Introduction
The number of patients with dementia increased to 35,600,000 in 2010 and is expected to double every 20 years, estimating that the number of dementia patients will be 65,700,000 in 2030 and 115,400,000 in 2050 (WHO, 2012). To reduce the behavioral and psychological symptoms of dementia (BPSD), various pharmacological and non-pharmacological treatments have been used for people with dementia (PWD). Some studies have reported that there was no effect of pharmacological interventions on the BPSD (Gitlin et al., Reference Gitlin2009), while a few studies found potential benefits to using non-pharmacological interventions for depression (Duru Aşiret and Kapucu, Reference Duru Aşiret and Kapucu2016), on cognitive function (Carrion et al., Reference Carrion, Aymerich, Baillés and López-Bermejo2013), and social activity (Akanuma et al., Reference Akanuma2011).
Reminiscence therapy was developed by Butler as a treatment for dementia using a process of “Life Review” (Butler, Reference Butler1963) in which an individual recalls their past events, activities, and experience to help give meaning to their lives. The life review process uses various memory triggers such as household items, objects from the past, photographs, and music (Woods et al., Reference Woods, Bruce, Edwards, Elvish and Hoare2012). Reminiscence therapy has been used broadly as a non-pharmacological intervention and can be conducted with a group or individually (Woods et al., Reference Woods, Spector, Jones, Orrell and Davies2005). Generally, group-based reminiscence therapy is commonly used since it allows participants to stimulate each other through conversation and increases their attention span. Individual reminiscence therapy also has benefits due to its focus on a person-centered approach.
Dementia describes a collection of symptoms that are caused by disorders affecting the brain. Since it was introduced in the early 1960s for patients with psychological signs and symptoms or in the frail elderly (Buchanan and Middleton, Reference Buchanan, Middleton and Bornat1994), reminiscence therapy has been reported to be effective in reducing depression (Duru Aşiret and Kapucu, Reference Duru Aşiret and Kapucu2016) for PWD. Reminiscence therapy could allow PWD to better manage BPSD (Tadaka and Kanagawa, Reference Tadaka and Kanagawa2007) and improve (O’Shea et al., Reference O’Shea2014) and slightly benefit on their quality of life (QoL) (Woods, et al., Reference Woods, O’Philbin, Farrell, Spector and Orrell2018), which can contribute to reduce the care burden on caregivers. In terms of QoL, there are only available measurements for participants with relatively high cognitive function, indicating a limitation to identify the effectiveness of reminiscence therapy.
Although BPSD as an effective outcome variable with reminiscence therapy is controversial by previous literature, BPSD is worthwhile to be examined because it is the factor causing the most stress on formal and informal caregivers of PWD. In addition, current scientific evidence is not sufficiently available for the occurrence and management of BPSD. Many researchers are trying to decrease the BPSD in PWDs by utilizing many different non-pharmacological interventions. Therefore, it must be valuable to examine the effect of reminiscence therapy on BPSD. Moreover, some other studies failed to prove the effectiveness of this therapy with regard to depression (Wang, Reference Wang2007), BPSD (Baines et al., Reference Baines, Saxby and Ehlert1987), and QoL (Lai et al., Reference Lai, Chi and Kayser-Jones2004) in PWDs. Therefore, it is uncertain whether reminiscence therapy has an effect in PWDs or what symptoms might be affected.
Because of this knowledge gap, a systematic review and meta-analysis of the published literature were performed to identify the overall effects of reminiscence therapy for PWD. A recent systematic review also reported that evidence of the therapeutic effect was not robust (Subramaniam and Woods, Reference Subramaniam and Woods2012; Woods et al., Reference Woods, Bruce, Edwards, Elvish and Hoare2012; Reference Woods, O’Philbin, Farrell, Spector and Orrell2018) because of the small number of studies. Huang et al. (Reference Huang2015) reported in their systematic review and meta-analysis that reminiscence therapy had an effect on depressive symptoms in PWDs, and they also tried to demonstrate the long-term effect of reminiscence therapy, although the study (Huang et al., Reference Huang2015) only examined depressive symptoms. Unlike pharmacological intervention, which tends to target only one symptom, we examined the effects of reminiscence therapy on various outcomes for PWDs including depression, QoL, and BPSD. We included a large number of RCTs to produce a robust result.
Regardless of this argument, reminiscence therapy has been known that there is practical advantages since it is a simple intervention with low risk for application to PWD (Haight et al., Reference Haight, Gibson and Michel2006; van Bogaert et al., Reference van Bogaert, Van Grinsven, Tolson, Wouters, Engelborghs and Van der Mussele2013). Non-pharmacological interventions that can slow disease progression are currently becoming increasingly important as an addition to pharmacological treatments (Ngo and Holroyd-Leduc, Reference Ngo and Holroyd-Leduc2014)
Therefore, this meta-analysis will identify the effects of reminiscence therapy on depression, quality of life, and BPSD among PWDs. In addition, this meta-analysis will identify the evidence as to whether individual or group reminiscence therapy can have better therapeutic effect, and what frequency of interventional sessions is suitable to obtain a therapeutic effect through the subgroup analysis.
Methods
A systematic search for randomized controlled trials (RCTs) was conducted from July to August 2016. The bibliographic databases CENTRAL, CDSR, DARE, MEDLINE, CINAHL, and ProQuest and the Korean databases RISS, KISS, and NDSL were used for the systematic search of RCTs. The keywords used in the search were as follows: (“reminiscence” OR “reminiscence therapy” OR “reminiscence intervention” OR “reminiscence treatment” OR “reminiscence program” OR “spiritual reminiscence” OR “structured reminiscence therapy” OR “memory reminiscence” OR “life review” OR “life story book”) AND (“randomized controlled trial”) AND (“dementia” OR “Alzheimer’s” OR “Alzheimer’s disease” OR “Alzheimer’s dementia” OR “vascular dementia”). Manual searching was also performed to include articles that might have been omitted from the electronic search.
A total of 157 original published studies were identified in the search, and 121 studies remained after deleting duplicates. Two authors independently reviewed the titles and abstracts of the studies and excluded studies that did not include reminiscence therapy, an RCT research design, and PWDs. Finally, 24 complete articles were included in the review to check for the level of evidence. The flow diagram of the study screening is shown in Figure 1. Two authors reviewed all of the papers to determine if the studies fit the inclusion criteria. When the two authors disagreed during the study evaluation, disagreement was resolved by discussion and/or consultation with a third author.

Figure 1. Flow diagram of the study screening.
Inclusion criteria
Only studies using randomized controlled trials as the primary study design were included; however, while reviewing the studies, we found that it was difficult to evaluate the blindness and randomization of studies on non-pharmacological interventions. There were unclear explanations on “random sequence generation” or “allocation concealment” when reviewing the studies; therefore, we included studies if the RCTs were applied using either “random sequence generation,” “allocation concealment,” or both based on the recommendation of CONSORT (Consolidated Standards of Reporting Trials) (Moher et al., Reference Moher, Schulz and Altman2001). If there was only a statement on applying random assignment without any explanation of the randomization or detailed randomization method, we categorized the potential study into the ‘unclear bias’ section of randomization.
The inclusion criteria for the participants were age over 60 years, diagnosis of dementia such as Alzheimer’s disease or vascular dementia, and cognitive decline. We mainly selected studies including participants with mild to moderate dementia. The severity of dementia was mostly evaluated by the Mini-Mental State Examination (MMSE) or Clinical Dementia Rating (CDR), and participants with an MMSE score between 10 and 24 or CDR score of 1–2 were selected for this study. If there were both dementia and non-dementia participants in a study, we only included the results from the patients diagnosed with dementia.
Studies using reminiscence therapy in which demented elderly participants recalled the past in groups or individually were included in the analysis. This therapy also uses aides such as photographs, music, and videos from the past. Studies with a minimum study period of four weeks were included. If the intervention used other therapies such as exercise, printing, music, gardening, or usual care, we only included the results from the reminiscence therapy. Interventions that did not influence the main variables such as no treatment, usual care, physical activity, or informal social contact were included in the control group (Akanuma et al., Reference Akanuma2011; Azcurra, Reference Azcurra2012; Kwon et al., Reference Kwon, Jung and Kim2012; Nakatsuka et al., Reference Nakatsuka2015). If studies had more than three groups such as an intervention, comparison, and control group, we analyzed the results from the reminiscence group and control group; however, if we were not able to extract the results from the reminiscence or control group, the study was excluded.
The outcome variables considered for the inclusion criteria were depression, QoL, and BPSD as primary outcomes to measure the effect of reminiscence therapy. Depression was typically measured by the self-reported Geriatric Depression Scale (GDS) and Cornell Scale for Depression in Dementia (CSDD) as an observed scale. Most studies assessed QoL with the Quality of Life in Alzheimer’s Disease (QoL-AD) measurement. BPSDs were measured by various instruments such as the Behavioral Rating Scale (BRS), Clifton Assessment Procedures for the Elderly (CAPE), and Cohen-Mansfield Agitation Inventory (CMAI).
Exclusion criteria
Exclusion criteria of this study were as follows: 1) studies including participants who were diagnosed with psychiatric disorders such as depression, 2) studies that did not extract results on the effect of reminiscence therapy including reality orientation or multi-sensory treatment, 3) qualitative or case control studies, 4) protocol and/or pilot studies that did not report results on the effect of reminiscence therapy, and 5) studies that were not written in English or Korean.
Assessment of the risk of bias
Evaluating the risk of bias (ROB) is one of the key steps in a systematic review and assessment the internal validity of the study, and eventually, evaluating ROB allows one to assess the strength of evidence (Hartling et al., Reference Hartling2012). Therefore, ROB was evaluated as a first step of a systematic review. However, this approach can only be used to interpret the quality of studies, not to merge the results as part of a quantitative evaluation. Therefore, we conducted a meta-analysis as the next step to identify the effect of reminiscence therapy on the selected variables.
Two of the study authors independently assessed the quality of the included studies using the “Risk of Bias (ROB)” tool developed by the Cochrane Collaboration, which is illustrated in Figure 2 (Higgins et al., Reference Higgins2011). The Risk of Bias tool assesses random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. We classified the ROB of the studies as “low risk,” “high risk,” or “unclear risk.”

Figure 2. The Cochrane Collaboration tool for assessing risk of bias.
Eighteen out of 24 RCTs were evaluated as having low risk of bias, which indicates a high quality study, and 6 RCTs were evaluated as having high risk of bias. When the random sequence generation method was assessed to evaluate the selective bias, 10 RCTs were evaluated as low risk of bias because they conducted a simple randomization using a computer or stratified randomization considering age, level of education, type of dementia, or type of institution. Six RCTs were evaluated as having high risk of bias. For example, if subjects with even numbers were assigned to the experimental group, the study was evaluated as having a high risk of bias because the odd or even numbers of a table list are a systematic or non-random approach compared to sequence generation by computer random number generation or coin tossing (Higgins et al., Reference Higgins2011). Studies without descriptions of selection bias were evaluated as unclear bias.
Blinding was evaluated based on whether the study participants and key study personnel were blinded to the study design and protocol. Due to the nature of reminiscence therapy as a non-pharmacological intervention, it is not possible for the study participants or key study personnel to be blinded (Ito et al., Reference Ito, Meguro, Akanuma, Ishii and Mori2007), so we evaluated all 24 RCTs as “high risk” of bias. In evaluation of the blinding of outcome assessment, we evaluated whether the measurement of outcome variables was performed by a third person and determined that 15 out of 24 RCTs were considered to have “low risk” of bias.
For evaluating incomplete outcome data, 11 out of 24 RCTs were evaluated as “low risk” of bias since more than 90% of the enrolled participants were included in the analysis. Studies with a low risk of bias were analyzed with the intention to treat (ITT) principal, mean value (Azcurra, Reference Azcurra2012; Wang, Reference Wang2007), or multiple imputation (O’Shea et al., Reference O’Shea2014; Woods et al., Reference Woods, Bruce, Edwards, Elvish and Hoare2012) in order to replace missing data.
The evaluation of reporting of selective outcomes was divided into either reported results or unreported results. Five out of 24 RCTs were evaluated as “high risk” of bias because the studies did not report results on all outcome variables mentioned in the methods section.
Overall risk of bias was evaluated, and studies with low risk of bias were considered as good quality studies if they included post-treatment assessment by assessors blind to treatment allocation (Woods et al., Reference Woods, Spector, Jones, Orrell and Davies2005). We also considered other bias such as detection, attrition, and reporting bias. Ultimately, 18 studies were evaluated to be of good quality.
Statistical analysis
A meta-analysis of 23 datasets from 24 studies was performed using Comprehensive Meta-Analysis Software® (Biostat, Englewood, NJ, USA) to estimate the overall effect size of the intervention. Among the 24 studies included in the ROB assessment, one was excluded because it did not report statistical information such as the mean and standard deviation, which were required to calculate the effect size for the meta-analysis. We divided the result of one study according to the type of dementia and showed that the separated results did not violate the independence assumption (Tadaka and Kanagawa, Reference Tadaka and Kanagawa2007). The overall effect size was determined using standardized mean differences (SMDs) and 95% confidence intervals. A test for quantitative heterogeneity was performed using Cochran’s Q statistics based on Chi-square test. According to the result of Cochran’s Q statistics, a random effect model was used, because Chi-square test ( p<0.1) indicated that the true effect size was not the same for all studies (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). In addition, we computed I 2 as the percentage of overall variance from the heterogeneity among the studies. According to I 2 value, we confirmed the use of a random effect model, because an I 2 value larger than 50% indicated heterogeneity among included studies (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). Funnel plots were generated to confirm the publication bias.
Results
Description of the included studies
Among 1763 participants in the 23 studies, the average ages of subjects in the intervention and control group were 85.2 and 85.3 years, respectively. Three studies included only Alzheimer’s disease, two studies included only vascular disease, one study included both Alzheimer’s and vascular dementia, and the rest of the studies included all types of dementia. Most of the studies screened the severity of dementia using either the MMSE or CDR and excluded subjects who had communication problems because of vision and/or hearing impairments.
In seven of the studies, the interventions were conducted in 12 sessions, which was the highest intervention dosage. Individual reminiscence interventions were conducted in five studies, while the rest of the studies conducted group reminiscence therapy. Interventions using social contact or counselling, exercise therapy, or usual care were included in the control group. Depression, cognition, behavioral problems, QoL, communication ability, functional status, and caregiver burden were assessed to measure the effects of the interventions. The results of the included studies are shown in Table 1.
Table 1. Summary of the included studies
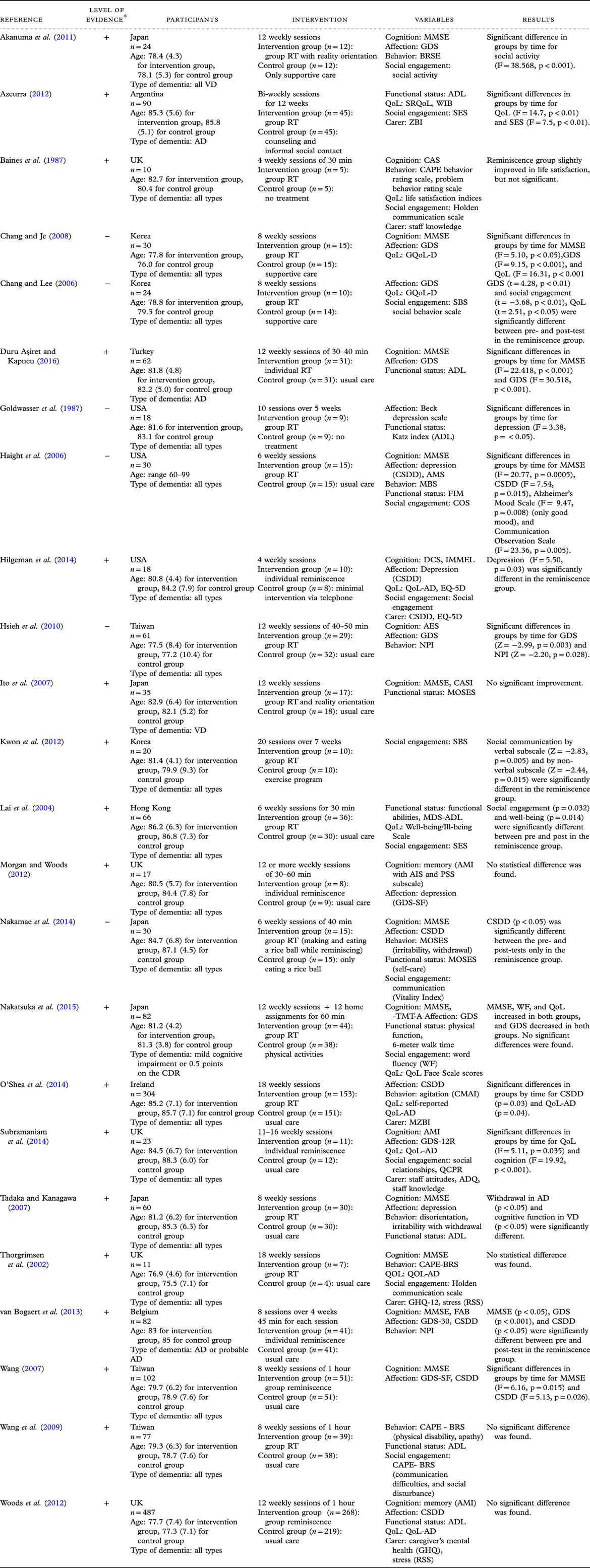
* Level of evidence = (+): Low risk of bias (−): High risk of bias.
Effects of the interventions
Depression, quality of life, and BPSD were selected to measure the effects of reminiscence therapy. Depression was measured in 16 RCTs. In 10 of these RCTs, depression was measured by the self-reported GDS, and the remaining 6 RCTs used the CSDD as an observed scale. With regard to the effects of reminiscence therapy on depression, Cochran’s Q was 91.37 (p<0.001), and I 2 value was 84%. The results indicated large heterogeneity, so a random effect model was used. The overall mean effect size for depression was −0.541 (95% CI: −0.847 to −0.234, Z = −3.730, p<0.001), indicating that depression was significantly decreased in the reminiscence group compared to the control group.
Based on the moderate heterogeneity indicated by the Cochran’s Q statistics ( p<0.1) and I 2 values (>50%) for the variables of QoL, and BPSD, a random effect model was used. The overall mean effect size from 11 RCTs including QoL was 0.376 (95% CI: 0.075 to 0.677, Z = 2.452, p=0.014), and the overall mean effect size from 10 RCTs including the BPSD variable was −0.327 (95% CI: −0.591 to −0.64, Z = −2.435, p=0.015). These results indicate that QoL significantly increased and BPSD significantly decreased in the reminiscence group compared to the control group. The results comparing depression, QoL, and BPSD between the reminiscence and control group are presented in Figures 3–5.
Sub-group analysis was conducted to compare the results from studies with high quality or studies with small sample size and the results of pooled analysis from 24 RCTs. The result for 18 studies with high quality presented a small to medium effect size (p<0.05), which was similar to the result of pooled analysis from 24 RCTs except for QoL (d=0.23, 95% CI:−0.054 to 0.53, Q=30.87, p<0.001, I2=74.08). In the result from 11 studies with small sample size (≤50), the effect size for cognition and BPSD showed homogeneity, and the effect size for depression, QoL, and BPSD was small (p<0.05). On the contrary, in 13 studies with large sample size (>50), the effect sizes for depression was significantly different, while those for BPSD and QoL were not significantly different between groups.

Figure 3. Comparison outcomes of reminiscence therapy versus control: depression.

Figure 4. Comparison outcomes of reminiscence therapy versus control: quality of life.

Figure 5. Comparison outcomes of reminiscence therapy versus control: BPSD.
With regard to the subgroup analysis for group versus individual reminiscence therapy, the overall mean effect size of the depression variable was –0.785 (95% CI: −1.191 to −0.378, Z=−5.451, p< 0.001) from 5 RCTs that conducted individual reminiscence therapy, and −0.466 (95% CI: −0.798 to −0.135, Z = −2.759, p=0.006) from 12 RCTs that conducted group reminiscence therapy.
A funnel plot method was conducted to assess publication bias. The distribution of studies examining depression, and QoL had a symmetric shape in the funnel plot, indicating that these results did not have any publication bias. However, the distribution of studies on BPSD showed an asymmetric shape, so Kendall’s tau statistic was calculated (Begg and Mazumdar, Reference Begg and Mazumdar1994). Kendall’s tau with continuity correction was −0.53 (z=2.14, p=0.03). Additionally, the imputed point estimate was 0.013 (95% CI:−0.258 to 0.285) using the trim and fill method, presenting that 6 studies are missing. Therefore, these statistics indicated publication bias in the studies included to calculate the effect size for BPSD. Funnel plots are presented in Figure 6.

Figure 6. Funnel plot of selected studies for effect size extraction. A = Depression; B = Quality of Life; C = BPSD.
Discussion
Assessment of the risk of bias
This study conducted a systematic review and meta-analysis to examine the effect of reminiscence therapy; however, there were some issues with the assessment of the risk of bias. The first issue was regarding the interpretation between the evaluation of “Risk of Bias” as qualitative information and the overall effect size as quantitative information calculated by the meta-analysis. In terms of the qualitative evaluation, only 18 out of the 24 RCTs had a low risk of bias (high methodological quality), and the rest had a high risk of bias. Thirteen of the studies had a large sample size more than 50 participants. There were only 2 trials which have more than 300 participants. Two trials that had a very large sample size (>300) or low risk of bias failed to identify significant differences between the reminiscence group and control group (O’Shea et al., Reference O’Shea2014; Woods et al., Reference Woods, Bruce, Edwards, Elvish and Hoare2012).
The result of meta-analysis is retrospective and is susceptible to several sources of bias. Studies with low methodological quality might have been included in the meta-analyses and could have altered the interpretation of the benefit of intervention. Therefore, it is very important to assess the quality of individual studies before performing meta-analysis (Chalmers, Reference Chalmers1991; Felson, Reference Felson1992). This current analysis only included studies belong to the inclusion criteria after conducting assessment of trial quality with ROB tool developed by the Cochrane Collaboration. This current study only included RCTs in order to exclude low quality studies such as case control study, cross sectional study, and protocol study. Although we tried to exclude studies with low quality, we also had to consider publication bias; therefore, we included three articles published in Korean, even though they did not clearly describe random sequence generation and allocation concealment related to selection bias.
The effect size for depression, QoL, and BPSD from 11 studies with small sample size was small. On the contrary, in 13 studies with large sample size, the effect sizes for depression was significantly different, while those for BPSD and QoL were not significantly different between groups. Our result was different from those of previous RCTs with a very large sample size or well-designed structure (O’Shea et al., Reference O’Shea2014; Woods et al., Reference Woods, Bruce, Edwards, Elvish and Hoare2012), so there is some argument. The purpose of RCTs is to present a strong treatment effect through the least biased estimates of treatment. On the contrary, systematic review based on meta-analysis aims to integrate individual effect size, not to summarize (Bailar, Reference Bailar1997).
Secondly, in terms of attrition bias, some studies applied the ITT principle, while other studies analyzed per-protocol or did not have any missing cases. ITT analysis includes every subject that is randomized for treatment assignment (Gupta, Reference Gupta2011). The purpose of the ITT approach maintains that the treatment group is similar to the original randomly assigned groups (Hollis and Campbell, Reference Hollis and Campbell1999). ITT analysis does not allow noncompliance, protocol deviations, or withdrawal and does not consider anything that occurs after randomization (Wertz, Reference Wertz1995). Thus, the Cochrane Handbook posits that, if ITT analysis was applied during a trial, attrition bias can be considered low (Hartling et al., Reference Hartling2012; Higgins and Altman, Reference Higgins, Altman, Higgins and Green2008). However, in ITT analysis, the estimate of treatment effect is generally conservative, and marked difference between compliance and non-compliance groups can complicate data interpretation. Therefore, ITT analysis has been criticized for being too cautious and more susceptible to type 2 error (Gupta, Reference Gupta2011; Hollis and Campbell, Reference Hollis and Campbell1999). Woods et al. (Reference Woods, Bruce, Edwards, Elvish and Hoare2012) reported that their study examined the differences in the results between analysis using the ITT method and complete case analysis and found no significant differences. Furthermore, Woods et al. (Reference Woods, Bruce, Edwards, Elvish and Hoare2012) reported that compliance analysis was significantly different in memory, quality of relationships, and QoL between reminiscence and control groups. Other studies also reported significant differences in outcomes between the ITT method and per-protocol analysis (O’Shea et al., Reference O’Shea2014; Tadaka and Kanagawa, Reference Tadaka and Kanagawa2007). Therefore, better application of ITT analysis is possible if complete outcome data are available for all randomized subjects (Gupta, Reference Gupta2011). Multiple imputation and maximum likelihood methods account for the missing data and the uncertainties they cause, and then a per-protocol technique could be used as a supportive method (CPMP, 2000). Because all of the trials used substandard methods with the potential for bias, we suggest a suitable method for dealing with missing data to increase the effects of non-pharmacological intervention, to reduce the attrition bias, and to increase the quality of evidence in RCTs.
Effectiveness of reminiscence therapy in elderly patients with dementia
Depression is the mostly commonly selected variable to identify the effectiveness of reminiscence therapy in individual studies on PWD. According to findings from this meta-analysis, reminiscence therapy for PWD had a medium effect size on depression, as demonstrated by significantly decreased depression in the reminiscence group compared to the control group. Depressed mood commonly occurs in PWD (Gottfries, Reference Gottfries2001). Chin (Reference Chin2007) reported that reminiscence therapy also has effects on happiness and depression in older adults without dementia. The American Psychiatric Association (APA) has described four different psychotherapeutic approaches for guiding a reminiscence therapy practice to treat patients with dementia. One of these psychotherapeutic approaches is an emotion-oriented approach that aims to improve mood using reminiscence therapy (APA, 2007). For patients with mood disturbance, a systematic review of dementia practice guidelines recommended that non-pharmacological management such as reminiscence therapy should be applied first (Ngo and Holroyd-Leduc, Reference Ngo and Holroyd-Leduc2014). The findings from the systematic review in this study are along the same lines as the APA description and dementia practice guidelines. This could be explained by the finding that the reduction in depressive symptoms could be related to other factors such as increased communication during the intervention. PWD were able to talk without being criticized while reminiscing, which increased their confidence after reminiscence therapy (Duru Aşiret and Kapucu, Reference Duru Aşiret and Kapucu2016). Therefore, the effectiveness of a non-pharmacological intervention should also consider that PWD are receiving a pharmacological intervention; furthermore, the non-pharmacological intervention should have a large effect on reducing the occurrence of depressive mood in PWD (van Bogaert et al., Reference van Bogaert, Van Grinsven, Tolson, Wouters, Engelborghs and Van der Mussele2013).
Lai et al. (Reference Lai, Chi and Kayser-Jones2004) reported that measuring changes in dementia patients is very difficult because there are limitations to addressing cognitive function or behavior on their own, so pleasure and wellbeing could be more suitable measures to identify the effects of interventions in the elderly with dementia. Nakatsuka et al. (Reference Nakatsuka2015) also reported that quality of life might be related to a good attitude and impressive experience during the intervention, so enjoyableness was important for the wellbeing of dementia patients regardless of the type of intervention. Stinson (Reference Stinson2009) also reported that reminiscence therapy increased adaptation to the present time, quality of life, and satisfaction because this intervention is based on remembering events experienced in the past. Therefore, our result was consistent with the Stinson (Reference Stinson2009) report.
Behavioral problems are recognized as important clinical features of dementia. These problems decrease patient quality of life and increase caregiver burden (Akanuma et al., Reference Akanuma2011). A systematic review for dementia practice guidelines recommended that pharmacological management should be used sparingly for BPSD only after non-pharmacological approaches have failed (Ngo and Holroyd-Leduc, Reference Ngo and Holroyd-Leduc2014). Overuse of antipsychotics in order to control the BPSD leads to high cost and harmful side effects, emphasizing the benefit of a combination approach of pharmacological and non-pharmacological treatments (Haight et al., Reference Haight, Gibson and Michel2006; van Bogaert et al., Reference van Bogaert, Van Grinsven, Tolson, Wouters, Engelborghs and Van der Mussele2013). Vascular dementia (VaD) patients, especially those with small vessel disease, present with social inactivity or apathy as a one of the major problems (Staekenborg et al., Reference Staekenborg2010); thus, psychosocial interventions and drug treatments should be encouraged. These results are consistent with those of our meta-analysis.
Our result showed that reminiscence therapy had a small to medium sized effect on depression, QoL, and BPSD in PWDs. Studies including in this current meta-analysis had various sample sizes, outcomes, countries of subject origin, and even different effect sizes. Many researchers and staff in long-term care settings have applied reminiscence therapy as a non-pharmacological intervention, regardless of its effectiveness in PWDs. One of the recent dementia practice guidelines (Ngo and Holroyd-Leduc, Reference Ngo and Holroyd-Leduc2014) recommended that pharmacological intervention to manage the BPSD should be used only after non-pharmacological intervention has failed. This guideline also recommended that non-pharmacological intervention such as reminiscence therapy be initially applied to manage mood symptoms. We expect that this guideline and the findings from this current study will encourage staffs in long-term care settings to develop and apply reminiscence therapy as a clinically appropriate method to decrease depression and BPSD and QoL for PWDs.
With regard to the result of the subgroup analysis for group versus individual reminiscence therapy, the overall mean effect size of the depression variable was –0.785 (95% CI: −1.191 to −0.378, Z=−5.451, p< 0.001) from 5 RCTs that conducted individual reminiscence therapy, and −0.466 (95% CI: −0.798 to −0.135, Z=−2.759, p=0.006) from 12 RCTs that conducted group reminiscence therapy. Therefore, we suggested that the individual approach had a stronger benefit to reduce depression than the group approach for PWDs, specifically the patient-focused intervention and patient centered interview (Hilgeman et al., Reference Hilgeman, Allen, Snow, Durkin, DeCoster and Burgio2014; Woods et al., Reference Woods, O’Philbin, Farrell, Spector and Orrell2018). Moreover, the life review process through reminiscence therapy needs to be developed as an individual approach, because the individual approach was relevant for individuals who wished to discuss one life stage for longer than anticipated (Morgan and Woods, Reference Morgan and Woods2012). In another subgroup analysis for length of study time, the overall mean effect sizes of the depression, QoL, and BPSD in reminiscence group with less than 8 sessions were significantly different, but the overall mean effect sizes of the QoL and BPSD in reminiscence group with less than 6 sessions or 4 sessions were not significantly different. Therefore, we suggested that reminiscence therapy of more than 8 sessions might be required to obtain the therapeutic effects on the QoL and BPSD.
There were some limitations in this study. First, we did not separately analyze the effects of group and individual reminiscence therapy. Second, the study setting was not distinguished, so some of the treatments occurred in care homes, while others were in community settings. Third, in this study, the study participants were not able to distinguish by neither the types of dementia nor diagnosis of dementia, therefore our results could not describe the differences in effects by reminiscence therapy; however, because Tadaka and Kanagawa (Reference Tadaka and Kanagawa2007) found differences in MMSE scores between vascular dementia and Alzheimer’s dementia, future study is suggested to examine the effects of reminiscence therapy on cognition as measured by MMSE according to the types of dementia.
Conclusion
This systematic review and meta-analysis provide the evidence that reminiscence therapy can have beneficial effects on depression, QoL, and BPSD in PWD. A heterogeneity test of effect size under a random effects model was performed. Depression and BPSD were significantly decreased, and QoL were significantly increased in the reminiscence group compared to the control group. Therefore, reminiscence therapy could be broadly used to control depression as an alternative to antipsychotics with harmful side effects or high cost.
Conflict of interest
There are no conflicts of interest to disclose.
Description of author’s roles
GR. Hong and K. Park designed the study, K. Park and S. Lee reviewed the selected articles and rated the risks of bias, J. Yang and T. Song reviewed the literature and organized them. K. Park was responsible for carrying out the statistical analysis. All authors drafted the article and critically reviewed it. All contributing authors have read and approved the final version of the manuscript.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science, ICT, & Future Planning: MSIP) (NO. NRF-2013R1A1A2073275).
Appendix: List of Abbreviations
- AD
= Alzheimer’s Disease
- ADL
= Activities of Daily Living
- ADQ
= Approaches to Dementia Questionnaire
- AES
= Apathy Evaluation Scale
- AIS
= Autobiographical Incidents Schedule
- AMI
= Autobiographical Memory Interview
- AMS
= Alzheimer Mood Scale
- BRS
= Behavioral Rating Scale
- BRSE
= Behavioral Rating Scale for the Elderly
- CAPE
= Clifton Assessment Procedures for the Elderly
- CAS
= Cognitive Assessment Scale
- CMAI
= Cohen-Mansfield Agitation Inventory
- COS
= Communication Observation Scale
- CPS
= Cognitive Performance Scale
- CSDD
= Cornell Scale for Depression in Dementia
- DCS
= Depressive Cognition Scale
- EQ-5D
= Euro Quality Of Life -5Dimensions
- FAB
= Frontal Assessment Battery
- FIM
= Functional Independence Measure
- GDS
= Geriatric Depression Scale
- GDS-SF
= Geriatric Depression Scale-Short Form
- GHQ
= General Health Questionnaire
- GQoL-D
= Geriatric Quality Of Life Dementia
- IMMEL
= Index for Managing Memory Loss
- NPI
= Neuropsychiatric Inventory
- MBP
= Memory Behavior Problem
- MBPC
= Memory & Behavior Problem Checklist
- MDS-ADL
= Minimal Data Set Activities of Daily Living
- MMSE
= Mini-Mental State Examination
- MOSES
= Multidimensional Observation Scale for Elderly Subjects
- MZBI
= Modified Zarit Burden Interview
- PSS
= Perceived Semantic Scale
- QCPR
= Quality of the Caregiving Relationship
- QoL
= Quality Of Life
- QoL-AD
= Quality of life in Alzheimer’s Disease
- RAID
= Rating Anxiety in Dementia
- RT
= Reminiscence Therapy
- RSS
= Relatives Stress Scale
- SBS
= Social Behavior Scale
- SES
= Social Engagement Scale
- SRQoL
= Self Reported Quality Of Life
- TMT-A
= Trail Making Test part A
- UK
= United Kingdom
- USA
= United States of America
- VD
= Vascular Dementia
- WF
= Word Fluency
- WIB
= Well/Ill -Being
- ZBI
= Zarit Burden Interview









