al. Reference Sandenbergh, Petruzzella and Coetzee2024; personal observations). “Escaping” cultivation has been reported as a main pathway for the establishment and subsequent invasion of I. pseudacorus (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021; Morgan et al. Reference Morgan, Berent and Fusaro2020; Sandenbergh et al. Reference Sandenbergh, Petruzzella and Coetzee2024).
Once dispersed, I. pseudacorus propagules, being disturbance adapted, can take advantage of undesirable conditions in novel environments. Disturbance favors I. pseudacorus establishment, with fire and flooding events aiding their dispersal and establishment (Stone Reference Stone2009). Anthropogenic disturbances such as eutrophication, habitat modification, and management activities can further promote the establishment of I. pseudacorus (Stone Reference Stone2009; Sutherland Reference Sutherland1990). Such events and the subsequent knock-on effects (i.e., indirect consequences) they have on plant communities can decrease the biotic resistance of the community while increasing resource availability, creating favorable conditions for I. pseudacorus establishment (Stone Reference Stone2009; Tu Reference Tu2003). In contrast, soil drainage and pasture improvement have been observed to hinder the spread of the invasion (Sutherland Reference Sutherland1990).
Growth and Development
Morphology
There are several diagnostic characteristics that describe the morphology of I. pseudacorus. In North America, identification can be confirmed in the reproductive stage, as I. pseudacorus is the only iris with completely yellow flowers naturalized in wet environments away from gardens (Goodridge et al. Reference Goodridge, Bixby, Winter-Gorsline, Tu, Anderson, Harber, Douglas, McMahan and Chan2011; Henderson Reference Henderson2002). Taxonomic descriptions from published floras vary slightly in size ranges of specific characters based on specimens from local regions, but descriptions for this species, particularly for floral and other reproductive traits, are comparable for identification in the native and invaded range (Flora Europaea 2022; Henderson Reference Henderson2002; Klinkenberg Reference Klinkenberg2010; Lui et al. Reference Lui, Butler, Allen, Snyder, da Silva, Brownson and Ecclestone2010; NatureGate 2022; Stone Reference Stone2009; Sutherland Reference Sutherland1990; Tutin et al. Reference Tutin, Heywood, Burges, Moore, Valentine, Walters and Webb1980; World Flora Organization 2022). Descriptions that follow reflect cross-referencing among published descriptions.
Iris pseudacorus is an emergent aquatic angiosperm from the rhizomatous, beardless subgenera Limniris sect. Limniris (Wilson Reference Wilson2006). Aboveground, the plant typically ranges in height from 0.5 to 1.5 m tall (Figure 1G). However, overall height of I. pseudacorus can vary considerably across environmental gradients, including in the native range, and has been observed to reach up to 2.17 m when growing in light-limited conditions inside forests (Gervazoni Reference Gervazoni2024). It produces a simple erect stem that is solid and often has one branch. The erect flattened green leaves, up to 10 per ramet, with a dominant raised midrib and parallel veins are primarily basal, linear to lanceolate; they emerge from the soil surface in a fan-shape arrangement, are typically 2- to 4-cm wide and 40-cm to 1.5-m long and sword-shaped with pointed tips and may be downward-curved near the top (Negrut et al. Reference Negrut, Nicolin and Neacșu2018; Sutherland Reference Sutherland1990).
The plant has bisexual pale to bright yellow flowers (8- to 10-cm diameter; flowers larger than 10 cm across are reported in Finland) (NatureGate 2022). Flowers are radially symmetrical, actinomorphic, and grouped as an inflorescence. The cyme-like inflorescences may each include 4 to 12 (often 5 to 10) flowers partially enclosed by inner and outer green spathes (bracts) with brown margins, the outer being strongly keeled and the inner unkeeled. There are 4 to 12 flowers per inflorescence, which are arranged on round, erect 2- to 5-cm-long peduncles (stalks) that are often branched (Figure 1 A–D). The flowers have six-clawed, yellow perianth segments that include two different whorls, including three large lanceolate to ovate or suborbiculate downward-spreading sepals (petal-looking) and three smaller erect upward petals that are narrowed in their midsection. External tepals are 40 to 80 by 20 to 45 mm, with a broadly ovate blade, sharply attenuated at the base and recurved, and bright yellow in color, with short radiating brownish lines. Internal tepals are 10 to 30 by 3 to 8 mm, erect, yellow, spatulate to oblanceolate, obtuse, and shorter than stigmatic blades (Lui et al. Reference Lui, Butler, Allen, Snyder, da Silva, Brownson and Ecclestone2010). The showiest parts of the flower are the external tepals and the petaloid styles (Figure 1B).
The inferior ovary is 12- to 20-mm long, triangular in cross-section with concave sides; the pedicel is 3- to 5-cm long. There are three keeled styles and three stigmas per flower. The style is filiform, ending in three petal-like yellow branches arched over the external tepals and a small rounded stigma with a prominent tongue on the underside (Figure 1E). The three stamens are hidden under the three style branches (Figure 1C). Floral tubes are 6- to 8-cm long with no constriction to the ovary. Each large yellow sepal has a darker yellow basal signal patch and short brown to purple lines or flecks that serve as pollinator nectar guides. Fruit type is a capsule (seed pod), prismatic to oblong-ovoid, 2.5- to 8.5-cm long with a 5-mm beak, and most often described as three-angled with an obvious groove at each angle (but see World Flora Organisation (2022) and Gleason and Cronquist (Reference Gleason and Cronquist1991) for reports of six-angled capsules). Developing capsules are yellow-green to green in color and can be dull to glossy in appearance, becoming dark brown and dehiscent when mature and the capsule splits to release seeds (Figure 1F). Each locule of the capsule contains rows of smooth, flattened, disk-shaped (6 to 7 mm), lustrous corky seeds. When mature, the capsule splits and releases seeds which, when growing in or near to waterbodies, disperse directly into or near the shallow water. Seeds have a hard seed coat enclosing a gas space, enhancing their buoyancy in water and dispersal by hydrochory. Chromosome numbers reported are 2n = 24, 30, 32, and 34 (Choi et al. Reference Choi, Weiss-Schneeweiss, Temsch, So, Myeong and Jang2020; Dyer et al. Reference Dyer, Ellis, Lithgow, Lowther, Mason and Williams1976; Henderson Reference Henderson2002).
Below the surface, I. pseudacorus can produce a woody crown below the leaf base, from which dense, freely branching pink rhizomes (1- to 5-cm diameter) form extensive clumps that can protrude at the surface and are often exposed by erosion. White adventitious roots can form above the soil at the base of the leaves (Jacobs et al. Reference Jacobs, Graves and Mangold2010a; Sutherland Reference Sutherland1990; Yu et al. Reference Yu, Yue, Wu, Yang, Fan, Wang and Hu2022). Fleshy roots (10- to 30-cm long) extend into the soil (Sutherland Reference Sutherland1990; Figure 1A). Belowground biomass, comprising rhizomes and roots, may represent greater than 99% of the total biomass, which is evidence of this weed’s resilience and ability to compete for space and resources (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021; Mopper et al. Reference Mopper, Wiens and Goranova2016; Sutherland Reference Sutherland1990). Rhizomes make up most of the belowground biomass, with roots representing just greater than 39% (Larue et al. Reference Larue, Korboulewsky, Wang and Mevy2010).
Stress Tolerance
Iris pseudacorus is an obligate wetland species that is often observed at the water’s edge of inland lakes, rivers, and canals, or in dry wetland soil, suggesting a degree of tolerance to water-level fluctuation. The species can tolerate water with low levels of oxygen and can survive under anoxic conditions for extended periods of time (up to and exceeding 8 wk) (Hetherington et al. Reference Hetherington, Hunter and Crawford1982; Hunter et al. Reference Hunter, Combs and George2001; Mulqueen and Gleeson Reference Mulqueen and Gleeson1988; Sutherland Reference Sutherland1990). Anoxia in plants like Iris pseudacorus typically occurs due to environmental conditions such as prolonged waterlogging, total submergence, or ice-encasement. However, Iris pseudacorus stores its carbohydrate reserves in the form of fructans, primarily within its rhizomes (Schlüter and Crawford Reference Schlüter and Crawford2001), and rhizomes and roots exposed to inundation have aerenchymous tissue that provides an adaptation to low-oxygen conditions in flooded environments (Yu et al. Reference Yu, Yue, Wu, Yang, Fan, Wang and Hu2022). Reserves in belowground tissues may also allow the plants to survive extended drought (Fitter and Hay Reference Fitter and Hay2012). Greater allocation of biomass to belowground structures than shoots could also provide some degree of protection to prevent juvenile plants from being washed away in high flows (Sutherland Reference Sutherland1990; Whitehead Reference Whitehead1971).
Deep water can prevent seed germination (Lenssen et al. Reference Lenssen, ten Dolle and Blom1998) and limit growth of seedlings (Coops and Van Der Velde Reference Coops and Van Der Velde1995), which explains its common occurrence in shallow water or wet soils. However, in New Zealand, the plant has occupied water depths from 0 to 0.8 m (Tanner et al. Reference Tanner, Clayton and Coffey1990), and in Montana, it was found in water depths up to 1 m (Preece Reference Preece1964).
Iris pseudacorus tolerates coastal habitats, including tidal freshwater, brackish water (Dutton and Thomas Reference Dutton and Thomas1991; Grewell et al. Reference Grewell, Gallego-Tévar, Gillard, Futrell, Reicholf and Castillo2021; Strong and Kelloff Reference Strong and Kelloff1994), and salt marshes (reviews by Sutherland Reference Sutherland1990; Tu Reference Tu2003). Sutherland and Walton (Reference Sutherland and Walton1990) observed that I. pseudacorus in high-elevation Irish tidal wetlands had more and longer leaves and high rhizome terminal bud survival compared with plants in low-elevation sites where frequency and depth of tidal inundation were higher.
Global warming and associated sea-level rise have raised questions about the physiological tolerances of invasive I. pseudacorus in estuarine wetlands in the naturalized range (Gerwing et al. Reference Gerwing, Thomson, Brouard-John, Kushneryk, Davies, Lawn and Nelson2021; Grewell et al. Reference Grewell, Gallego-Tévar, Gillard, Futrell, Reicholf and Castillo2021). Grewell et al. (Reference Grewell, Gallego-Tévar, Gillard, Futrell, Reicholf and Castillo2021) conducted greenhouse experiments to evaluate the response of pre-reproductive I. pseudacorus populations (the colonizing life stage) from the San Francisco Bay delta estuary to increasing salinity, inundation, and their interaction. Growth, biomass allocation, and morphological, physiological, and biochemical traits were evaluated in response to freshwater to marine salinity levels. Results indicated that I. pseudacorus populations at the colonizing life stage were highly vulnerable to increasing salinity, even at 17 ppt brackish concentration. While the species showed tolerance to inundation, increasing salinity limited its capacity to acclimate to greater inundation. Experimental results from the greenhouse study with California populations inform risk assessments in light of climate change and suggest efforts to control invasive estuarine populations should prioritize freshwater tidal habitat, because successful growth and spread is best supported in these areas (Grewell et al. Reference Grewell, Gallego-Tévar, Gillard, Futrell, Reicholf and Castillo2021). However, dense populations of I. pseudacorus have been observed in marine wetlands of the Punta Lara Reserve in Argentina (PG, personal observations), suggesting possible genotypic variation in salinity tolerance among invaded ranges.
Phenology and Reproduction
The timing of phenological development and life stage transitions of I. pseudacorus can be expected to vary widely given climate and other environmental conditions across the broad extant range from 68°N to 28°S, and with differences in altitude (sea level to 1,315 m asl; Welsh et al. Reference Welsh, Atwood, Goodrich and Higgins1987), climate, and hydrological regimes all playing a role. Even so, there are many common aspects of this species’ life history.
The perennial life cycle of a newly establishing I. pseudacorus plant begins with the germination of a seed or the sprouting of an established rhizome fragment. Riverine and other wetland types occupied by I. pseudacorus are subjected to regular disturbance regimes (flooding, bank erosion, etc.) that create gaps that promote its rapid colonization of systems (Barrat-Segretain and Bornette Reference Barrat-Segretain and Bornette2000; Grewell et al. Reference Grewell, Futrell, Iannucci and Drenovsky2019; Pyšek and Prach Reference Pyšek and Prach1993).
Water-dispersed seeds are often deposited along high-water lines where they are most likely to germinate and establish as seedlings (Tu Reference Tu2003). During the seedling stage, inundation reduces I. pseudacorus seedling growth, but seedlings recover soon after (Coops and Van Der Velde Reference Coops and Van Der Velde1995; Lenssen et al. Reference Lenssen, Menting, van der Putten and Blom1999). Thomas (Reference Thomas1980) found that I. pseudacorus plants experiencing short inundation had higher growth than plants with long inundation on the Potomac River near Washington, DC, while in Montana, Preece (Reference Preece1964) observed more vigorous growth in I. pseudacorus growing in 1-m-deep water than plants that were not inundated.
For established perennial stands, new seasonal growth of I. pseudacorus commences with resprouting from rhizome bud banks or new emergence from the seedbank during the early spring season (Fitter and Hay Reference Fitter and Hay2012; Jacobs et al. Reference Jacobs, Graves and Mangold2010b). Annual rhizome growth typically continues through each growing season until branching begins after flowering (Jacobs et al. Reference Jacobs, Graves and Mangold2010b). Sutherland (Reference Sutherland1990) studied the species in Ireland and reported that when rhizomes reach about 10 yr in age, they fragment and disperse via hydrochory to form new clones. However, in the native range, many stands are observed to persist and flower for 30 to 40 yr with continued incremental growth (Whitehead Reference Whitehead1971), and some extant naturalized population patches in the invasive California range have been present for at least 50 yr (Consortium of California Herbaria 2022; BG, personal observations). While genetic studies reveal the primary dispersal and colonization is from seeds (Gaskin et al. Reference Gaskin, Pokorny and Mangold2016), local spread by radially spreading clones produces dense stands that displace resident vegetation (Falińska Reference Falińska1986; Preece Reference Preece1964; Thomas Reference Thomas1980).
During pre-reproductive growth, the plants also store carbohydrates in roots, leaf bases, and pre-flowering shoot tissues (Grewell et al. Reference Grewell, Gallego-Tévar, Gillard, Futrell, Reicholf and Castillo2021; Sutherland Reference Sutherland1990). Seasonal leaf growth proceeds from the leaf base–rhizome interface. However, in most temperate and colder areas, leaves die back seasonally. Iris pseudacorus plants remain in a pre-reproductive life stage through their early years of colonization when there is significant investment of resources toward belowground growth and carbon reserves (Jacobs et al. Reference Jacobs, Graves and Mangold2010a; Sutherland Reference Sutherland1990).
Following emergence of fan-shaped leaf clusters, mature plants begin to flower. Like all other life-stage transitions, timing of flowering each year is dependent on local climate and hydrologic conditions. In areas with mild winter climates, leaf growth from rhizomes can occur all year (Jacobs et al. Reference Jacobs, Pokorny, Mangold and Graves-Medley2011). Pollination begins during flowering, with capsules expanding and filling with seeds. The seeds then mature and disperse, completing the plants’ seasonal life cycle.
Floral Biology
Sexual reproduction in I. pseudacorus occurs by obligate outcrossing (Fryxell Reference Fryxell1957), and as in many other species in the same genus, flowers are adapted for large pollinators. The nectar produced by the flower is situated outside the whorl of stamens (Sutherland Reference Sutherland1990). When insects visit the flowers, they pass between the stamens and outer tepals, making contact with petaloid stigmas and stamens depending on the insect size and the flower morph: bombophila and syrphophyla (Sutherland Reference Sutherland1990). In the bombophila flower, the petaloid stylar branches are situated 6 to 10 mm above the corresponding outer perianth segment and are pollinated by bumblebees large enough to enter in contact with stamens and stigma (Figure 1 A and B). In the syrphophyla morph, the petaloid stylar branches are situated close to the outer perianth segment, thus much smaller insects, such as syrphid flies, act as effective pollinators (Good Reference Good1986).
Among the floral visitors of this species, bees (Hymenoptera) and long-tongued flies (Diptera), are the most frequently mentioned (McGrannachan and Barton Reference McGrannachan and Barton2019; Sutherland Reference Sutherland1990). Observations made in the native range included mainly bumblebees of the genus Bombus, as well as Apis mellifera (Apidae) and Osmia rufa (Megachilidae) bees. Syrphid flies, including Rhingia campestris, Episyrphus balteatus, and Eristalis spp., were also included, as were the scathophagids Scatophaga stercoraria; the Hepialidae moth Hepialus humuli; and the Noctuidae moths Apamea monoglypha, Noctua pronuba, Ochropleura plecta, and Apamea crenata (Good Reference Good1986). In the introduced range, the pollinators associated with I. pseudacorus include Bombus bumblebees, A. mellifera bees, the soldier flies Hedriodiscus pulchur (Stratyomidae), and some coleopteran species, including the coccinellid predator Eriopis connexa (Stone Reference Stone2009).
Seed Production
In the native range, an average of five capsules per plant has been documented by Sutherland (Reference Sutherland1990), along with a mean seed production per capsule that varied between 32 and 46 at different sites. Additionally, Coops and Van Der Velde (Reference Coops and Van Der Velde1995) reported a mean of 47 seeds per reproductive stem. In the Afrotropics (South Africa), a mean of 7.9 flowers and 2 seed capsules were produced per reproductive stem, with 42.5 (± 1.9) seeds produced per seed capsule, resulting in 773.5 seeds produced per square meter (Sandenbergh et al. Reference Sandenbergh, Petruzzella and Coetzee2024). In the Neotropical invaded range (Argentina), preliminary results show an average of 3.44 flowers and 4.32 capsules per stem, and a seed production of 65.54 (± 32.71) seeds produced per capsule (PG, unpublished data).
These results show an increased production of flowers, capsules, and seeds per capsule for I. pseudacorus in the introduced range. Enhanced reproductive potential for this species in Argentina and South Africa could be explained by different hypotheses, including the more effective use of resources by invasive species in the introduced range as a result of “escaping” predation by the natural enemies with which they have coevolved (Keane and Crawley Reference Keane and Crawley2002; Liu et al. Reference Liu, Stiling, Pemberton and Peña2006; Puliafico et al. Reference Puliafico, Schwarzlaender, Harmon and Hinz2008).
Seedbanks
Iris pseudacorus forms soil seedbanks, but longevity of these belowground banks is uncertain, likely varies with environmental conditions, and may be impacted by global environmental changes. Sutherland (Reference Sutherland1990) did not observe seedlings in most native habitats visited, but it is possible that conditions during the short period of observation were not sufficient for seeds to break dormancy and emerge. In France, I. pseudacorus was abundant in a wet meadow, but was absent from the soil seedbank (Vecrin et al. Reference Vecrin, Grevilliot and Muller2007), while in the Netherlands, I. pseudacorus emerged from 25% of soil seedbank studies in an emergence assay, although the species was present in standing vegetation at 84% of sampled fens (van der Valk and Verhoeven Reference van der Valk and Verhoeven1988). In the invaded range, Leck and Leck (Reference Leck and Leck2005) recorded seedbank emergence in freshwater tidal wetlands in Delaware, USA. Along Vancouver Island’s Courtenay River in British Columbia, Canada, I. pseudacorus has formed “considerable viable seed banks” that continuously recruit thousands of emergent seedlings that have been targeted for removal by weed managers (Evergreen 2007).
Seed Viability and Germination
Iris pseudacorus allocates significant resources to seed production. Seed germination is a critical life stage that is often overlooked, but can be key to the spread of invasive plants (Gioria and Pyšek Reference Gioria and Pyšek2017). Contradictory accounts in the literature, typically reported in reviews or anecdotal accounts, have often not been supported with methodological details (see Gillard et al. Reference Gillard, Castillo, Mesgaran, Futrell and Grewell2022). For example, Baskin and Baskin (Reference Baskin and Baskin2014) claim the species has morphophysiological dormancy that requires cold stratification without scarification. However, Guppy (Reference Guppy1912) reported that I. pseudacorus seeds from England did not present dormancy and germinate rapidly, although Suzuki and Yamagata (Reference Suzuki and Yamagata1980) achieved germination only after removing the seed cap or damaging the seed coat. Crocker (Reference Crocker1906) documented 97% germination of seeds with caps removed within 1 mo. A review by Sutherland (Reference Sutherland1990) suggests seeds from the Netherlands achieved 25% germination during 6 wk in drained soil, while 40% to 48% germination was recorded for non-scarified seeds from the United Kingdom. Germination of I. pseudacorus is hypogeal and cryptocotylar (unpublished data). Vivipary has been observed in the field in California (Gillard et al. Reference Gillard, Castillo, Mesgaran, Futrell and Grewell2021), Argentina, and South Africa (Gervazoni Reference Gervazoni2024; Sandenbergh Reference Sandenbergh2021; Figure 6), whereby seeds have germinated inside recently dehisced seed capsules, providing evidence that dormancy may not be required. Accordingly, in Argentina, a germination experiment showed that seeds with a cold pretreatment for dormancy breaking, had a lower germination percentage than the control (Gervazoni Reference Gervazoni2024; Gervazoni et al. Reference Gervazoni, Sosa, Coetzee, Cabañas and Franceschini2023).

Figure 6. Vivipary in (A) South African, (B) Argentinian, and (C) Californian Iris pseudacorus seed capsules (Photos: E Sandenbergh, Johannesburg, 2020; P Gervazoni, Misiones, 2023; J Futrell, Brannon Island, 2018).
The germinability of I. pseudacorus seeds has been tested by different authors in different regions under varying conditions. In the native range (Netherlands), germination of I. pseudacorus was assessed by Coops and Van Der Velde (Reference Coops and Van Der Velde1995) at 20 to 25 C, with a photoperiod of 12-h light/12-h dark, and was reported as relatively low, with only around 25% of the tested seeds germinating. A more recent study conducted in Germany showed a germination percentage of 100% under alternating temperature conditions of 22/14 C and 14 h of light, although this percentage decreased to 19% under constant temperature conditions (22 C) (Rosbakh et al. Reference Rosbakh, Phartyal and Poschlod2020).
In the invaded range, greenhouse experiments performed in California with an average temperature of 21.4 ± 5.1 C, showed that, under freshwater conditions, approximately 96% of seed could germinate—a proportion that decreased with increased aqueous salinity (Gillard et al. Reference Gillard, Castillo, Mesgaran, Futrell and Grewell2021). Additional tests performed in the same region determined 28.2 ± 0.5 C as optimal and 41.0 ± 1.7 C as the maximum temperature at which germination could occur. The study showed that although a combination of fluctuating temperatures and light is a key factor to achieve high germination rates, seeds can also germinate in conditions of constant temperature, as well as in the dark (Gillard et al. Reference Gillard, Castillo, Mesgaran, Futrell and Grewell2022). Regarding other regions of the invaded range, Sandenbergh et al. (Reference Sandenbergh, Petruzzella and Coetzee2024) also reported high germination rates (approximately 83% of germination with a cold pretreatment) for seeds collected at different sites across South Africa. In Argentina, 60.56% of germination was obtained for seeds with the cold pretreatment and 84.17% for the control group. Seed viability was assessed by Gaskin et al. (Reference Gaskin, Pokorny and Mangold2016), who reported 99.1% seed viability of seeds from Montana.
These results demonstrate that I. pseudacorus is a plant with a broad capacity for germination, being able to produce seedlings under different environmental conditions. Additional germination experiments are being conducted in the introduced (Argentina) and native (Belgium) ranges in order to achieve a more comprehensive dataset for the germination potential of I. pseudacorus (unpublished data).
Sexual reproduction can be expected to be increasingly more important as climate change drives changes in water levels that promote increased seedbank emergence from greater exposure of moist soil in wetlands. Iris pseudacorus seeds germinate best in moist soil, rather than water-logged soils (Coops and Van Der Velde Reference Coops and Van Der Velde1995; Lenssen et al. Reference Lenssen, ten Dolle and Blom1998; Thomas Reference Thomas1980). Climate warming is altering cues that drive germination, prompting the need for a better understanding of how I. pseudacorus will respond to continuing environmental changes. Gillard et al. (Reference Gillard, Castillo, Mesgaran, Futrell and Grewell2022) experimentally evaluated the effects of stratification, light, seed coat presence or absence, and constant versus alternating temperatures on the germination of I. pseudacorus seeds from California and used the results in a thermal time model. Prior exposure to cold or warmth was not a prerequisite for germination, seeds could germinate with or without the seed coat, and in light or dark conditions. The highest germination rates were achieved with exposure to diurnally fluctuating temperatures (Gillard et al. Reference Gillard, Castillo, Mesgaran, Futrell and Grewell2022). At high temperatures (36 C), seeds from multiple study populations proved viable and germinated. Collectively, results reveal a broad capacity of I. pseudacorus for germination that will likely support continued invasiveness where environmental conditions are changing, including under higher temperatures predicted with global warming (Gillard et al. Reference Gillard, Castillo, Mesgaran, Futrell and Grewell2022).
Vegetative Reproduction
Vegetative reproduction by I. pseudacorus occurs through the fragmentation of rhizomes, as detailed in “Dispersal and Establishment” and “Phenology and Reproduction.”
Population Dynamics
Population Genetics
At present, the genetic and geographic origins of invasive I. pseudacorus populations are not known (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021), but a few studies regarding the population genetics of I. pseudacorus are available (Gallego-Tévar et al. Reference Gallego-Tévar, Grewell, Gaskin and Castillo2024; Gaskin et al. Reference Gaskin, Pokorny and Mangold2016; Lamote et al. Reference Lamote, Roldán-Ruiz, Coart, De Loose and Van Bockstaele2002). As an invasive aquatic plant, I. pseudacorus is expected to demonstrate low levels of genetic variation, as many aquatic invasive angiosperms primarily reproduce asexually. However, Lamote et al. (Reference Lamote, Roldán-Ruiz, Coart, De Loose and Van Bockstaele2002), Gaskin et al. (Reference Gaskin, Pokorny and Mangold2016), and Gallego-Tévar et al. (Reference Gallego-Tévar, Grewell, Gaskin and Castillo2024) found populations of I. pseudacorus to be more genotypically diverse than previously anticipated, suggesting the species may employ sexual modes of reproduction to a much higher degree than was thought to be the case. While Lamote et al. (Reference Lamote, Roldán-Ruiz, Coart, De Loose and Van Bockstaele2002) observed distinct grouping patterns in Bulgarian I. pseudacorus populations as a result of geographic barriers, their results suggest that both sexual and asexual reproduction is occurring in each of the separate populations. Gaskin et al. (Reference Gaskin, Pokorny and Mangold2016) reported that I. pseudacorus populations in the northwest United States reproduce almost solely by seed, with 98% unique genotypes observed throughout the invasion, and Gallego-Tévar et al. (Reference Gallego-Tévar, Grewell, Gaskin and Castillo2024) reported high rates of intrapopulation genetic variance within both native (Spain) and introduced (California) I. pseudacorus populations, with the former demonstrating higher levels of genetic diversity than the latter.
Similar results were found for South African I. pseudacorus populations, with ∼98% unique genotypes observed and a high level of genetic diversity present between and within populations (Sandenbergh Reference Sandenbergh2021). Population genetics studies are being conducted on I. pseudacorus populations in Argentina and New Zealand to elucidate aspects of the genetic composition and diversity of populations in other regions of the introduced range (Sandenbergh Reference Sandenbergh2021). The results thus far are in agreement and suggest that I. pseudacorus spreads primarily through the production and dispersal of sexually produced seeds. As I. pseudacorus was formerly thought to reproduce predominantly by clonal rhizome fragmentation, these results provide important information for management and control organizations, whose efforts should be focused on preventing or reducing seed production in the field.
Patch Composition and Competition
Depending on the stage of invasion, in the introduced range, I. pseudacorus populations can occur as solitary plants, small patches, and large, monospecific stands. In the eastern United States with a long history of invasion, I. pseudacorus occurs in red maple (Acer rubrum L.)-river birch (Betula nigra L.)-green ash (Fraxinus pennsylvanica Marshall) swamp forest associations; shrub–swamp communities with bog-myrtle (Myrica gale L.), swamp rose (Rosa palustris Marshall), and hazel alder ]Alnus serrulata (Aiton) Willd.]; natural and constructed freshwater marshes in association with rice cutgrass [Leersia oryzoides (L.) Sw.], pickerelweed (Pontederia cordata L.), and Typha spp.; marshes and swamps with emergent macrophytes such as Appalachian arrowhead [Sagittaria australis (J.G. Sm.) Small] and Carex spp. and with trees typical in swamp forests (see review in Stone Reference Stone2009). In West Virginia, southern USA, it occurs in diverse fringed sedge table wetlands with mostly native Carex spp., club-rushes (Scirpus spp.), and soft rushes (Juncus spp.); in regularly inundated floodplain and riparian sycamore(Platanus sp.)–birch (Betula sp.) forests; and riverine wetlands with wild celery (Vallisneria americana Michx.)-pondweed (Potamogeton spp.) associations (Suiter and Evans Reference Suiter and Evans1999). In Central and Northern Plains states, I. pseudacorus is reported to occur in monocultures or intermixed with sandbar willow (Salix interior Rowlee)-broadleaf cattail (Typha latifolia L.) communities (Preece Reference Preece1964 in Stone Reference Stone2009). Iris pseudacorus invasions in freshwater wetlands of California and Oregon impact native emergent T. latifolia, broadleaf arrowhead (Sagittaria latifolia Willd.), and clubrush (Schoenoplectus spp). communities. In tidal wetlands of North American Pacific estuaries, I. pseudacorus occurs with baltic rush (Juncus balticus Englem.), hardstem bulrush (Schoenoplectus acutus (Muhl. ex Bigelow) Á. Löve & D. Löve), narrowleaf cattail (Typha angustifolia L.), water parsley (Oenanthe sarmentosa C. Presl ex DC.), and associates (Gallego-Tévar et al. Reference Gallego-Tévar, Grewell, Whitcraft, Futrell, Bárcenas-Moreno and Castillo2022). Given the shorter history of invasion, there is very little information available regarding plant communities associated with I. pseudacorus in the Southern Hemisphere. However, it is known that I. pseudacorus often co-occurs with Typhaceae species, including T. latifolia (in South Africa and Argentina), common bulrush (Typha capensis Rohrb.) (in South Africa), and Asian bulrush (Typha orientalis Presl.) (in New Zealand), as well as with species from the Cyperaceae and Pontederiaceae families, among others (Gervazoni Reference Gervazoni2024; McGrannachan and Barton Reference McGrannachan and Barton2019; personal observations).
Due to its ability to form dense rhizomatic mats that exclude co-occurring species, I. pseudacorus has been described as an aggressive competitor, capable of engineering ecosystems and drastically reducing native plant diversity (Lamote et al. Reference Lamote, Roldán-Ruiz, Coart, De Loose and Van Bockstaele2002; Thomas Reference Thomas1980). Hayasaka et al. (Reference Hayasaka, Fujiwara and Uchida2018) recorded richness of vascular plants in the presence of I. pseudacorus in Japan, finding a negative relationship between the cover of I. pseudacorus and species richness. The results showed a significantly lower number of other species when the coverage of I. pseudacorus was more than 50%. Displacement of native populations was also observed in New Zealand with T. orientalis and Carex sp. (CM, personal observations), and in South Africa with T. capensis and Pontederia cordata L. (Sandenbergh Reference Sandenbergh2021). In Argentina, sites with advanced stages of invasion have been reported, where the cover of I. pseudacorus can be observed up to the horizon, with preexisting native vegetation having been displaced (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020; PG, personal observations; Figure 7).

Figure 7. Iris pseudacorus invasion in Buenos Aires Province, Argentina. (Photo: A Faltlhauser).
Management Options
Mechanical/Physical Control
Iris pseudacorus control by mechanical or manual means is often problematic, as such processes are time-intensive and laborious, and the plant’s capacity for vegetative reproduction allows swift recovery (Tu Reference Tu2003). This is particularly concerning in riparian habitats, as small fragments of rhizome can be dislodged and carried by moving water to establish new populations downstream (USDA-APHIS 2013). However, mechanical or manual methods are only effective for small infestations (Ramey Reference Ramey and Peichel2001). Methods such as clipping or mowing the flower heads before seed production may help to reduce viable seed capacity and prevent cross-pollination, but do not kill the plant (DiTomaso and Kyser Reference DiTomaso and Kyser2016; Tu Reference Tu2003). Annual management of an invaded site by hand pulling, digging, and cutting can weaken and eventually kill targeted plants, but this process is both time- and labor-intensive, and requires repeated efforts over several years (Tu Reference Tu2003). Benthic barriers such as rubber matting have proven effective against partly submerged populations, with rhizomes killed within 70 d and no detection of regrowth after 200 d (Tarasoff et al. Reference Tarasoff, Streichert, Gardner, Heise, Church and Pypker2016). Likewise, Tarasoff and Gillies (in review) found that cutting stems to the base of the plant and submerging them in 5 cm of water is sufficient to kill I. pseudacorus rhizomes (personal communication). This is consistent with Stoneburner et al. (Reference Stoneburner, Meiman, Ocheltree, Nissen and Bradfield2021), who found that cattle trampling combined with inundation was an effective treatment to reduce the height and density of I. pseudacorus.
Chemical Control
Chemical control methods are often used to manage invasive I. pseudacorus populations, and the herbicides glyphosate, imazapyr, and metsulfuron have shown certain levels of effectiveness (DiTomaso and Kyser Reference DiTomaso and Kyser2016; Global Invasive Species Database 2022; Wildland Consultants 2011). Glyphosate is commonly used in a 5% to 8% solution with a surfactant during late spring or early summer, to prevent seed development. During fall, a 2% to 8% solution has also been shown to be effective according to weed managers (Jacobs et al. Reference Jacobs, Pokorny, Mangold and Graves-Medley2011). DiTomaso and Kyser (Reference DiTomaso and Kyser2016) found that drizzle application of imazapyr gave significantly better control (99.2%) compared with glyphosate (86.6%) and was as effective as boom-sprayer treatments with both herbicides. However, non-target effects after herbicide applications are difficult to avoid, particularly in aquatic systems (DiTomaso and Kyser Reference DiTomaso and Kyser2016), and as there is no species-specific herbicide registered against I. pseudacorus, it is likely that the herbicides used will also affect co-occurring indigenous plant species. These effects will depend on the herbicide used, rate of application, and the life form of the co-occurring indigenous plant species.
The financial costs associated with chemical control of I. pseudacorus can be substantial, with labor, time, equipment, and chemical costs all contributing to high expenditures. In New Zealand, chemical control costs (including labor and herbicide) of I. pseudacorus were estimated to be NZ$100 to NZ$340 (± US$60 to $210) per hectare for isolated patches, and more than NZ$1,350 (± US$830) per hectare when I. pseudacorus cover exceeds 40% (Wildland Consultants 2011).
Biological Control
Iris pseudacorus has a wide distribution (GBIF 2022) with a broad ecological tolerance and high competitive ability. Coupled with its ability to reproduce rapidly by both rhizome fragmentation and seed dispersal, these attributes make I. pseudacorus a challenging species to control mechanically and chemically. As such, biological control may be the most feasible option to manage and control I. pseudacorus infestations both effectively and sustainably. However, cooccurrence of a wide range of native and horticulturally valuable Iris species may pose challenges to the adoption of a biological control program.
The aim of any biological control program is to identify and select potential agents based on the exploration of the weed’s natural enemies in the native range, as well as to test the specificity of the enemy to its host, thus reducing environmental risks and associated economic costs (Briese Reference Briese2004; van Klinken and Raghu Reference van Klinken and Raghu2006). The invertebrate fauna associated with I. pseudacorus in the native range was surveyed in several countries, including the United Kingdom, Belgium, and Italy (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021; Sutherland Reference Sutherland1990). Approximately 65% of the herbivore assemblages in the native range are represented by Coleoptera, whereas the remaining were species in the Hemiptera, Orthoptera, Lepidoptera, and Hymenoptera (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021). Almost all of these herbivorous species are leaf miners and defoliators, while some are associated with flowers, fruits, and rhizomes (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021; Sutherland Reference Sutherland1990).
The assessment of the prioritization process, considering geographic distribution of the insects, impact of the plant damage, and inferred host specificity, evidenced that most of the herbivorous insects of the assemblages were incidental visitors and polyphagous feeders, and hence considered unsuitable as potential biocontrol agents. Three herbivorous species, the flea beetle Aphthona nonstriata (Coleoptera: Chrysomelidae) (Figure 8A), the seed weevil Mononychus punctumalbum (Coleoptera: Curculionidae) (Figure 8B), and the sawfly Rhadinoceraea micans (Hymenoptera: Tenthredinidae) (Figure 8C), are being evaluated as potential biocontrol agents due to their unique association with species in the genus Iris and their potential to cause relevant plant damage to I. pseudacorus (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021).

Figure 8. Representation of damage caused to Iris pseudacorus by three potential biological control agents. (A) Aphthona nonstriata (Coleoptera: Chrysomelidae), (B) Mononychus punctumalbum (Coleoptera: Curculionidae), and (C) Rhadinoceraea micans larvae (Hymenoptera: Tenthredinidae).
Larvae of the sawfly R. micans are highly damaging and can completely defoliate their host. This species is considered an interesting option for release in big wetland areas of Argentina invaded by I. pseudacorus (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021) due to its highly specific oviposition preference on the host plant. The larvae are believed to sequester and store secondary plant metabolites, which could be an effective defense against generalist predators, as well as the strongly hydrophobic cuticle which allows them to move on the water’s surface to reach new plants (Boevé et al. Reference Boevé, Voigt and Gorb2013; Voigt et al. Reference Voigt, Gorb and Boevé2011). The occurrence of this species is associated with temperate and cold areas (GBIF 2021b), which could limit its establishment in subtropical wetland areas of the introduced range where I. pseudacorus has a considerable level of invasion (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Falthauser, Fuentes-Rodriguez and Martínez2020).
The weevil M. punctumalbum is an interesting candidate due to intensive feeding by the adults on flowers and fruit and because the larvae bore the seeds, pupating within the mature fruit (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021; Sutherland Reference Sutherland1990). Because I. pseudacorus has high seed production, viability, and dispersion rates, this candidate could be effective in limiting the spread and colonization of new habitats. However, considering its current distribution in the native range (GBIF 2022), its establishment could be limited mainly to temperate and cold habitats (GBIF 2021a).
The flea beetle A. nonstriata, is a common and abundant insect species in its native range (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021), and its occurrence is linked to a range of climates and habitats (GBIF 2021c). Adults feed on leaves and overwinter among leaf litter, whereas larvae are stem borers that feed on the rhizome. Currently, a quarantine population of A. nonstriata is under evaluation in South Africa, and preliminary results indicate that it is feasible for rearing to be conducted under controlled conditions, and that larvae cause significant damage to rhizomes and roots (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021). A recent study predicts the highest climatic suitability for A. nonstriata across northeast Argentina, Uruguay, southern Brazil, southern South Africa, southeast Australia, and New Zealand. Therefore, these wetland areas should be prioritized when releasing A. nonstriata to allow for the its establishment and to allow for the agent to perform optimally (Minuti et al. Reference Minuti, Stiers and Coetzee2022).
Preliminary surveys of the insect fauna associated with I. pseudacorus in Argentina and South Africa show that invertebrate assemblages include insects belonging to a range of orders, including the Hemiptera, Coleoptera, Diptera, Dermaptera, Hymenoptera, Orthoptera, Lepidoptera, Blattodea, Psocoptera, Thysanoptera, and Ephemeroptera (Gervazoni Reference Gervazoni2024; Gervazoni et al. Reference Gervazoni, Fuentes-Rodriguez, Sosa, Del Río, Sabater and Franceschini2021). Currently, the candidate agents from the native range have not been found in field surveys in Argentina, South Africa, and New Zealand, and the presence of native analogue species is under evaluation (Gervazoni Reference Gervazoni2024; Gervazoni et al. Reference Gervazoni, Fuentes-Rodriguez, Sosa, Del Río, Sabater and Franceschini2021, Reference Gervazoni, Fuentes-Rodríguez, Sosa, Coetzee, Bertucci, Kramarz and Chayasa2022). Preliminary assessment of herbivory in Argentinian wetlands shows that, occasionally, flowers have feeding spots on tepals that could be attributed to weevils. Generalist ants may occasionally damage margins of the tepals and other flower structures. No significant damage has been recorded in the rhizome and roots in the introduced range (unpublished data). Fruits and seeds present consistent damage by borer insects, but taxonomic identification and their inferred host range are under evaluation (Gervazoni Reference Gervazoni2024; Gervazoni et al. Reference Gervazoni, Fuentes-Rodríguez, Sosa, Coetzee, Bertucci, Kramarz and Chayasa2022). Damage to leaves could be frequent in populations of some wetland areas of Argentina, but the percentage damage is low, with no significant impact to the foliage (unpublished data).
It is important to understand the full implications of current management options for I. pseudacorus on the aquatic ecosystems it invades, and what that means for the sustainability and provision of freshwater ecosystem services. The aim of biocontrol against invasive macrophytes is to diminish invasive populations and restore access to clear freshwater dominated by native biodiversity, but in the absence of addressing the drivers of these invasions, aquatic systems are highly susceptible to secondary invasion by submerged and emergent exotic aquatic plant species.
General Outlook
Iris pseudacorus is an invasive macrophyte that is difficult to manage in affected ecosystems. The risk of introductions to new areas is high given its horticultural value, particularly in the wetland/aquatic landscaping field, for example, golf courses and trout farms. To minimize the impacts of new I. pseudacorus invasions, prevention of continued introduction and early warning will be the most effective strategy through public awareness campaigns in vulnerable regions. As invasions continue to spread, research must focus on understanding the mechanisms facilitating new invasions and devising successful integrated management strategies that address those mechanisms. Recent studies highlighting the efficacy of the potential biocontrol agent, A. nonstriata, in reducing seedling establishment are promising, because the main pathway of spread is through seed dispersal and seedling establishment (GM, unpublished data). Further suppression of seed production could be realized through the release of the seed-feeding weevil, M. punctumalbum, but the potential non-target effects on Iris species in the horticultural industry will have to be weighed carefully, as this weevil is an Iris specialist. Such strategies must also address ecosystem-level responses to control, to improve the chances of long-term success. Traditionally, intervention has been aimed at restoring invaded ecosystems by removing the invader and relying on natural restoration processes. However, when restoration is considered in the context of regime shifts between degraded stable states, there is a clear need to adopt an all-inclusive approach focused on active restoration. It is important, therefore, to consider the effects that invasive species such as I. pseudacorus have upon the multitrophic interactions that define ecosystem structure and functioning, which could elucidate the drivers that determine levels of success and failure in the establishment of this species.
Finally, an effective weed management plan for I. pseudacorus should include the collaboration of all social actors, the scientific community, citizens, and governments, considering activities of environmental education, training for the economic sector related to gardening, landscaping, and nursery, as well the generation of laws and regulations that aim to prohibit its commercialization and avoid new areas of invasion.
Acknowledgments
We thank technician Pedro Quaranta (CECOAL-CONICET-UNNE) for the plant drawings.
Funding statement
This research was funded by The South African National Research Foundation and Department of Forestry, Fisheries and the Environment, and Argentina’s National Scientific and Technical Research Council (CONICET: PIP KA11220200102296CO), the Secretariat of Science and Technology of the National University of the Northeast (SGCyT-UNNE: PI-17Q003), National Agency for Scientific and Technological Promotion of Argentina (PICT 2020 SERIE A-035-65). The Ph.D project of GM was funded by a strategic basic research fellowship of the Research Foundation–Flanders (FWO).
Competing interests
The authors declare no competing interests in undertaking this work.
Appendix
Species Distribution Model
Occurrence records for I. pseudacorus were sourced from the Global Biodiversity Information Facility portal (GBIF.org 2022a; GBIF.org 2022b). These were then cleaned by removing duplicate records, correcting erroneous or imprecise coordinates, and omitting records assigned to political centroids and biodiversity institutions. The remaining occurrences were visualized in QGIS software v. 3.14 (QGIS Development Team 2020), and suspicious records that could not be confirmed or corrected were excluded. The dataset was then filtered to minimize the influence of spatial autocorrelation (Boria et al. Reference Boria, Olson, Goodman and Anderson2014). These analyses were performed using the packages CoordinateCleaner (Zizka et al. Reference Zizka, Silvestro, Andermann, Azevedo, Duarte Ritter, Edler and Antonelli2019), ecospat (Di Cola et al. Reference Di Cola, Broennimann, Petitpierre, Breiner, d’Amen, Randin and Guisan2017), and spThin (Aiello-Lammens et al. Reference Aiello-Lammens, Boria, Radosavljevic, Vilela and Anderson2015) in R software v. 3.6.1 (R Development Core Team 2019).
Bioclimatic predictors were obtained from WorldClim 2.1 (Fick and Hijmans Reference Fick and Hijmans2017) at a resolution of 2.5 arcmin. Climatic data were then extracted from all raster layers at each occurrence point, and Pearson’s correlation coefficients were computed for each pair of variables. A selection was made, among highly correlated variables (|r| > 0.75), based on their model contribution (i.e., jackknife analysis) and in the authors’ opinion regarding their biological relevance to the distribution of I. pseudacorus. Variables known to limit plant species distribution, such as thermal extremes, water stress, and their interaction, were prioritized. The bioclimatic variables chosen as predictors for the model were: minimum temperature of the coldest month (bio6), mean temperature of the warmest quarter (bio10), annual precipitation (bio12), precipitation seasonality (bio15), and precipitation of the warmest quarter (bio18).
The analyses were performed in Maxent 3.4.1 (Phillips et al. Reference Phillips, Anderson, Dudík, Schapire and Blair2017). Occurrences from both the native and introduced range of I. pseudacorus were used for model training and testing. This method allows one to take into account the climatic variability acquired by the species in newly colonized areas and is believed to yield more reliable outputs than using native or invaded range records alone (Beaumont et al. Reference Beaumont, Gallagher, Thuiller, Downe, Leishman and Hughes2009). Maxent modeling settings were as follows: convergence = 105; number of iterations = 500; prevalence = 0.5; regularization multiplier = 1; features = automatic. Model output was set as logistic, thus expressing suitability scores ranging from 0 (no suitability) to 1 (maximum suitability). Ten bootstrap replicates were computed, each allocating 70% of occurrences (n = 798) to model calibration and the remaining 30% (n = 342) to model evaluation. The average of all replicate models was used as final output. The minimum training presence threshold was used for graphical representation, as it includes all known areas where climate could potentially allow the species to establish, an important aspect in risk management of invasive species.
As I. pseudacorus has a wide geographic distribution, both in its native range and at a global scale, the background used for modeling was defined based on broad bioclimatic zones (Hill and Terblanche Reference Hill and Terblanche2014). Background data representative of the climate of the study area were drawn from a customized mask generated by selecting Köppen-Geiger climate zones (Beck et al. Reference Beck, Zimmermann, McVicar, Vergopolan, Berg and Wood2018) containing at least one occurrence record for the species (for details, see Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stiers2021). The Multivariate Environmental Similarity Surface (MESS) was used to assess coverage of environmental gradients upon model projection and identify areas of uncertainty (Elith et al. Reference Elith, Kearney and Phillips2010). Where detected, the respective “out-of-range” environmental variables were extrapolated from the dissimilarity maps (MoD) provided by the program output (Elith et al. Reference Elith, Phillips, Hastie, Dudík, Chee and Yates2011). The Continuous Boyce Index (CBI) was employed as a measure of model accuracy (Hirzel et al. Reference Hirzel, Le Lay, Helfer, Randin and Guisan2006). This threshold-independent metric is considered more reliable than Area Under the Curve (AUC) when it comes to validating predictions and transferability of models built with presence-only data (Manzoor et al. Reference Manzoor, Griffiths and Lukac2018). CBI values were calculated using the package ecospat (Di Cola et al. Reference Di Cola, Broennimann, Petitpierre, Breiner, d’Amen, Randin and Guisan2017) in R software v. 3.6.1.











