Introduction
Mild Cognitive Impairment (MCI) is a syndrome characterized by a decline in cognitive functions greater than expected for age and education, but that does not interfere notably with daily life activities (Gauthier et al., Reference Gauthier2006). Prevalence in the elderly general population (>65 years) ranges from 3% to 19%, and more than half of the affected patients develop dementia within 5 years (Gauthier et al., Reference Gauthier2006). Patients with MCI can be considered as a risk state for dementia and represents a strategic intervention point (Burns and Zaudig, Reference Burns and Zaudig2002; Petersen and Morris, Reference Petersen and Morris2005). As a consequence, patients affected by MCI are increasingly involved in clinical trials aimed at testing new anti-dementia treatments and interventions and often receive dementia-related medication (Ilhan Algin et al., Reference Ilhan Algin, Dagli Atalay, Ozkan, Ozbabalik Adapinar and Ak Sivrioz2017; Ma et al., Reference Ma2017; Petersen et al., Reference Petersen2005).
Several studies reported impaired decisional capacity to consent to treatment or research in this group of patients (Appelbaum, Reference Appelbaum2010; Griffith et al., Reference Griffith2010; Han et al., Reference Han, Boyle, James, Yu and Bennett2015; Jefferson et al., Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008; Reference Jefferson2012; Lui et al., Reference Lui2012; Mittal et al., Reference Mittal2007; Okonkwo et al., Reference Okonkwo2007; Reference Okonkwo2008a; Reference Okonkwo2008b; Simpson, Reference Simpson2010). Hence, patients with MCI are a clinical population at risk of decisional incapacity, whose treatment requires clinicians to carefully find the proper balance between respecting the right of capable patients to make choices (even if clinicians do not approve) about their treatment or participation in clinical trials and the right of incapable patients to be protected from the possible harmful consequences of their improper decisions.
Meeting three criteria is required to ensure an adequate informed consent acquisition, namely, full information disclosure, voluntariness, and patient’s capacity to make a decision (Appelbaum, Reference Appelbaum2007). Following the model of Grisso and Appelbaum decisional capacity consists of four elements, i.e. understanding, appreciating, reasoning, and expressing a choice (Appelbaum, Reference Appelbaum2007). The capacity threshold to consent to clinical research has different characteristics from that for treatment, including understanding and manipulating information related to the possibility to receive a placebo or not benefitting directly from the experimental intervention, as well as the possibility to withdraw from the study at any time without negative consequences, or the risk for serious or unknown adverse events (Dunn and Jeste, Reference Dunn and Jeste2001; Parmigiani et al., Reference Parmigiani, Mandarelli, Dacquino, Pompili, Lelli Chiesa and Ferracuti2016). Patients involved in clinical research moreover might fail to acknowledge the distinction between research and usual care, and assume that decisions about their care will be made only for their individual benefit, a process defined as therapeutic misconception (Dunn et al., Reference Dunn, Palmer, Keehan, Jeste and Appelbaum2006b). In addition, requirements for decisional capacity can vary by jurisdiction and according to different research protocols or medical treatments (the higher the risk, the higher is the required decisional capacity level).
When performing decisional capacity evaluations, physicians tend to rely on their clinical judgment; however the reliability of clinician-based capacity evaluation criteria has been questioned, especially when unstructured evaluations are performed (Raymont et al., Reference Raymont2004). Such an issue is of particular concern especially when assessing patients whose cognitive functioning is half-way between being completely intact and grossly compromised (Appelbaum, Reference Appelbaum2010), such as for patients affected by MCI.
To overcome this issue, several tools have been developed, which are aimed at guiding and supporting the clinician when assessing decisional capacity to consent to treatment or research (Appelbaum and Grisso, Reference Appelbaum and Grisso2001; Dunn et al., Reference Dunn, Nowrangi, Palmer, Jeste and Saks2006a; Grisso et al., Reference Grisso, Appelbaum and Hill-Fotouhi1997; Janofsky et al., Reference Janofsky, McCarthy and Folstein1992; Jeste et al., Reference Jeste2007; Marson et al., Reference Marson, Ingram, Cody and Harrel1995; Parmigiani et al., Reference Parmigiani, Mandarelli, Dacquino, Pompili, Lelli Chiesa and Ferracuti2016), among which the most widely used are the MacArthur Competence Assessment Tool for Treatment (MAcCAT-T) (Grisso and Appelbaum, Reference Grisso and Appelbaum1998) and the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) (Appelbaum and Grisso, Reference Appelbaum and Grisso2001).
Decisional capacity to consent to medical treatment (Appelbaum, Reference Appelbaum2007; Hamann et al., Reference Hamann2011; Lui et al., Reference Lui2012; Martin et al., Reference Martin2008; Moye et al., Reference Moye, Karel, Gurrera and Azar2006; Okonkwo et al., Reference Okonkwo2007; Reference Okonkwo2008a; Reference Okonkwo2008b; Palmer et al., Reference Palmer, Dunn, Appelbaum and Jeste2004; Raymont et al., Reference Raymont2004) and to clinical research (Jefferson et al., Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008; Reference Jefferson2012; Mittal et al., Reference Mittal2007) are negatively associated with an executive (Koren et al., Reference Koren, Poyurovsky, Seidman, Goldsmith, Wenger and Klein2005; Mandarelli et al., Reference Mandarelli, Parmigiani, Tarsitani, Frati, Biondi and Ferracuti2012) and global cognitive dysfunctions (Karlawish et al., Reference Karlawish, Casarett and James2002; Mandarelli et al., Reference Mandarelli2014; Reference Mandarelli2018; Reference Mandarelli2019; Palmer et al., Reference Palmer2005; Palmer and Savla, Reference Palmer and Savla2007; Stroup et al., Reference Stroup2005).
Previous reviews have investigated the prevalence of mental incapacity to consent in patients affected by cognitive impairment and Alzheimer’s disease (AD) (Appelbaum, Reference Appelbaum2010; Kim et al., Reference Kim, Karlawish and Caine2002; Simpson, Reference Simpson2010; van Duinkerken et al., Reference van Duinkerken, Farme, Landeira-Fernandez, Dourado, Laks and Mograbi2018), but the clinical, demographical, and neuropsychological factors associated with the decisional capacity in patients with MCI remains elusive. We deem that identifying these factors would inform strategies to develop interventions tailored at enhancing decisional capacity in this vulnerable group of patients. The objective of this systematic review and meta-analysis was to identify which clinical, demographic, and treatment-related variables were associated with informed consent to treatment and research in patients with MCI, and to investigate the differences in the decisional capacity to consent to treatment and research between patients with MCI, AD, and healthy comparisons (HCs).
Materials and methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Liberati et al., Reference Liberati2009).
Literature search
We used a systematic search strategy to identify relevant papers. We conducted a two-step literature search on 19 October 2019. As a first step, the Medline/Pubmed, CINAHL, PsycINFO, Web of Science, and Scopus databases were searched, with the following string: (“Mild Cognitive Impairment” OR “mild neurocognitive disorder”) AND (“decision making” OR “informed consent” OR “decisional capacity” OR “mental competency” OR “mental capacity”). The second step involved the implementation of an additional electronic search based on a manual search of the reference lists of the retrieved papers by two investigators (G.P. and B.B.). Abstracts of papers identified through these two steps were then screened for eligibility, and papers surviving this screening were assessed for eligibility based on a full-text reading. Discrepancies were resolved through consensus with a third author (A.D.), and eventually, Delphi rounds with all other authors. The protocol for this review has been registered in the international prospective register of systematic reviews (PROSPERO registration number CRD42020158692).
Inclusion and exclusion criteria
Papers were included if dealing with consent to treatment or research, in patients affected by MCI. We excluded papers written in languages other than English, Italian, Greek, Hebrew, or Spanish, reviews, retrospective studies, or case reports, and those papers whose full text was unavailable even after contacting the corresponding author.
Data extraction (selection and coding)
Titles and abstracts were screened by two reviewers independently, in duplicate, to determine whether retrieved studies met the above-outlined inclusion criteria.
Full texts were obtained for studies apparently meeting inclusion criteria or where a decision could not be made from the title and/or abstract alone, for a detailed review against inclusion criteria. Full-texts were independently assessed for eligibility by two reviewers. Discrepancies were resolved by an initial discussion with a third reviewer, when required, and possibly, with Delphi rounds, until complete consensus was reached.
A standardized form was used to extract data from the included studies to assist in study quality and evidence synthesis. Extracted information included: the focus of the study, participant characteristics, screening tools/neuropsychological assessments performed, the decisional ability construct the authors were referring to, the criterion used to validate the final judgment (capable/incapable), and authors conclusions, as well as information required for assessment of the risk of bias. Extraction was completed by two reviewers independently, in duplicate. A third reviewer was consulted when needed.
Quality evaluation
The assessment of observational study quality was conducted using the Newcastle–Ottawa scale (NOS) (Deeks et al., Reference Deeks2003). Two reviewers independently assessed the methodological quality of the studies using the NOS for case–control study in three domains: (1) subject selection: a score of 0–4, (2) comparability of the case and control groups: a score of 0–2, and (3) the ascertainment of either the exposure or outcome of interest: a score of 0–3 (Deeks et al., Reference Deeks2003). Disagreements were resolved through Delphi rounds until full consensus was reached.
Statistical analyses
The Review Manager software version 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration) and the Comprehensive Meta-Analysis software version 2 (Biostat, Inc., Englewood, New Jersey, USA) were used to perform the meta-analysis. We performed four meta-analyses to examine the psychometric properties of the tools used to assess decisional capacity to consent to treatment or research, including Understanding, Reasoning, Appreciation, and Expression of a Choice. The Cochran’s Q-statistic test and the I 2 statistic were used to evaluate the heterogeneity of the studies. A P-value of Q text <0.10 or I 2 > 50% indicated significant heterogeneity (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). A subgroup analysis was performed on studies sharing the same decisional capacity instrument. The outcome measures from the individual studies were combined using a random-effects model. Since scores on the decisional capacity subscales were continuous data obtained from different scales (MacCAT-T, Capacity to Consent to Treatment Instrument – CCTI, MacCAT-CR, University of California Brief Assessment of Capacity to Consent – UBACC), standardized mean difference (SMD) with 95% confidence intervals (CIs) was used to analyze the studies. We considered P < 0.05 (two-tailed) to be statistically significant. Publication bias was tested by visually inspecting the funnel plot and performing the Egger’s linear regression test. Sensitivity analysis was conducted by removing each study individually to evaluate the quality and consistency of the results.
Results
We identified 109 potentially eligible studies from 1672 records obtained from the selected databases and one from alternative sources. After reviewing the full content of the papers, 102 papers were excluded for several reasons: 49 did not investigate the capacity to consent to treatment or research, 26 were case descriptions, editorials, or reviews, 16 examined a different population or did not provide data separately for MCI, 7 did not provide the needed data, even after their authors were contacted, and 4 contained duplicate data. The process of identifying eligible studies is outlined in Figure 1. For the list of the excluded studies see the Supplementary Data file published as supplementary material online attached to the electronic version of this paper.

Figure 1. The flow diagram of the study screening, conducted according to the PRISMA guidelines.
Studies, participants, and treatment characteristics
The characteristics of the included studies are summarized in Table 1. Of the seven studies, one study used the MacCAT-CR (Jefferson et al., Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008), one used the UBACC (Duron et al., Reference Duron2013), three studies used the CCTI (Griffith et al., Reference Griffith2010; Okonkwo et al., Reference Okonkwo2007; Reference Okonkwo2008b), and two studies used the MacCAT-T (Lui et al., Reference Lui2012; Oshima et al., Reference Oshima2020).
Table 1. Studies on decisional capacity to consent to treatment or research
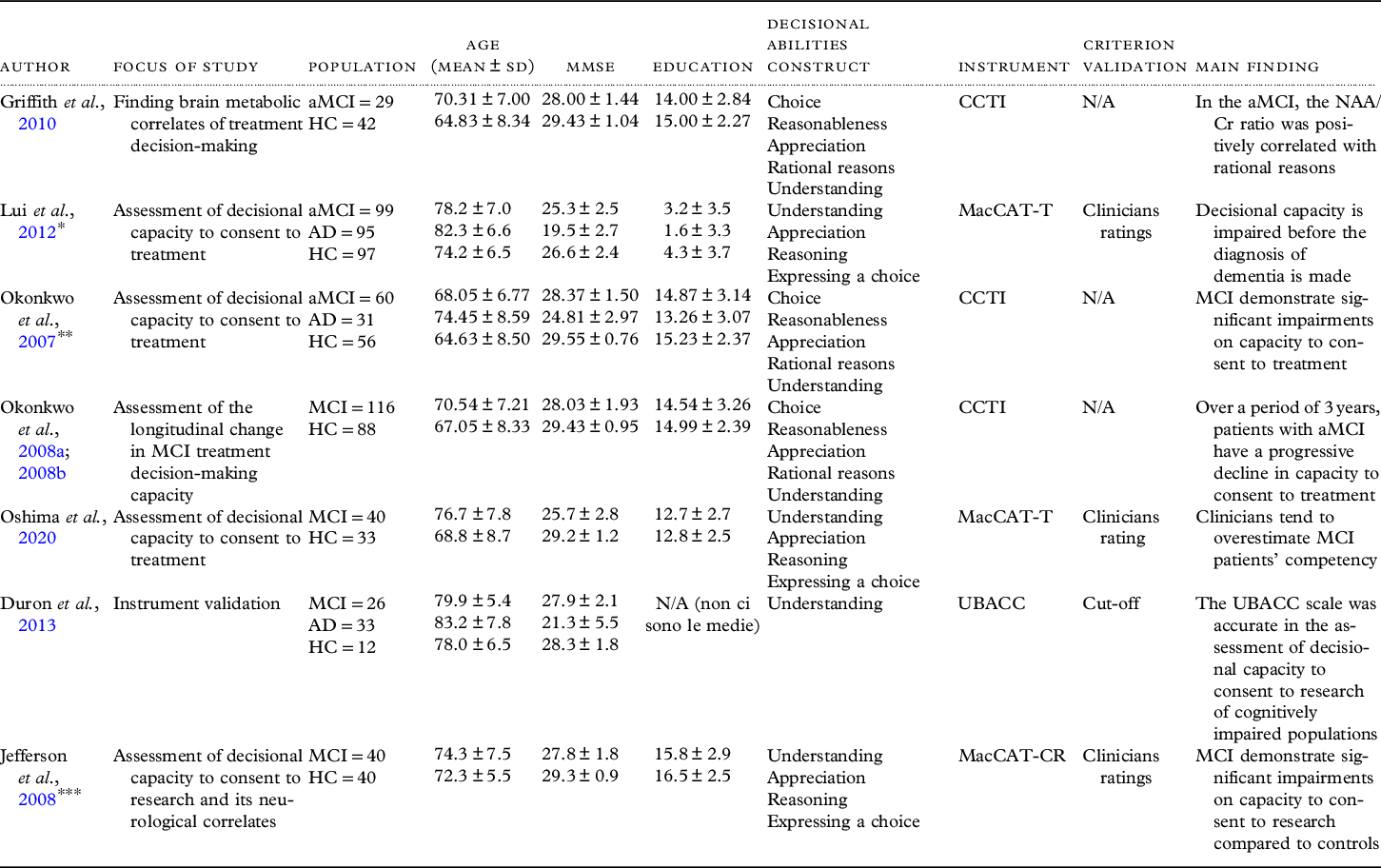
aMCI = amnestic Mild Cognitive Impairment; AD = Alzheimer’s disease; MMSE = Mini Mental State Examination; CCTI = Capacity to Consent to Treatment Instrument; MacCAT-CR = MacArthur Competence Assessment Tool for Clinical Research; MacCAT-T = MacArthur Competence Assessment for Treatment; SD = standard deviation; UBACC = University of California Brief Assessment of Capacity to Consent; NAA/Cr = N-acetylaspartate (NAA)/Creatine (Cr); *Incorporates data of Lui et al. Reference Lui2013, and Lam et al. Reference Lam, Chiu, Leung, Appelbaum and Karlawish2013; **Incorporates data of Okonkwo et al. Reference Okonkwo2008a; Reference Okonkwo2008b; ***Incorporates data of Jefferson et al., Reference Jefferson2012.
The MacCAT-T is a semi-structured interview that assesses the main facets of treatment-related decision-making, reflecting commonly applied legal standards for competence to consent to treatment. The subscales investigate the understanding of the disclosed information about the disorder and the treatment’s main features, as well as presumed associated risks and benefits (rated 0–6); appreciation, i.e. the patient’s ability to appreciate his/her own diagnosis and treatment (rated 0–4); the patient’s reasoning ability, including consequential and comparative thinking, and logical consistency (rated 0–8); and the ability to clearly express a choice (rated 0–2). In the included studies it was used to assess the capacity of subjects to consent to medical treatment, based on a hypothetical situation (Lui et al., Reference Lui2012) and hypothetical vignettes (Oshima et al., Reference Oshima2020).
The CCTI is a vignette-based assessment measure of medical decision-making capacity, structured on five consent standards: expressing a treatment choice (S1; range: 0–4), making a reasonable treatment choice (S2; %), appreciating the consequences of a treatment choice (S3; range: 0–8), providing rational reasons for a treatment choice (S4; range: 0–12), and understanding the treatment choices (S5; range: 0–78). However, the capacity of making a reasonable treatment choice (S2) is not a clinically accepted consent standard because of concerns about the arbitrariness of the operative term reasonable (Okonkwo et al., Reference Okonkwo2007). The CCTI consists of two clinical vignettes that each present a hypothetical medical problem (A: neoplasm, B: cardiovascular disease) and symptoms, and two treatment alternatives with associated risks and benefits.
The MacCAT-CR is a semi-structured interview, which relies on the same MacCAT-T multidimensional capacity model but comprises 21-items assessing four abilities underlining competence to consent to clinical research: understanding of the research project’s purpose, risks, potential benefits, and procedures (range 0–26); appreciation of the effect of research participation on one’s own condition (range 0–6); reasoning about the consequences of participation (range 0–8); and expression of a choice in a consistent way (range 0–2). In the included study (Jefferson et al., Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008) was used to assess subjects’ decision-making to consent to a hypothetical clinical trial.
The UBACC is a 10-item scale, developed as a screening instrument, and focuses on capacity to provide informed consent to a research protocol. It investigates the understanding of the research project’s purpose, risks, benefits, and nature (research or treatment) (range 0–8); appreciation of the effect of research participation on one’s own condition (range 0–10); and reason about the motive to participate (range 0–2). Duron et al. (Reference Duron2013) used this instrument on a subsample of patients enrolled in a real clinical trial to assess the relationship between IGF-1 and IGFBP-3 serum levels and cognitive impairment, in a large sample of older subjects evaluated in memory clinics.
Among the included studies, only one (Griffith et al., Reference Griffith2010) mentioned that a portion of patients affected by MCI was taking pharmacological treatment (cholinesterase inhibitors) during the study period. Jefferson et al. (Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008) referred to slightly deviate the MacCAT-CR administration from the manual (by allowing participants to retain a copy of the consent disclosure while answering all items) to enhance the ecological validity of the consent process.
A total number of 410 patients with MCI, 149 patients with AD, and 368 healthy subjects were included. Four studies were conducted in the United States (Griffith et al., Reference Griffith2010; Jefferson et al., Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008; Okonkwo et al., Reference Okonkwo2007; Reference Okonkwo2008a; Reference Okonkwo2008b), one in France (Duron et al., Reference Duron2013), one in Japan (Oshima et al., Reference Oshima2020), and one in China (Lui et al., Reference Lui2012). The mean age ranged from 68.05 to 79.9 years for the MCI patient group, from 74.45 to 83.2 years for the AD patient group, and from 64.63 to 78 years for the HCs. Mean years of education ranged from 3.17 to 15.8 for the MCI patient group, from 1.62 to 13.26 for the AD patient group, and from 4.3 to 16.5 for the HCs. The mean MMSE total score ranged from 25.3 to 28.37 for the MCI patient group, from 19.5 to 24.81 for the AD patient group, and from 26.6 to 29.55 for the HCs.
Quality evaluation
The NOS scores of the seven studies ranged from five to four points (Table 2). Patients from four out of seven studies had adequate definitions with independent validation. Four studies performed an independent decisional capacity evaluation by a physician blind to capacity assessment (Duron et al., Reference Duron2013; Jefferson et al., Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008; Lui et al., Reference Lui2012; Oshima et al., Reference Oshima2020). Reasons for increased bias included inadequate representativeness of patients (no study enrolled consecutive patients), and the lack of age- and education-matching between patients with MCI, AD, and HCs.
Table 2. Quality assessment through the Newcastle–Ottawa scale (NOS)

Decision-making capacity in Mild Cognitive Impairment
A total number of 410 patients with MCI and 368 HCs from 7 studies were compared on Understanding scores. Using random-effects models, patients scored significantly lower on Understanding (SMD = −1.13, 95% CI: −1.41 to −0.84, P < 0.001; I 2 = 67%, P = 0.006). Due to subscale heterogeneity, a sensitivity analysis was conducted; we detected a significant decrease in the Understanding subscale score of patients with MCI compared with their control counterparts (SMD = −1.04, 95% CI: −1.31 to −0.77, P < 0.001; I 2 = 52%, P = 0.07, Figure 2A).

Figure 2. Meta-analysis of the standardized mean difference in Understanding (A), Appreciating (B), Reasoning (C) and Expressing a choice (D) of patients with MCI versus HCs.
A total number of 384 patients with MCI and 356 healthy subjects from 6 studies were compared on the scores of Appreciating, Reasoning, and Expression of a choice. Using random-effects models, patients scored significantly lower on Appreciating (SMD = −0.51, 95% CI: −0.66 to −0.36, P < 0.001; I 2 = 0%, P = 0.97), and on Reasoning (SMD = −0.62, 95% CI: −0.77, −0.47, P < 0.001; I 2 = 0%, P = 0.46), but no significant differences were found in the Expression of a Choice subscale (SMD = −0.19, 95% CI: −0.52, 0.14, P = 0.27; I 2 = 77%, P = 0.002), compared with the control group (Figures 2B, 2C and 2D).
To overcome the limitations of comparing studies using different decisional capacity measures, we performed a subgroup analysis on 205 patients with MCI and 186 healthy subjects from 3 studies that used the same instrument (CCTI) on the score of Understanding, Appreciating, Reasoning, and Expression of a choice. Using random-effect models, patients scored significantly lower on Understanding (MD = −15.12, 95% CI: −17.24 to −13, P < 0.001; I 2 = 2%, P = 0.36), on Appreciating (MD = −0.60; 95% CI: −0.83 to −0.37, P < 0.001; I 2 = 0%, P = 0.77), and on Reasoning (MD = −2.54, 95% CI: −3.25 to −1.84, P < 0.001; I 2 = 0%, P = 0.42), but no significant differences were found in the Expression of a Choice subscale (MD = 0.01; 95% CI: −0.07 to 0.10, P = 0.74; I 2 = 0%, P = 0.62) compared with the HC group (see Figure S1 published as supplementary material online attached to the electronic version of this paper.
A total number of 185 patients with MCI and 149 patients with AD from 3 studies were compared on their scores on Understanding. Using random-effects models, patients with AD scored significantly lower on Understanding (SMD = 1.50, 95% CI: 0.91, 2.09, P = 0.01, I 2 = 78%, P = 0.001; Figure 3).

Figure 3. Meta-analysis of the standardized mean difference in Understanding of patients with MCI versus AD.
Due to the dearth of available data, further analysis could not be conducted to identify which clinical, demographic, and treatment-related variables were associated with informed consent to treatment and research in patients with MCI.
Publication bias and sensitivity analysis
Visual inspection of the funnel plot and the Egger’s regression test did not show any publication bias for the subscales of Understanding (t = 0.50; P = 0.64), Appreciation (t = 1,739; P = 0.157), Reasoning (t = 0.538; P = 0.619), and Expression of a Choice (t = 0.64; P = 0.567) in the comparison between patients affected by MCI and HCs. Similarly, the Egger’s regression test did not show any publication bias for the subscale of Understanding (t = 5.725; P = 0.11) in the comparison between patients with MCI and with AD. However, we should be cautious in excluding the presence of publication bias, considering the low statistical power of the test in a meta-analysis based on a limited number of trials (Eggers et al., Reference Egger, Davey Smith, Schneider and Minder1997; Higgins et al., Reference Higgins2020). After removing each study one by one, the results were not altered compared to the primary analysis, suggesting the consistency of the results.
Discussion
This meta-analysis of seven studies showed significantly lower scores in capacity to consent to treatment and research in patients with MCI compared to HCs in understanding, appreciating, and reasoning, while they scored significantly higher compared to AD patients on understanding. The magnitude of the effects was medium to large across different decisional capacity subscales. Regarding the Understanding subscale, the confidence in the estimate of the effect is moderate despite the large effect size because of some inconsistency due to the moderate and high degree of heterogeneity between studies in the comparison between patients with MCI and HC and with MCI and with AD, respectively. Overall, our review is consistent with impaired decision-making in patients with MCI (Appelbaum, Reference Appelbaum2010; Griffith et al., Reference Griffith2010; Han et al., Reference Han, Boyle, James, Yu and Bennett2015; Jefferson et al., Reference Jefferson, Lambe, Moser, Byerly, Ozonoff and Karlawish2008; Reference Jefferson2012; Lui et al., Reference Lui2012; Mittal et al., Reference Mittal2007; Okonkwo et al., Reference Okonkwo2007; Reference Okonkwo2008a; Reference Okonkwo2008b; Simpson, Reference Simpson2010), and highlights the need for accurate and efficient capacity evaluations in this vulnerable group of patients. Patients with MCI are increasingly treated with dementia-related medications and are also often involved in clinical trials, and the assessment of their decisional capacity to consent to treatment or research can represent a challenge for physicians who tend to rely on their clinical judgment. For example, a study by Marson et al. (Reference Marson, McInturff, Hawkins, Bartolucci and Harrell1997) found that the achieved agreement among five physicians who reviewed videotapes of capacity assessments and rated the competence of patients with mild AD, without the use of any systematic and structured interview, was not better than chance (kappa statistic, 0.14). A recent study by Oshima et al. (Reference Oshima2020) found that clinicians tend to overestimate MCI patients’ competence to consent to treatment. This is an issue that needs to be addressed, considering that treatment or research in the absence of informed consent, except in an emergency or exceptional circumstance contrasts with the principle of autonomy and may determine physician professional liability issues (Appelbaum, Reference Appelbaum2010). Moreover, the elevated risk for reduced decisional capacity in elderly patients affected by cognitive impairment often raises uncertainty as to whether they can provide valid consent or consent must be obtained from a substitute decision-maker (Appelbaum, Reference Appelbaum2010).
Regarding decisional capacity to consent to research, S. Y. Kim (Reference Kim2011) suggested introducing the concept of authenticity, referring to the congruence between a person’s values (including beliefs, commitments, and relationships) and his/her decision. According to this author, in contrast to an autonomous decision, which can only be made by the patient, a decision can be authentic even when made by a surrogate, because authenticity does not require an intact capacity for self-determination, but only that the decision conforms to the individual’s values.
Although decisional capacity is negatively associated with executive and cognitive dysfunctions, to date, empirical literature does not provide a basis for drawing firm conclusions about the role of specific cognitive domains on specific aspects of healthcare decision-making (Palmer and Harmell, Reference Palmer and Harmell2016). Despite the strong correlation reported between cognitive abilities and decisional capacity components, no clear pattern of differential association has emerged (Palmer and Harmell, Reference Palmer and Harmell2016). Specifically, the Understanding subscale has shown associations with episodic memory (Okonkwo et al., Reference Okonkwo2008a; Palmer et al., Reference Palmer, Dunn, Appelbaum and Jeste2004), executive functioning (Dymek et al., Reference Dymek, Atchison, Harrell and Marson2001; Mandarelli et al., Reference Mandarelli, Parmigiani, Tarsitani, Frati, Biondi and Ferracuti2012), verbal memory, and phonemic fluency (Gerstenecker et al., Reference Gerstenecker, Meneses, Duff, Fiveash, Marson and Triebel2015), and between processing speed and episodic memory (Okonkwo et al., Reference Okonkwo2008a; Reference Okonkwo2008b). Regarding the Appreciation subscale, correlations have been reported for working memory (Palmer et al., Reference Palmer, Dunn, Appelbaum and Jeste2004), processing speed (Okonkwo et al., Reference Okonkwo2008a; Reference Okonkwo2008b), and episodic memory (Okonkwo et al., Reference Okonkwo2008a; Reference Okonkwo2008b). Finally, the Reasoning subscale showed associations with working memory (Moye and Karel, Reference Moye and Karel1999; Palmer et al., Reference Palmer, Dunn, Appelbaum and Jeste2004), and executive functions (Dymek et al., Reference Dymek, Atchison, Harrell and Marson2001; Marson et al., Reference Marson, Chatterjee, Ingram and Harrell1996). Further studies are needed to understand which cognitive functions are associated with the decisional capacity to consent to treatment and research. This is a topic that needs to be addressed because it would allow the suggestion of supplementary tools to evaluate patients’ competency as well as to develop targeted interventions to enhance patients’ decision-making.
To avoid depriving patients of their right to make healthcare decisions, it has been suggested to maximize patients’ decisional capacity through several means, like conducting the clinical evaluation in the patient’s native language, detecting and addressing potentially treatable conditions associated with impaired capacity (such as fever, sedation, dehydration, depression, and anxiety) before obtaining consent; by manipulating the environment where assessment occurs (for example, by performing the evaluation in quiet places to avoid distractions or by assessing patients affected by mild dementia early in the day to avoid sundowning), by enhancing the informed consent process to make the decisional task easier (Appelbaum, Reference Appelbaum2010).
Among the above-mentioned strategies, a promising field of research, regrettably neglected, is represented by the possibility to enhance informed consent by compensating for cognitive deficits. In fact, to the best of our knowledge, studies aimed at improving decisional capacity to consent to research in AD and MCI are few and have led to contrasting results (Mittal et al., Reference Mittal2007; Palmer et al., Reference Palmer2018; Rubright et al., Reference Rubright, Sankar, Casarett, Gur, Xie and Karlawish2010). A study on the use of multimedia disclosure and cognitive feedback to improve decisional capacity to consent to research among patients with AD and non-psychiatric comparison participants found no significant effect of the enhanced consent procedure relative to the routine consent (Palmer et al., Reference Palmer2018). Another study comparing two enhanced consent procedures (a PowerPoint presentation and an enhanced printed consent form) in patients with AD and MCI found an improvement in patients’ understanding of both conditions (Mittal et al., Reference Mittal2007). However, the small sample size of this study (19 patients with AD and 16 with MCI) and the absence of a routine consent comparison condition prevent the generalization of its results. Finally, a study by Rubright and colleagues (Reference Rubright, Sankar, Casarett, Gur, Xie and Karlawish2010), employing a memory and organizational aid added to a standard consent procedure, found an improvement in capacity to consent to research in patients with AD. Moreover, to the best of our knowledge, to date, there is no study that has specifically tested the efficacy of a specific cognitive training aimed at enhancing decisional capacity in informed consent to treatment or research participation. We deem that this a gap that needs to be addressed, especially for those patients whose decisional capacity, although not yet deeply compromised, may benefit from the support and enhancing interventions. This view is also supported by the UN Convention on the Rights of Persons with Disabilities (CRPD; UN General Assembly, 2006) that holds that the full and equal enjoyment of legal capacity for disabled people requires a shift to supported decision-making paradigms and the abolition of substituted decision-making that allows forced treatment (Craigie, Reference Craigie2015; Craigie et al., Reference Craigie2019; Szmukler and Bach, Reference Szmukler and Bach2015; Szmukler et al., Reference Szmukler, Daw and Callard2014).
Limitations
Several limitations need to be acknowledged. First, the sample sizes of the included studies as well as the overall sample were relatively small, the studies were all observational and with a moderate risk of bias. Second, due to the dearth of studies, we pooled together studies using different tools and evaluating capacity to consent to treatment and capacity to consent to research. This was possible because, despite their different capacity thresholds, these are very similar concepts and refer to the same four dimensions (understanding, appreciating, reasoning, and expressing a choice). However, some differences need to be acknowledged. For example, the UBACC has been developed as a screening instrument and employs just one item to assess reasoning; the MacCAT-T and the MacCAT-CR differ in the appreciation dimension as the possibility to quit the research at any time and without consequences is a central aspect. In addition, a study comparing the agreement between three treatment decisional capacity assessment instruments (MacCAT-T, CCTI, HCAI) in mild-to-moderate dementia found a fair agreement for overall capacity and very good for understanding, while poor for Appreciating, Reasoning, and Expression of a choice (Gurrera et al., Reference Gurrera, Karel, Azar and Moye2007). Finally, considering that four different tools for assessing patients’ decisional capacity were used across studies, we used random-effects models and effect size of SMDs.
With these caveats in mind, we deem that our study raises awareness and prompts clinicians and researchers to pay more attention to decisional capacity to consent to treatment or research of patients affected by MCI.
Conflict of interests
All authors declare to have no conflict of interest regarding this work.
Source of funding
This work did not receive private or public grants or funds.
Description of authors’ roles
G. Parmigiani, G. Mandarelli, and S. Ferracuti designed the study, G. Parmigiani and B. Barchielli reviewed the selected papers and rated the risk of bias, G. Kotzalidis, F. D’Antonio, A. Di Vita, and C. de Lena reviewed the literature and organized them. G. Parmigiani and A. Del Casale were responsible for carrying out the statistical analysis. All authors drafted the article and critically reviewed it. All contributing authors have read and approved the final version of the manuscript.
Acknowledgments
We gratefully acknowledge the contribution of Ms Mimma Ariano, Ms Ales Casciaro, Ms Teresa Prioreschi, and Ms Susanna Rospo, Librarians of the Sant’Andrea Hospital, School of Medicine and Psychology, Sapienza University, Rome, for rendering precious bibliographical material accessible.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1041610220004056







