Introduction
Dealing with dementia is currently one of the greatest challenges for health and social care (Winblad et al., Reference Winblad2016; Livingston et al., Reference Livingston2020). The prevalence of apathy in people living with dementia (PWD) is high, and not only is apathy the most common neuropsychiatric symptom in dementia (Brodaty and Burns, Reference Brodaty and Burns2012), but it is also accompanied by greater functional and cognitive decline (Robert et al., Reference Robert2009) and negatively associated with quality of life (Nijsten et al., Reference Nijsten, Leontjevas, Smalbrugge, Koopmans and Gerritsen2019). Considering unsatisfactory pharmacological treatment options, there is a growing interest in non-pharmacological interventions for managing apathy in PWD (Zucchella et al., Reference Zucchella2018; Theleritis et al., Reference Theleritis, Siarkos, Katirtzoglou and Politis2017). A variety of promising non-pharmacological interventions have been investigated, such as music therapy (Raglio et al., Reference Raglio2010; Holmes et al., Reference Holmes, Knights, Dean, Hodkinson and Hopkins2006), activity interventions (Treusch et al., Reference Treusch, Majic, Page, Gutzmann, Heinz and Rapp2015), and environmental stimulation (Jao et al., Reference Jao, Liu, Williams, Chaudhury and Parajuli2019). However, studies in this field are heterogenous and there is a lack of standardized and systematic methodological approaches (Theleritis et al., Reference Theleritis, Siarkos, Politis, Katirtzoglou and Politis2018; Goris et al., Reference Goris, Ansel and Schutte2016). Moreover, most studies on non-pharmacological interventions for PWD do not focus on apathy as a primary outcome (Theleritis et al., Reference Theleritis, Siarkos, Politis, Katirtzoglou and Politis2018).
A recent meta-analytic study confirmed that non-pharmacological interventions can generally improve activities of daily living and depression in nursing home residents living with moderate to severe dementia (Na et al., Reference Na2019). In light of these positive findings, evidence-based treatment guidelines have included recommendations for non-pharmacological interventions as primary treatment of both cognitive and non-cognitive symptoms in dementia (Pink et al., Reference Pink, O’Brien, Robinson and Longson2018; Dyer et al., Reference Dyer, Harrison, Laver, Whitehead and Crotty2018). However, considering the immense workload and limited resources in everyday nursing home settings, adequate delivery of guideline-based non-pharmacological interventions can be especially challenging in care facilities (Staedtler and Nunez, Reference Staedtler and Nunez2015; Bennett et al., Reference Bennett2020).
Previous research has indicated that interventions for PWD are more effective when tailored to the specific needs of the targeted person (O’Connor et al., Reference O’Connor, Ames, Gardner and King2009). Information and Communication Technologies (ICT) such as tablet computers can be viewed as innovative tools for supporting delivery of non-pharmacological interventions (Hitch et al., Reference Hitch, Swan, Pattison and Stefaniak2017; Tyack and Camic, Reference Tyack and Camic2017). ICT-based interventions can utilize adaptive algorithms, animated features, and simplified interfaces to increase the individual fit of an intervention to a specific user (D’Onofrio et al., Reference D’Onofrio2017) and may enhance beneficial effects of conventional interventions (Subramaniam and Woods, Reference Subramaniam and Woods2016; Hung et al., Reference Hung2018). However, there is a lack of controlled studies in this field (Van der Roest et al., Reference Van der Roest, Wenborn, Pastink, Droes and Orrell2017). ICT also allow new possibilities for assessing state variables in PWD. Ecological momentary assessments (EMA) focus on the PWD’s current state and are administered repeatedly over a certain period of time (Shiffman et al., Reference Shiffman, Stone and Hufford2008). It has been argued that situational EMA may be more suited to assess fluctuations in mood or quality of life, as retrospective self-reports and questionnaires can prove challenging and may not capture subtle changes related to specific events and situations (Beerens et al., Reference Beerens2016; Schall et al., Reference Schall, Tesky, Adams and Pantel2018).
The objective of the present study PflegeTab (English translation: CareTab) was to evaluate a novel ICT-based intervention for activating nursing home residents with dementia in a cluster-randomized controlled trial (cRCT). We hypothesized the multicomponent tablet-based intervention (TBI) would lead to a decrease in apathy (primary outcome) compared to an active control group receiving conventional individual activity sessions (CAS). Effects on secondary outcomes, quality of life, and depressive and neuropsychiatric symptoms were also investigated. Further, we assessed effects of the intervention on momentary quality of life with EMA before and after each activity session.
Methods
Study design
The study was designed as a two-arm prospective longitudinal cRCT and carried out in 10 nursing homes in Berlin, Germany, from June 2016 to May 2017. Randomization was performed at nursing home level to avoid contamination across groups (cluster-randomization, parallel design) and stratified according to total number of residents per unit. A member of the research team randomly assigned the units in each stratum to the TBI or CAS (each five nursing homes) group using opaque sealed envelopes (1:1 randomization). Assessments of primary and secondary outcomes were conducted before the intervention and after 8 weeks. EMAs were recorded in both groups before and after each session throughout the intervention period. Study assessors, participants, and staff members were blinded to the allocation until after the collection of baseline data. Effective blinding was not possible during the intervention, as TBI administers received tablet computers and training. The study was conducted and reported in accordance with CONSORT and approved by the Ethics Committee of the Medical University Berlin (EA1/013/16).
Participants and recruitment
All participants were long-term residents from the included nursing homes. Consent was first obtained from legal guardians, PWD were then asked to give consent. PWD and guardians were thoroughly informed about the trial, and study information was provided in plain language writing. Inclusion criteria were dementia diagnosis or cognitive impairment meaning a Mini-Mental State Examination (MMSE) score of less than 24 points (Folstein et al., Reference Folstein, Folstein and McHugh1975). Exclusion criteria were other mental and behavioral disorders and short-term residency of less than 4 weeks.
Intervention
The multicomponent TBI comprised seven applications specifically developed for PWD. Based on results of a pilot study (Nordheim et al., Reference Nordheim, Hamm, Kuhlmey and Suhr2015), the aims were (a) to stimulate cognitive and functional abilities and (b) to support emotional regulation. All components of the TBI were developed within a participatory and iterative framework including several pretests. Four applications (Quiz, Spelling game, Show me, and Move me) targeted cognitive and functional abilities. Task difficulty was adapted based on task performance: exercises became more difficult as performance improved and vice versa (Cha et al., Reference Cha2019). Three applications (Interactive Cat, Picture Gallery, Color and Sound) were designed to support emotional self-regulation. Task difficulty was not adapted for these applications, as they were mainly designed to enhance communication and well-being (Figure 1).
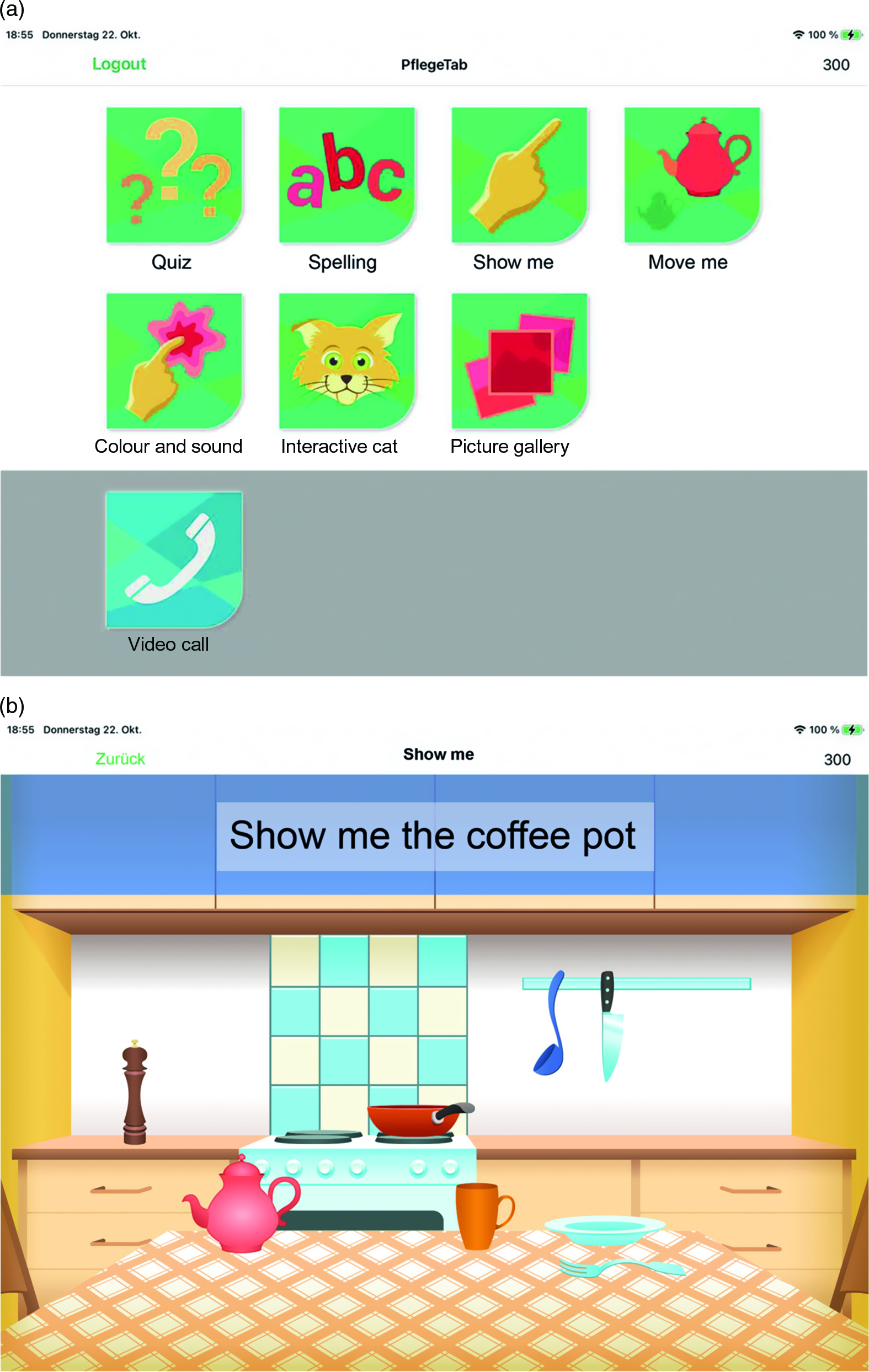
Figure 1. Panel A: PflegeTab launch screen with all seven applications for: (a) stimulating cognitive and functional abilities (Quiz, Spelling, Show me, and Move me) and (b) supporting emotional regulation (Interactive Cat, Color and Sound, and Picture Gallery). Panel B: Task in the Move me application. The applications were designed especially for older and inexperienced tablet users. They were developed for the purpose of this research and are currently not available to the public. Interested researchers may contact us for a demo version. The TBI was executed on Apple iPads version Air 2 (Model A1567) and the application was programmed in Swift. The copyright for the depicted images is owned by the authors.
Trained staff members guided participants throughout each TBI session and provided assistance whenever needed. Within each session, several activities were selected according to participants’ current preferences and needs. Instructions were provided both visually and auditory via tablet, and participants also received motivational feedback. Staff members sat with participants throughout the entire session and were instructed to encourage and reinforce them. The main purpose of the intervention was to engage PWD in a stimulating activity and to provide a positive and enjoyable experience. Therefore, feedback was based on user interactions rather than user performance, meaning that every interaction with the tablet was rewarded, regardless if an action was carried out correctly or not.
Participants from the five CAS units received the same amount of individual activity sessions as participants in TBI facilities. No specifications were made about the nature of the activities, except that no ICT devices should be involved. Staff members documented the activities in a logbook.
Procedure
A 2-hour training session was conducted on-site in each TBI facility. Additionally, a user manual was provided and a support hotline was set up. Members of the occupational therapy staff were to engage participants in three 30-minute individual sessions per week, resulting in a planned goal criterion of 24 activity sessions per participant. Two trained research assistants visited each unit and collected informant and self-rated data. The intervention phase commenced for each participant as soon as their baseline data were fully collected. Post-assessments were then collected 8 weeks later. Informant data on participants were assessed from care professionals who knew the participant well. None of the informants participated in the activity sessions. EMA were collected immediately before and after each individual activity session and recorded via tablet for TBI and on paper for CAS.
Measurements
The primary outcome apathy was assessed with the Apathy Evaluation Scale – Informant version (AES-I) (Marin et al., Reference Marin, Biedrzycki and Firinciogullari1991) at baseline and after 8 weeks. The AES-I consists of 18 items rated on a 4-point Likert scale. The total score ranges from 18 to 72; higher scores reflect higher levels of apathy. The subscale Apathy of the Neuropsychiatric Inventory – Nursing Home Version (NPI-NH) (Cummings et al., Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein1994) was used to determine convergent validity of the main outcome scale AES-I. Correlation between the NPI-NH subscale Apathy and the AES-I scores was moderate (Spearman’s r = .52).
Informant reports of global quality of life were assessed with the QUALIDEM scale (Ettema et al., Reference Ettema, Droes, de Lange, Mellenbergh and Ribbe2007) consisting of 37 items rated on a 4-point Likert scale with a total score ranging from 0 to 111. Self-rated quality of life was assessed with the Quality of Life in Alzheimer’s Disease (QOL-AD) questionnaire (Logsdon et al., Reference Logsdon, Gibbons, McCurry and Teri2002). Participants are asked to rate 13 different aspects of their lives on a 4-point Likert scale resulting in a total score from 13 to 39. Higher scores reflect higher quality of life levels in both measures. An eight-item version of the QUALIDEM was used to conduct EMA of momentary quality of life. Psychometric properties of the QUALIDEM short version have been published elsewhere (Junge et al., Reference Junge2020). Neuropsychiatric symptoms were measured with the informant-based NPI-NH questionnaire (Cummings et al., Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein1994). NPI-NH evaluates 12 neuropsychiatric symptoms using standardized interview questions. Informants rate the frequency and severity of each symptom, resulting in a total NPI-NH score from 0 to 144. Higher scores represent more neuropsychiatric symptoms. We assessed the prescription of psychotropic medications as a further indicator of neuropsychiatric symptoms (Maust et al., Reference Maust, Langa, Blow and Kales2017). Information on prescribed medication was derived from medical records and medication lists at the time of baseline assessment and again at post-assessment 8 weeks later. Type of medication, current dosage, and intake intervals were recorded. Depressive symptoms were measured using the Geriatric Depression Scale (GDS) (Yesavage and Sheikh, Reference Yesavage and Sheikh2008). GDS is a 15-item questionnaire in a yes/no format with total scores from 0 to 15. Higher total scores indicate a higher risk of depression. Further covariates were age, gender, functional status assessed with Barthel Index (BI) (Mahoney and Barthel, Reference Mahoney and Barthel1965), and dementia stage measured with the Functional Assessment Staging (FAST) (Sclan and Reisberg, Reference Sclan and Reisberg1992). FAST is comprised of seven stages and nine substages, which were transformed into a consecutive score ranging from 1 to 16 for further analysis. Higher scores represent higher dementia severity.
Sample size calculation
Sample size was estimated with G-Power (Version 3.1; test family: two-sample t-test) and based on expected differences in QOL-AD scores (Hoe et al., Reference Hoe, Hancock, Livingston, Woods, Challis and Orrell2009). Previous research has suggested that effects of interventions on quality of life and apathy are comparable (Nijsten et al., Reference Nijsten, Leontjevas, Smalbrugge, Koopmans and Gerritsen2019). The final estimate was n = 240 PWD (i.e. 120 per group). This calculation was based on a significance level of 5% (two-sided), 80% power, a medium effect size of Cohen’s d=.5, and an expected attrition rate of 20% (Hoe et al., Reference Hoe, Hancock, Livingston, Woods, Challis and Orrell2009). Taking the nested structure of the data into account, we anticipated small intracluster correlations between nursing homes with an intraclass correlation coefficient of .005 (Adams et al., Reference Adams, Gulliford, Ukoumunne, Eldridge, Chinn and Campbell2004).
Statistical analysis
Linear mixed-effects models (LMM) fit by restricted maximum likelihood Estimation were applied using an intention-to-treat approach, including all available data regardless of loss to follow-up. When using LMM in incomplete data, power issues because of reduced sample size as well as bias in results due to selection of cases with more complete data might arise, therefore multiple data imputation (MI) was used (Jakobsen et al., Reference Jakobsen, Gluud, Wetterslev and Winkel2017). Especially if the missing data mechanism is missing at random and the probability of missingness is related to observed characteristics, one cannot rule out bias. MI based on chained equations and predictive mean matching was performed at item level for primary and secondary outcome measures and covariates, and scale scores were then computed. We analyzed 10 imputed datasets separately and combined the results following Rubin’s rules (Rubin, Reference Rubin2004). The number of scale scores including imputed data at item level were AES-I (n = 28; 17%), QOL-AD (n = 71; 44%), QUALIDEM (n = 28; 17%), GDS (n = 102; 64%), NPI-NH (n = 28; 17%), FAST (n = 31; 19%), and MMSE (n = 74, 46%). All individual scale items, age, gender, group (TBI vs CAS), years of education, nursing home, and medication were used for the imputation process.
Change scores were computed by subtracting baseline scores from post-intervention scores. Baseline outcome measures were included as fixed covariates and a random intercept was added at nursing home level to account for clustering of participants. P-values are reported for unadjusted models and additionally for models adjusted for age, gender, neuropsychiatric symptoms (NPI-NH), and dementia stage (FAST). Generalized estimating equations (GEE) were used where more robust estimation methods lead to more stable models. For the purpose of a sensitivity check, differently specified LMM analyses were conducted based on a three-level hierarchy with the repeated measure time points nested in participants who were grouped in different nursing homes (random intercepts). Fixed factors were group (TBI vs CAS), time (baseline vs post-intervention), and a group x time interaction. Time was modeled as a repeated measure with an autoregressive covariance structure. LMM for analyzing momentary quality of life included the factor group (TBI vs CAS) and covariates for pre-session EMA measurements (baseline EMA), age, gender, neuropsychiatric symptoms (NPI-NH) and dementia stage (FAST), and the time-varying covariate session. Clustering at nursing home level was accounted for (random intercept). No adjustment for multiple testing was applied for secondary hypotheses analyses. In this exploratory study, interpretation of results of secondary hypotheses analyses is based on effect estimates and 95% CI and not on p-values.
All statistical analyses were performed using IBM SPSS software (IBM SPSS Statistics for Windows, Version 26.0, IBM Corp., Armonk, NY, USA).
Results
Participant characteristics
A total of 203 residents were deemed eligible after initial screening, and n = 162 (80%) were included in the study. The most common reason for non-inclusion was failure to reach the legal guardian (Figure 2).

Figure 2. Flow chart of trial participants.
Post-intervention data were collected from 134 (83%) of the 162 participants at baseline. On average, participants were aged 85 years (SD = 7.1, range = 53–100) and reported lower secondary education (mean = 10.5 years of education, SD = 4.2, range = 0–19). The majority were women (74%) and in need of substantial care (53% w/care level 4 “most severe impairment”). The mean FAST score was 9.1 (SD = 1.8, range = 4–16), which reflects moderately severe dementia (FAST stage 6d). The overall mean AES-I score was 48.8 (SD = 10.6, range = 20–69). For a total of 61 participants (38%), substantial clinical apathy was reported at baseline with the subscale Apathy of the NPI-NH (mean = 2.1, SD = 3.1, range = 0–12). An average intake of 2.0 psychotropic substances per day (SD = 1.5, range = 0–7) was reported. The average GDS score of 3.4 (SD = 2.6, range = 0–11) was below the clinical cutoff for depression of five points. Chi-square, Mann–Whitney U-test, and t-test confirmed that cluster randomization was successful, as no differences were revealed between intervention and control group in most characteristics at baseline. Lower levels of neuropsychiatric symptoms were reported for the TBI group at baseline (U = 2536.50, p = .017). In subsequent adjusted LLM analyses, the NPI-NH score was controlled for. All descriptive analyses are based on original data (Table 1).
Table 1. Baseline characteristics for total cohort, TBI and CAS group, M (SD) or n (%), n = 162

FAST stages (1–7) and substages (6a–e and 7a–f) were transformed into a consecutive score ranging from 1 to 16.
Note: TBI = tablet-based intervention; CAS = conventional activity sessions; M = mean; SD = standard deviation; BI = barthel index; FAST = functional assessment staging; AES-I = apathy evaluation scale – informant version; QOL-AD = quality of life in Alzheimer’s disease; NPI-NH = neuropsychiatric inventory – nursing home version; GDS = geriatric depression scale; ns = non-significant.
Dose of the intervention and attrition rate
Overall, the majority of participants (85%) failed to reach the goal of 24 sessions over 8 weeks. On average, the TBI group (n = 80) received 12.7 sessions (SD = 8.7, range = 0–36) and completed 53% of the scheduled intervention sessions, while the CAS group (n = 82) received 15.7 sessions (SD = 7.1, range = 0–30) and completed 66% of the scheduled intervention sessions. The most frequent CAS were memory training, life story work, and physical activity (i.e. short walks). The sample-wide attrition rate was 17% (28 participants). Post-intervention data could not be collected from 24 PWD (30%) in the TBI group and 4 PWD (5%) in the CAS group. The most frequent reason for discontinuing the study was lack of motivation and mental overload in the TBI (13 participants) and death in the CAS group (3 participants).
Impact of the intervention on primary and secondary outcomes
Unadjusted LMM analysis showed no significant group differences in change of the primary outcome apathy (AES-I score) (β = .25; 95% CI −3.89, 4.38, p = .91). This corresponds to a standardized effect size (Cohen’s d) of .02. Overall, the levels of apathy decreased slightly in both groups with an estimated mean decrease in AES-I scores of .61 points (95% CI −3.54, 2.33) in the TBI group and .36 points (95% CI −3.27, 2.55) in the CAS group. Baseline AES-I scores were negatively associated with change scores of AES-I. Higher AES-I scores at baseline were associated with a decrease in apathy rates, while lower baseline scores were associated with an increase in apathy rates (association of baseline and post-intervention AES-I: β = −.43; 95% CI −.57, −.29, p<.001). Further exploratory analyses to test for a differential intervention effect between participants with and without clinically relevant apathy did not yield any different results.
No substantial group differences in change scores were revealed by the unadjusted models in secondary outcomes QOL-AD (β = .12; 95% CI −1.23, 1.47, p = .86), NPI-NH (β = −.91; 95% CI −6.35, 4.54, p = .74) and GDS (β = .003; 95% CI −.74, .73, p = .99). For the secondary outcome QUALIDEM, we saw a statistically nonsignificant group difference in QUALIDEM change scores (β = 2.04; 95% CI −.86, 4.94, p = .17). Estimated average QUALIDEM scores increased by .81 points (95% CI .71, 4.99) in the TBI group compared to an increase of 2.85 points (95% CI −1.02, 2.64) in the CAS group. Furthermore, the analysis for psychotropic medication revealed a group difference (β = .42; 95% CI .15, .69, p<.01) in favor of a greater reduction in the TBI group. Estimated mean change scores showed an average reduction of .41 substances (95% CI −.61, −.22) in the TBI group compared to an average change of .01 substances (95% CI −.17, .19) in the CAS group. This effect remained stable in models adjusted for gender, age, and dementia stage (FAST) and neuropsychiatric symptoms (NPI-NH) (β = .43; 95% CI .11, .76, p<.01). Table 2 shows the estimated post hoc means and group differences of primary and secondary outcome scores. There were no substantial differences in findings for any models with and without imputation.
Table 2. Estimates of primary and secondary outcome post-intervention means for CAS and TBI group, adjusted for mean baseline values of particular outcome (n = 162)
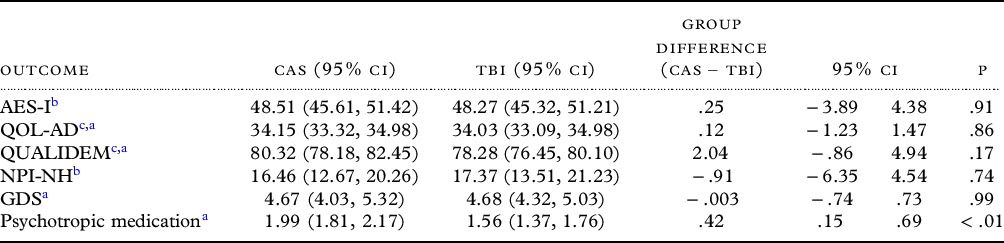
TBI = tablet-based intervention; CAS = conventional activity sessions; 95% CI = 95% confidence interval; AES-I = apathy evaluation scale – informant version; QOL-AD = quality of life in Alzheimer’s disease; NPI-NH = neuropsychiatric inventory – nursing home version; GDS = geriatric depression scale.
Note: Group means and differences were estimated with generalized estimating equations.
a (GEE) and linear mixed models.
b (LMM). All models are adjusted for particular baseline measures. Clustering of measurements in nursing homes and participants were accounted for. Positive difference values indicate smaller means in the TBI group compared to the CAS group.
c Denotes measures where higher scores show improvements.
For a sensitivity check, additional LMM analyses including a fixed factor for measurement timepoints (baseline vs post-intervention) were conducted. Analyses revealed an overall post-intervention improvement of QUALIDEM scores (collapsed over groups) (β = 3.36; 95% CI .49, 6.23, p = .022). Analyses based on imputed data also revealed a group x time interaction (β = −5.44; 95% CI −10.05, −.84, p = .021). Post-intervention improvement of informant rated quality of life was greater in the CAS group (EM = 3.73; 95% CI .97, 6.49) than in the TBI group (EM = .68; 95% CI −2.50, 3.86). These findings on QUALIDEM remained stable in models adjusted for gender and baseline values of age, dementia stage (FAST), and neuropsychiatric symptoms (NPI-NH). The adjusted model for QUALIDEM revealed associations of QUALIDEM scores with NPI-NH scores and gender. Higher levels of quality of life were associated with less neuropsychiatric symptoms (β = −.53; 95% CI −.63, −.43, p = <.001) and female gender (β = 3.76; 95% CI .04, 7.48, p = .048).
Ecological momentary assessments of quality of life
Over all sessions and participants, a total of 2264 pre-session EMAs and 2150 post-session EMAs were recorded. Sessions without post-session EMA recordings were omitted from further analyses. Across both groups, LMM analyses revealed a general post-session improvement of .32 points in mean EMA of quality of life (β = −.11; 95% CI −.20, −.01, p = .03). Further analyses with EMA change scores as outcomes and adjusted for EMA pre-session values revealed a group difference, and the post-session improvement was greater for the CAS compared to the TBI group (β = .43; 95% CI .30, .56, p<.001). The LMM estimated change in the intervention group was .02 (95% CI −.07, .12) and .46 (95% CI .37, .54) in the CAS group (Figure 3). This finding remained stable after adjusting for gender and baseline values of age, FAST, and NPI-NH.

Figure 3. Overall observed means for pre- and post-session EMA scores for TBI and CAS group. Note: Error bars represent standard deviations of observed mean EMA scores.
Discussion
This study investigated effects of a multicomponent TBI for activating nursing home residents with dementia. We hypothesized that regular, guided, and tailored TBI sessions would improve the primary outcome apathy, and secondary outcomes quality of life, and neuropsychiatric and depressive symptoms, compared to CAS. However, we did not find a positive effect of TBI on apathy. Improvements in quality of life (measured with QUALIDEM) were observed in both groups and these were larger in the CAS compared to the TBI group. EMA recorded before and after each activity session also rendered short-term post-session benefits on quality of life in the CAS group. A reduction of psychotropic medication was found for TBI compared to CAS.
Although we expected the tailored TBI would increase engagement and reduce apathy, our findings do not support this notion. While it is clear that apathy plays an important role in dementia, research on the impact of non-pharmacological interventions on apathy has yielded mixed results (Goris et al., Reference Goris, Ansel and Schutte2016; Theleritis et al., Reference Theleritis, Siarkos, Politis, Katirtzoglou and Politis2018). Previous studies have also failed to detect clinically meaningful effects on apathy in the long term. Treusch et al. (Reference Treusch, Majic, Page, Gutzmann, Heinz and Rapp2015) found an increase in apathy levels in a control group compared to a group with a weekly occupational and sport intervention. However, this effect faded 12 months after the termination of the intervention, suggesting that long-term and ongoing interventions are necessary to achieve a meaningful impact on apathy in PWD. Cohen-Mansfield (Reference Cohen-Mansfield2018) observed increased engagement levels in nursing home residents with dementia during group activities compared to a control condition with unstructured time, while Raglio et al. (Reference Raglio2010) reported beneficial effects of a music-based intervention. Future studies on ICT-based interventions should incorporate these activity types to gain more knowledge on effective strategies for reducing apathy in PWD. Moreover, considering our finding that higher levels of apathy at baseline were associated with a decrease in apathy, future studies that aim to address apathy in PWD should strive to include participants with high levels of apathy at baseline and define appropriate inclusion criteria prior to study entry (Cummings et al., Reference Cummings2015).
In line with previous findings, informant-rated quality of life improved in both groups (Ballard et al., Reference Ballard2018). However, the observed improvement was smaller for the TBI than the CAS group. In contrast, self-rated quality of life did not change markedly over the intervention period. This finding could be related to known challenges regarding self-reported outcomes in PWD (Robertson et al., Reference Robertson, Cooper, Hoe, Hamilton, Stringer and Livingston2017). We also observed improvements in momentary quality of life in the CAS group, whereas a ceiling effect was observed in the TBI group. Previous research has also reported situational improvements of quality of life in PWD (Schall et al., Reference Schall, Tesky, Adams and Pantel2018).
A reduction of psychotropic medication was found in the TBI compared to CAS group. Although we did not expect this specific result, previous studies have reported similar findings. A cRCT conducted by Joranson et al. (Reference Joranson, Pedersen, Rokstad and Ihlebaek2016) reported a significant decrease in prescribed psychotropic medication related to a robot-assisted intervention for nursing home residents with severe dementia. Ballard et al. (Reference Ballard2016) argue that effective non-pharmacological interventions should be implemented alongside antipsychotic review in order to reach sustainable benefits for PWD in nursing home care.
Possible reasons for the absence of group differences may be related to: (1) design of the study; (2) implementation of the intervention; and (3) content of the intervention.
All participants received substantial one-on-one time from occupational therapists, which may have led to benefits for participants in both groups. The activities conducted in the CAS group were chosen individually, essentially meaning that this group also received a tailored intervention. Evidence-based recommendations for cognitive interventions in dementia have established that control group activities should match those of intervention groups in duration, intensity, and socio-physical environment (Ibanez et al., Reference Ibanez, Richly, Roca and Manes2014). Therefore, we chose an active control group as opposed to a comparison group receiving treatment as usual. Methodological issues concerning active control group trials have been discussed elsewhere (Temple and Ellenberg, Reference Temple and Ellenberg2000; Makuch and Johnson, Reference Makuch and Johnson1989). The absence of group differences within our study design could either mean that both treatments were equally effective (i.e. noninferiority), or that no treatment had an effect. Previous research on individualized activity interventions for PWD has demonstrated that tailored interventions directed toward individual needs and abilities of PWD are associated with better clinical outcomes (Vernooij-Dassen et al., Reference Vernooij-Dassen, Vasse, Zuidema, Cohen-Mansfield and Moyle2010; Ballard et al., Reference Ballard2018). Our finding that global quality of life, on average, marginally improved in both groups after 8 weeks, combined with the ceiling effect in momentary quality of life in the TBI group and the improvement of momentary quality of life we observed in the CAS group, may suggest that in fact both groups received potentially effective treatments. Furthermore, there were considerable individual differences in change of quality of life around the mean change in our study, and future studies should thus investigate which time-variant individual factors account for improved treatment effects.
Overall, only 59% of the intervention sessions were carried out. One important reason for the poor implementation was a lack of time and staff resources as well as high staff turnover rates in some of the participating units. Occupational stress in nursing home staff has been a much-researched topic (Costello et al., Reference Costello, Walsh, Cooper and Livingston2019). This unforeseen reduction of the intervention dose could have affected our results, as previous research has pointed out that the frequency and intensity of interventions are important factors (Kim and Park, Reference Kim and Park2017). Conversely, the dose of the intervention exceeded the number of planned sessions in some participants. This too entails a methodological problem and could have impacted our findings. Previous studies have also reported inconsistent delivery of technology-based interventions (Godwin et al., Reference Godwin, Mills, Anderson and Kunik2013). We also found lower rates of delivered activity sessions for TBI units compared to CAS. Despite our efforts to boost acceptance, there may have been persistent ICT-related inhibitions in some of the participating staff. Perceived usefulness and perceived ease of use are pivotal factors for acceptance or rejection of new technologies in healthcare settings (Rahimi et al., Reference Rahimi, Nadri, Lotfnezhad Afshar and Timpka2018; Gagnon et al., Reference Gagnon2012).
Finally, we must address the fact that 13 participants in the TBI group terminated the study because the TBI was too mentally challenging and stressful. Although reduced levels of cognitive functioning and inexperience of PWD were considered when designing the applications, we cannot rule out that the fact of simply being introduced to an unfamiliar device itself may have been overwhelming and excessively demanding for some participants. Hung et al. (Reference Hung2020) reported similar implementation barriers related to novel technologies.
Strengths and practical implications
Studies of non-pharmacological interventions have confirmed that changes in mood, cognition, and behavior seldom persist in PWD after cessation of the intervention (Kim and Park, Reference Kim and Park2017). This may be linked to the progressive nature of dementia, making it difficult to establish long-lasting and sustainable improvements in the absence of ongoing interventions. It can also be methodologically challenging to quantify intervention effects on global outcomes such as apathy or quality of life. A strength of our study lies in the assessment of momentary quality of life in addition to conventional global outcome measures. This way, we were able to detect changes in both global and momentary states associated with the activity sessions. Even though short-term improvements may not impact global outcomes, temporary benefits can be extremely meaningful for PWD and nursing home staff. Future studies should utilize situational EMA to investigate the effectiveness of ongoing interventions in PWD.
It has been widely acknowledged that the prevalence of polypharmacy and inappropriate psychotropic medication are high in PWD (Jester et al., Reference Jester, Molinari, Zgibor and Volicer2021). Our results indicate that non-pharmacological interventions may have the potential to reduce psychotropic drugs in nursing home residents with dementia. However, we specifically underline that this finding cannot be interpreted any further in the context of our study. It remains unclear if the reduced psychotropic medication can be attributed to less neuropsychiatric symptoms or other factors, as we did not find a corresponding decrease of NPI-NH scores in the TBI group. Further research is needed to investigate the possible impact of ICT-based interventions on prescription of psychotropic medications.
While interventions such as ours may not reduce costs or replace staff, they could absorb some of the workload for nursing home staff and enrich the repertoire of available activity options. ICT devices are small, easy to operate, and pervasive in today’s modern society. One single device can be used to engage numerous PWD, either simultaneously or individually. Therefore, we strongly recommend further research on meaningful ICT-based interventions for PWD.
Another strength of our study was that the activity sessions were executed by nursing home staff under “real-world” conditions, as recommended by Bennett et al. (Reference Bennett2020). Future research on ICT-based interventions in nursing homes should consider barriers concerning workplace conditions, user acceptance and digital infrastructure. We recommend extensive staff training prior to introduction of novel interventions and close monitoring of ongoing interventions to ensure a successful implementation and increase user acceptance of ICT-based interventions in nursing homes.
Limitations
Our study design does not allow an unambiguous interpretation of the results. Future studies should incorporate a third study arm to unravel effects associated with new interventions. Secondly, while baseline measurements were carried out by blinded study assessors, this approach was not feasible for the collection of EMA. EMA were conducted by the person who carried out the activity session, meaning that rater bias cannot be fully ruled out. This also may have amplified the ceiling effect observed in the TBI group. A third limitation stems from the fact that we were unable to collect self-reported data in some participants with higher dementia stages, resulting in higher proportions of missing data on self-report instruments. We cannot rule out inflated Type I error rates, since we did not adjust for multiple testing in the analyses of secondary hypotheses. P-values should be interpreted cautiously for secondary hypotheses. Finally, our study was underpowered which may have made it more difficult to detect a difference in our primary outcome.
Conclusion
Tablet computers can support delivery of non-pharmacological interventions in nursing homes and facilitate regular assessments of fluctuating momentary states in residents with dementia. Although the improvements in global quality of life observed in our study may not be specific to TBI, we believe they are related to the individualized and tailored activity sessions. We also found that EMA collected directly before and after activity sessions revealed subtle and short-term benefits. Non-pharmacological interventions could have a more meaningful impact on momentary states of nursing home residents with dementia than on their global conditions. These findings can be of high clinical relevance and underline the importance of individualized activity interventions in nursing home care. However, further research is needed to determine effective intervention components and unravel short- and long-term benefits of ICT-based interventions in PWD.
Conflicts of interest
None.
Source of funding
German National Association of Statutory Health Insurance Funds.
Description of authors’ roles
JLO, PG, JNVA, SM, AK, and JN designed and conducted the study. JLO, SL, and JN conducted review of the literature. JLO was the main contributor in writing the manuscript. SL and JN made contributions to the manuscript. JLO, UG, and PG analyzed the data, and all authors were involved in reviewing and interpreting the data. JLO, SL, PG, UG, JNVA, SM, AK, and JN critically revised the current manuscript for submission. All authors read and approved the final version of the manuscript.
Acknowledgments
We would like to thank Laura Jordan and Sophie Guinet for their efforts in collecting the data. We thank Jacqueline Wienholtz, Marco Reichert, and Ines Jesse for their support in organizing the study. We also acknowledge contributions from Britta Hesse, Sonia Sobol, and Verena Anton throughout the project.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1041610221000818.







