What is already known on this subject: The majority of people are aware that they should be more physically active but it is difficult to motivate people. Much effort has been expended by clinical public health and others to encourage people to undertake more physical activity. Walking is an excellent mode of physical activity, and more may take part if the social side of walking in groups was promoted.
What this study adds: This systematic review demonstrates that walking in groups is more effective than inactivity to increase physical activity in physically healthy people. Far less evidence is available on walking in groups compared with walking alone, but the trend was improved physical activity at follow-up for participants walking in groups.
The World Health Organisation physical activity strategy recommends that adults undertake 150 minutes of moderate aerobic physical activity, such as cycling or fast walking (3–5 miles per hr), or 75 minutes of vigorous activity or a mix of moderate and vigorous activity every week, plus muscle-strengthening exercises on 2 or more days per week that work all of the major muscles in the body (1;2). However, only a relatively small proportion of adults meet these guidelines. In the United States, in 2014, 49.2 percent adults met the physical activity guidelines for aerobic physical activity and 20.8 percent adults met the physical activity guidelines for both aerobic physical and muscle-strengthening activity (3). The equivalent proportions meeting the physical activity guidelines for aerobic physical activity are: 24 percent of men and 21 percent of women in Canada (4), 40 percent of adults in Australia (5), and 67 percent of men and 55 percent of women in the United Kingdom (Reference Townsend, Wickramasinghe, Williams, Bhatnagar and Rayner6).
Dropout rates for exercise initiatives are known to be high (Reference Gidlow, Johnston, Crone and James7;Reference Stiggelbout, Hopman-Rock, Crone, Lechner and van Mechelen8). However, there is good evidence that exercise adherence is enhanced through the use of social support (Reference Wing and Jeffery9;Reference Campbell, Holmes and Everson-Hock10). A recent mixed-methods systematic review on community-based group exercise interventions for older adults found that increased social connectedness, wellbeing gains, and an empowering environment were themes associated with above average long-term adherence rate (Reference Farrance, Tsofliou and Clark11). This study concluded that incorporating participants’ views into exercise program designs could provide guidance for innovative interventions, which would lead to sustained adherence.
Walking is a highly accessible form of physical activity and is associated with a range of positive health benefits (Reference De Moor12;Reference Lee and Buchner13). Governments have strongly encouraged the public to increase physical activity through walking. For example, the U.K. government aimed to invest £7 million between 2008 and 2011 in a program of innovative campaigns to encourage people to walk more (Reference Milton and Grix14;15), and the U.S. Department of Health and Human Services advocates walking as the principle component of its Active Living (16;17) initiative (one of seven priorities in the National Prevention Strategy) (18). And, as mentioned above, the World Health Organization physical activity recommendations include walking.
There have been three recent systematic reviews evaluating the effectiveness of walking groups to enhance health (Reference Hanson and Jones19) and increase physical activity (Reference Kassavou, Turner and French20;Reference Blank, Jones, Buckley Woods and Payne21). They included forty-two studies (Reference Hanson and Jones19), nineteen studies (Reference Kassavou, Turner and French20), and ten studies (in the led walks section) (Reference Blank, Jones, Buckley Woods and Payne21), and all have strengths and weaknesses. For example, two (Reference Hanson and Jones19;Reference Kassavou, Turner and French20) included both randomized and nonrandomized studies, but the other (Reference Blank, Jones, Buckley Woods and Payne21) included randomized controlled trials (RCTs) only. All three included studies with physically and/or mentally healthy participants and studies with participants with a variety of physical conditions that may impede walking (such as knee osteoarthritis), and did not meta-analyze results for different participant groups separately. Also studies included in earlier systematic reviews were not included in later systematic reviews. One (Reference Kassavou, Turner and French20) included more than one effect size estimate per study, thus double counting results from some participants. One (Reference Blank, Jones, Buckley Woods and Payne21) did not conduct meta-analyses, and one (Reference Hanson and Jones19) had a physical functioning (6-min walk test) meta-analysis of two included studies in nonhealthy patients. None of the reviews looked at the specific impact that being part of a group had on adherence to the intervention.
This systematic review evaluates the effectiveness in physically healthy adults of walking in groups compared with inactive controls and/or individuals walking alone, focusing on any measure of physical activity or quality of life at follow-up. By also including walking alone as a comparison group, we examine whether being part of a group is more likely to lead to greater benefits than walking alone.
METHODS
We developed and registered a protocol for this systematic review (Prospero registration number CRD 42016033752). The predefined inclusion criteria were comparative group studies in any language with physically healthy adults taking part in led walks or community group walks with an aspect of social interaction in addition to walking. We defined physically healthy as free from reported physical conditions or pain that would impede walking. We accepted a maximum of 20 percent in any group with pre-existing physical conditions so as not to exclude useful information, because many participants were likely to be older and not all would be completely physically healthy. Any forms of walking groups were compared with either (a) standard care, waiting list or any other nonactive interventions such as physical activity advice or lectures on diet or nutrition (Set 1), or (b) walking alone (Set 2). Outcomes of interest were any measure of physical activity at follow-up and/or any measure of generic quality of life or wellbeing. Outcomes could be measured at any time at or after the end of the intervention.
The following databases were searched between 2010 and March 2016: Medline, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central and Web of Science, Science Citation Index. Search terms included walk*, groups, program*, club, community, healthy, physical activity and exercise. Both MESH terms and keywords were used (see Supplementary Table 1). Search terms were piloted to ensure that searches were sufficiently sensitive to find known includable studies. Reference lists of included studies and systematic reviews (Reference Hanson and Jones19–Reference Blank, Jones, Buckley Woods and Payne21) were checked for includable studies. Because there had been three relevant published systematic reviews with very comprehensive searches, with dates up to 2011–12, our searches were started in 2010 to ensure no studies were missed during the overlapping period. All relevant titles and abstracts were transferred to Endnote for assessment.
Two reviewers (C.M. and J.E.) checked study eligibility independently. Both also independently extracted data from studies into standardized, predesigned extraction tables in Microsoft Word. Disagreements were resolved through discussion. Quality of included studies was assessed using likelihood of selection, performance, attrition, and detection biases because of the variety of study designs included. Specific quality checklists evaluate these biases tailored to different study designs and as we had a variety of study designs included, going back to fundamental quality assessment was considered to be more useful than using a mixture of different checklists.
We tabulated the characteristics and results of all the included studies; analysis was quantitative. Numerical results were presented as point estimates of effect sizes (means, medians) with any reported measures of spread (standard deviations, standard errors, ranges, confidence intervals). Where standard errors, ranges, or 95 percent confidence intervals (CIs) were provided, standard deviations were calculated using standard formulae from the Cochrane Handbook (Reference Higgins and Green22). Review Manager (version 5.3, The Cochrane Library) was used for meta-analyses. Where medians and ranges were given, these were only converted into means and SDs if the ranges were not skewed. We used random effects models because of heterogeneity of participants, interventions, and outcome measures of physical activity. Where categorical measures were reported, meta-analyses used odds ratios (OR). Most outcomes, however, were continuous measures, and we used standardized mean differences (SMD) as outcomes had differing measurement scales.
In one of the continuous outcome measures, a lower score was a better result (time taken to walk 1 mile), so these results were reversed for the meta-analysis. Several of the studies had more than one measure of physical activity, so we conducted two continuous measures meta-analyses, one using the lowest values (smallest effect size) and one using the highest values (largest effect size). Where only one measure of physical activity was reported this is used in both meta-analyses. There was insufficient evidence to warrant further investigation of heterogeneity by meta-regression. Risk of publication bias was assessed using a funnel plot.
Role of the Funding Source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Searches found 1,404 titles and abstracts. After removing duplicates, 1,047 remained for screening, of which 1,000 were excluded. Full papers for seventy-nine articles were assessed for inclusion (forty-seven from database searches and thirty-two from reference lists) (see Supplementary Figure 1). For a full list of excluded studies and reasons for exclusion, see Supplementary Table 2. There were eighteen studies included in the qualitative synthesis: fourteen used an inactive control (Set 1) (Reference Avila and Hovell23–Reference Takahashi, Miyashita and Kawanishi36) and four compared group walking interventions to walking alone (Set 2) (Reference Cox, Burke and Beilin37–Reference Thomas, MacFarlane and Guo40). One study from Set 1 (Reference Kriska, Bayles and Cauley30) had a second publication reporting long-term follow-up (Reference Pereira, Kriska and Day41). There were ten studies from Set 1 in the quantitative syntheses (meta-analyses). It is possible that there might be an effect from publication bias suggesting that small trials with no significant effects have not been published, or their physical activity results not published (see Supplementary Figure 2).
Characteristics of included studies are presented in Supplementary Table 3. The majority of studies (14 of 18) were RCTs or cluster RCTs; there was also one nonrandomized experimental study (Reference Takahashi, Miyashita and Kawanishi36), two case–control (Reference Lee, Lee, Jeon, Hong and Park38;Reference Nguyen, Gauvin, Martineau and Grignon39) and one cohort study with a local population comparator (Reference Krieger, Rabkin, Sharify and Song29). The number of participants in studies varied between 17 and 605 participants; seven of the studies had fewer than 50 participants. Most studies included older participants (older than 65 years) but participants’ ages ranged from 18 to 91 years. Participants were community volunteers in eight studies (Reference Avila and Hovell23;Reference Fisher and Li24;Reference Hamdorf and Penhall26;Reference Maki, Ura and Yamaguchi32–Reference Palmer34;Reference Takahashi, Miyashita and Kawanishi36;Reference Cox, Burke and Beilin37); recruitment was by means of general practices or community centers in six studies (Reference Gusi, Reyes, Gonzalez-Guerrero, Herrera and Garcia25;Reference Isaacs, Critchley and See Tai27;Reference Lamb, Bartlett, Ashley and Bird31;Reference Resnick35;Reference Lee, Lee, Jeon, Hong and Park38;Reference Thomas, MacFarlane and Guo40), from specific housing areas in two studies (Reference Krieger, Rabkin, Sharify and Song29;Reference Nguyen, Gauvin, Martineau and Grignon39), and from random population sampling in one study (Reference Jancey, Lee, Howat, Clarke, Wang and Shilton28). In the remaining study, the recruitment method was unclear (Reference Kriska, Bayles and Cauley30).
The interventions were all led walks or walking in groups. In some studies, the intervention consisted of encouraging participants to walk in a group, facilitated by advertising locally and training an individual to lead the walks, in others the intervention entailed leading the group in the walks. Interventions studied lasted between 5 and 90 minutes on 1 to 7 days per week, for between 8 weeks and 1 year. The frequency and duration of walking was tailored to the ages of the sample participants.
The comparators in Set 1 were usual activities, cancer screening, fitness testing, advice, educational lectures, no walking group encouragement, waiting list, no intervention, routine care or unspecified inactive controls. The comparators in Set 2 were usual care with encouragement to walk but no access within the study to a walking group (Reference Isaacs, Critchley and See Tai27;Reference Jancey, Lee, Howat, Clarke, Wang and Shilton28), being a former walking club member but still walking (Reference Nguyen, Gauvin, Martineau and Grignon39), and not being paired with a “buddy” to walk with (Reference Thomas, MacFarlane and Guo40). Follow-up was at the end of the intervention only for most of the studies, three studies had additional follow-ups at between 3 months and 10 years (Reference Avila and Hovell23;Reference Isaacs, Critchley and See Tai27;Reference Kriska, Bayles and Cauley30). One case–control study (Reference Nguyen, Gauvin, Martineau and Grignon39) had no follow-up, as the comparator was retrospective. Outcomes measured were of a wide variety of categorical and continuous physical activity measures; no study used the same physical activity measure.
Quality of included studies varied (see Supplementary Table 4); nine studies were classified as being at high risk of bias and five medium and four low risk of bias. Several of the studies gave insufficient details to assess all aspects of quality, so classification may not be accurate. An intervention such as this cannot be blinded to the participant, but blinding of investigators and outcome assessment should have been possible, but it was not apparent whether this had been done in the majority of the studies (Reference Fisher and Li24–Reference Hamdorf and Penhall26;Reference Jancey, Lee, Howat, Clarke, Wang and Shilton28;Reference Moore-Harrison, Speer, Johnson and Cress33–Reference Resnick35;Reference Takahashi, Miyashita and Kawanishi36;Reference Lee, Lee, Jeon, Hong and Park38–Reference Thomas, MacFarlane and Guo40). For the cluster RCTs, in Thomas et al. (Reference Thomas, MacFarlane and Guo40), it was clear that participants knew they were part of a trial, whereas in Fisher and Li (Reference Fisher and Li24) and Jancey et al. (Reference Jancey, Lee, Howat, Clarke, Wang and Shilton28), this was unclear.
Physical Activity Outcomes
Numerical results are shown in Table 1. For Set 1 (inactive controls), meta-analysis of the continuous measure of physical activity showed that walking in groups increased physical activity at follow-up compared with inactive controls (nine RCTs, highest value SMD 0.58 [95 percent CI, 0.34–0.82; I2 = 76 percent] and lowest value SMD 0.43 [95 percent CI, 0.20–0.66; I2 = 73 percent]) (see Figure 1a and b). Removing the nonrandomized experimental study (Reference Takahashi, Miyashita and Kawanishi36) reduced the SMD from 0.58 (95 percent CI, 0.34–0.82) to 0.51 (95 percent CI 0.28 to 0.74) (Reference Takahashi, Miyashita and Kawanishi36).
Table 1. Numerical Physical Activity Results

*p = 0.05 or less.
a Details from Blank et al. (Reference Blank, Jones, Buckley Woods and Payne21).
b Estimated values for SD.
CI, confidence interval; IPAQ, International Physical Activity Questionnaire; IQR, inter-quartile range; kcal, kilocalories; km, kilometre; LSI, Large Scale Integrated; MET, metabolic equivalent; mins, minutes; MVPA, moderate or vigorous physical activity; NB, nota bene; NR, not reported; N/A, not applicable; SD, standard deviation; SE, standard error; WG, walking group; wk, week.
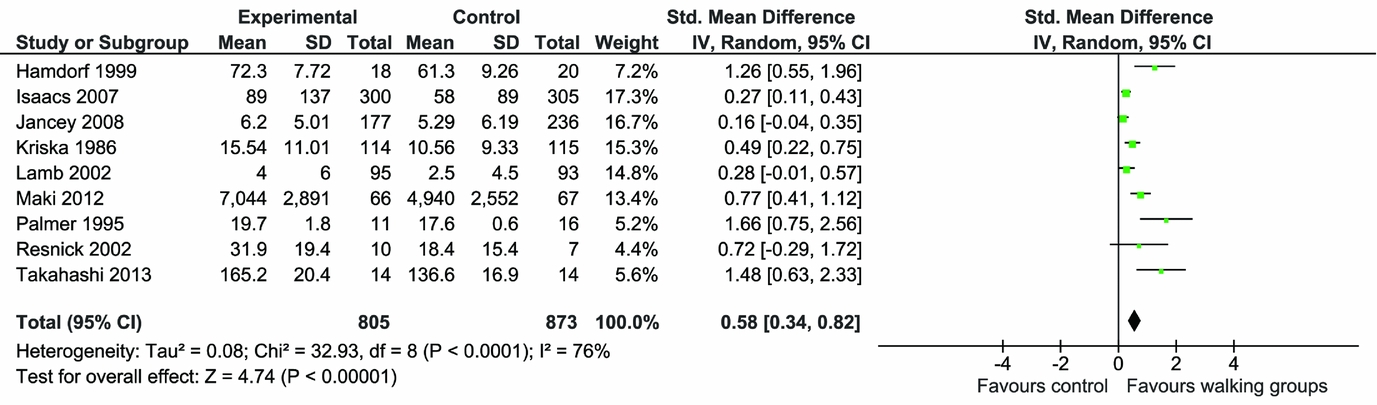
Figure 1. a: Meta-analysis of continuous physical activity outcomes (higher values).
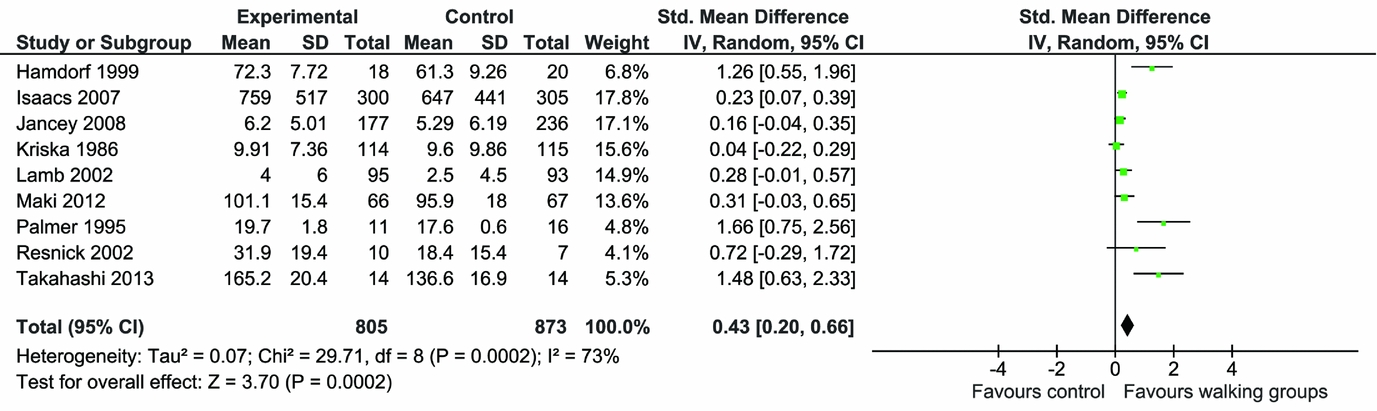
Figure 1. b: Meta-analysis of continuous physical activity outcomes (lower values).
When the two studies that undertook follow-up beyond the end of the intervention (22 months and 3.5 months after participating in intervention) (Reference Isaacs, Critchley and See Tai27;Reference Kriska, Bayles and Cauley30) are taken out of the lowest value meta-analysis, the SMD increases from 0.43 (95 percent CI, 0.20 to 0.66) to 0.66 (95 percent CI, 0.30 to 1.02), suggesting that physical activity gains associated with participating in walking groups diminished over time (Reference Isaacs, Critchley and See Tai27;Reference Kriska, Bayles and Cauley30). Two studies measured categorical outcomes for physical activity. The meta-analysis found that the risk of participants being physically active at the end of the intervention was significantly higher in the intervention group compared with the comparators (relative risk 1.44 [95 percent CI, 1.22–1.70; I2 = 0 percent]) (Supplementary Figure 3).
For Set 2 (walking alone controls), studies were too few and too heterogeneous to conduct meta-analysis. For Cox et al. (Reference Cox, Burke and Beilin37), there was no difference in 1.6 km walk time between intervention and control groups at both 6 months and 1 year follow-ups. In Lee et al. (Reference Lee, Lee, Jeon, Hong and Park38), exercise frequency and duration were statistically significantly improved for the intervention group compared with controls at the end of the intervention (12 weeks). For Nguyen et al. (Reference Nguyen, Gauvin, Martineau and Grignon39), there was a higher percentage of participants walking 1 km or less in the intervention group compared with the controls. In Thomas et al. (Reference Thomas, MacFarlane and Guo40), those receiving the buddy intervention had higher mean physical activity levels at 12 months than controls, although the numerical results for the control group were not explicitly reported.
Quality of Life Outcomes
Seven of the Set 1 and none of the Set 2 studies measured quality of life and wellbeing (see Table 2). Studies used a variety of measures for quality of life and wellbeing including Euroqol EQ-5D, Nottingham Health Profile (NHP), SF-36, and SF-12. All scores except NHP had higher scores indicating better quality of life. For NHP, higher scores indicated greater number and severity of problems. In five of the seven studies (Reference Fisher and Li24–Reference Hamdorf and Penhall26;Reference Maki, Ura and Yamaguchi32;Reference Moore-Harrison, Speer, Johnson and Cress33), the walking group intervention groups showed statistically significantly improved scores compared with controls in at least one of the outcomes measured. In the remaining two studies (Reference Isaacs, Critchley and See Tai27;Reference Resnick35), there were no significant differences found, including in NHP scores. None of the outcomes measured showed significantly worse quality of life or wellbeing for the walking group interventions compared with controls.
Table 2. Quality of life and wellbeing results (all self-report)
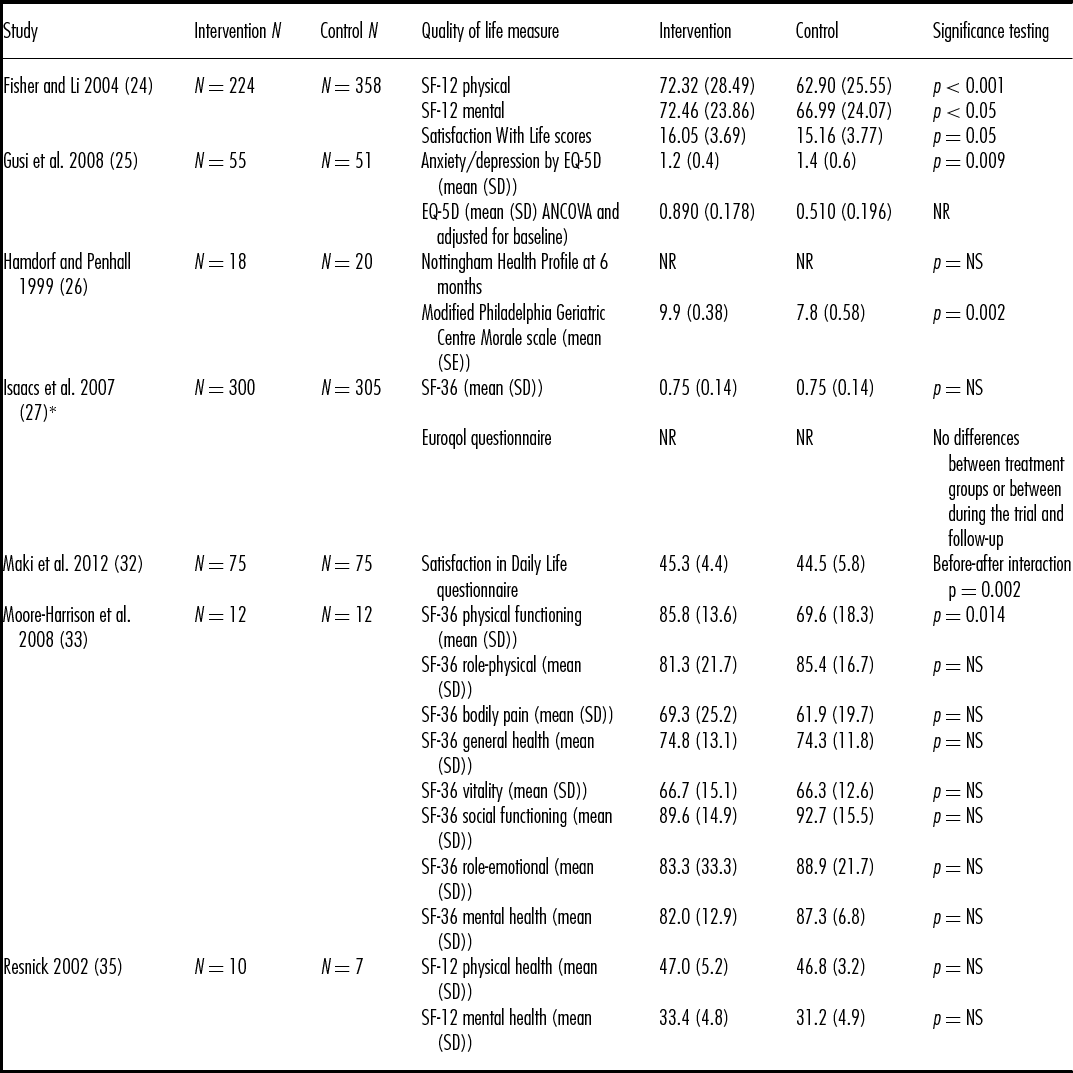
*Follow up 2 reported for intervention group only N = 300, SF-36 mean (SD) = 0.77 (0.15), Abbreviations: EQ-5D – Euroqol 5 Dimensions, NR – not reported, NS – no significant difference between groups, SD – standard deviation, SE – standard error, SF – short form
Scale ranges – SF-12 - range 0 to 100 for physical and mental health components, where a zero score indicates the lowest level of health and 100 indicates the highest level of health. Satisfaction with Life – range 5 to 35, with a score of 20 representing neutral and between 5–9 indicating extreme dissatisfaction with life, and between 31–35 indicating extreme satisfaction. EQ-5D (Euroqol) – range 0 to 1 where 0 is death and 1 is perfect perceived health. Nottingham Health Profile – range 2 to 200 where the higher the score, the greater the number and severity of problems. Modified Philadelphia Geriatric Centre Morale scale - range 0 to 17 where a higher score indicates higher morale, Satisfaction in Daily Life – range unavailable but higher score indicates better quality of life, SF-36 - - range 0 to 100 for eight scales where a zero score indicates the lowest level of health and 100 indicates the highest level of health.
Other Outcomes
Retention rates are shown in Supplementary Table 4 and include retention rates for all participants, or retention rates by group where reported. Ten of the studies reported retention rates separately for the intervention groups compared with controls (eight in Set 1 and two in Set 2). Seven had higher rates for the intervention groups, whereas three had higher rates for the control groups. In several instances, the rates were very similar. Many of the studies found that retention rates dropped gradually over time. There was insufficient information to determine whether different types of control had any impact on retention rates.
Three studies in Set 1 and no studies in Set 2 reported numerical results for measures of social network or sociableness. Jancey et al. (Reference Jancey, Lee, Howat, Clarke, Wang and Shilton28) used a categorical measure of “Having no friends nearby” in Generalized Estimating Equations and found that it had a significant negative effect (p = .037) on total physical activity times, suggesting that fewer friends nearby was correlated with less total physical activity. Krieger et al. (Reference Krieger, Rabkin, Sharify and Song29) measured the number of neighbors the participant knew well enough to say hello to. They reported before and after results for the intervention group only and found a significant increase in the mean number of neighbors that participants knew well enough to say hello to while walking (4.3 [95 percent CI, 2.0–6.7] p = .001). Maki et al. (Reference Maki, Ura and Yamaguchi32) measured the Lubben Social Network Scale and found that there was no significant difference in mean scores between the intervention and control groups (16.3 [SD 5.7] versus 16.8 [SD 5.2] p = .16).
DISCUSSION
Main Findings
The main finding was that physical activity in physically healthy adults improved at follow-up for the walking group intervention compared with inactive controls. This is based mostly on self-report physical activity outcomes, and only one study used accelerometry (Reference Takahashi, Miyashita and Kawanishi36), but this study was small, with fourteen participants in each group. This physical activity improvement was strongest immediately following completion of the intervention and reduced somewhat at longer follow-ups. Walking in groups tended to increase quality of life measures and may increase social connectedness, but the evidence for this was uncertain. There was insufficient evidence to indicate whether walking in groups was more effective than walking alone for increasing physical activity and no evidence on the impact on quality of life.
Retention rates tended to be higher in the intervention groups. No included study reported the proportion of participants meeting the recommended guidelines for physical activity of 30 minutes moderate intensity physical activity five times per week (42). In general, the quality of the evidence found was mixed, with seven of thirteen studies in Set 1 and two of four studies in Set 2 considered to be at high risk of bias.
Comparison to Previous Work
Previous systematic reviews found that walking groups, compared with a variety of active and inactive controls provided wide-ranging health benefits (Reference Hanson and Jones19) and that they were effective in increasing physical activity (Reference Kassavou, Turner and French20), including for leisure and travel (Reference Blank, Jones, Buckley Woods and Payne21). However, this is the first systematic review to quantify this effect in physically healthy people compared with inactive controls through meta-analyses. Also, this is the first systematic review to attempt to compare the sociable side of walking in groups to people walking alone.
Strengths and Limitations
This systematic review has several strengths in that it is both more comprehensive than previous systematic reviews as it included adult participants of any ages, and more focused as it only included mainly physically healthy participants, rather than mixing participants with conditions likely to impede the ability to walk, such as knee arthritis, with participants without such difficulties. In the included studies, participants varied but were mostly older adults, particularly older women and it is women in the age group of 55- to 74-year-olds that form the majority of walkers in walking groups (Reference Coleman, Kokolakakis and Ramchandani43). As many participants were older, not all will be completely physically healthy, so a pragmatic decision was made to limit the proportion of physically unhealthy participants in any group to 20 percent or less, so as not to exclude useful information.
Extensive searches of reference lists from previous systematic reviews, included studies and policy documents were made, in addition to database searches, to find all eligible studies. All included studies were listed in one or more of the three systematic reviews (Reference Hanson and Jones19–Reference Blank, Jones, Buckley Woods and Payne21). It is clear from the fact that the previously published systematic reviews (Reference Hanson and Jones19–Reference Blank, Jones, Buckley Woods and Payne21) were not comprehensive that searching for these types of studies is not straightforward. One reason is that, when searching for studies, the term “walking group” can refer to one arm of a comparative study rather than where people were walking in groups. Therefore, a relatively large number of full texts were read thoroughly to ascertain the exact nature of the walking intervention and whether it had any kind of social interaction. Physical activity interventions are difficult to search for by means of databases alone, for example another systematic review of physical activity interventions found twice as many studies by means of other sources than by means of database searches (Reference Waters, Reeves, Fjeldsoe and Eakin44). Also definitions of physical activity, exercise, and physical fitness can vary, so in this study, we use descriptions defined by Caspersen et al. (Reference Caspersen, Powell and Christenson45).
There were some studies where full papers were unavailable that could have been includable in the systematic review. Every effort was made to use all available data including extracting information from existing systematic reviews. The included studies were very heterogeneous in terms of participants, interventions, comparators, follow-up lengths, and study designs, so it could be argued that studies should not have been meta-analyzed. Also, some studies had imbalances at the start of the study, for example, the cluster RCT by Jancey et al. (Reference Jancey, Lee, Howat, Clarke, Wang and Shilton28). However, random effects models were used to mitigate these factors to some extent, but this gives more weight to smaller studies than fixed effects meta-analysis. Given that most of the included studies were relatively small, this weighting may be a strength rather than a weakness.
We included any comparative studies rather than RCTs only, and it could be argued that the different study designs should not have been meta-analyzed. Also no two physical activity outcomes were the same. Most were by self-report, which can be inaccurate; few used objective measures; and only one used accelerometry (Reference Takahashi, Miyashita and Kawanishi36). However, they were all measuring physical activity in some way, which meant that they could be meta-analyzed. This approach assumes that an SD change in one physical activity measurement scale is equivalent to an SD change on another, which may not be true. Some numerical results were missing, which meant that not all studies could be entered in the meta-analyses. We had to estimate SD from other measures of spread in three studies (Reference Hamdorf and Penhall26;Reference Isaacs, Critchley and See Tai27;Reference Lamb, Bartlett, Ashley and Bird31), but in one other (Reference Krieger, Rabkin, Sharify and Song29), there was no measure of spread given, so it could not contribute to the meta-analysis result. Because of all these factors, we consider our meta-analyses exploratory, and we conducted sensitivity analyses by altering the physical activity outcomes entered into the meta-analyses to generate highest and lowest effect size estimates.
We did not include the time spent in physical activity in the meta-analyses, although this is reported in Tables 1a and 1b. It might be that longer walking duration is a better predictor of physical activity outcomes, and this could be established through meta-regression. However, we chose not to conduct meta-regression because of the wide variation in physical activity outcome measures used in the included studies, and because there were only nine studies that could contribute to the calculation. In addition, some of the studies included warming up and cooling off, whereas others did not report this. These times are often opportunities for social interaction, which would not be captured if duration of exercise was used only. Social connectedness outcome measures were not well reported, and the measures used were not well validated.
Implications for Policy
This systematic review aims to inform public policy on group walking promotion. As high levels of moderate intensity physical activity (60 to 75 min per day) seem to eliminate any increased risk of death associated with lack of physical activity, the more that people can be encouraged to undertake physical activity, the better it will be for them, the health services and the public purse (Reference Ekelund, Steene-Johannessen and Brown46;Reference Ding, Lawson and Kolbe-Alexander47). The lack of strong evidence demonstrating that group walking participation enhanced physical activity compared with walking alone means that there is no strong driver as yet for governments to adopt coherent strategic plans or to invest in this area of physical activity behavior change. Walking in groups is a safe and inexpensive intervention that can be delivered easily and successfully in the community and has consistency with expectations and the public's perception of walking.
Implications for Research
There needs to be further research clearly evaluating the benefits for physically healthy people in taking part in group walking compared with walking alone, particularly measuring physical activity over the longer term. The activity measure should be that recommended by the World Health Organization, that is, the proportion meeting the physical activity guidelines. Other outcomes should include generic quality of life and wider societal costs. Capturing any adverse events is also important. There also needs to be evaluation of the best ways to motivate people to continue with walking once the initial enthusiasm wanes and the officially organized activity is discontinued. It is possible that sociable aspects of group walking may enhance persistence in maintaining physical activity participation.
There needs to be encouragement to the physical activity research community to standardize physical activity measurement (following the COMET initiative) (Reference Williamson and Clarke48), so that all studies measure physical activity consistently. This would enable results of various interventions to be compared across studies.
CONCLUSIONS
The bulk of the empirical evidence base for walking in groups consists of small studies comparing this activity to inactive controls and there is good evidence that walking in groups is more effective than inactivity. However, there is far less evidence on walking in groups compared with walking alone, yet research has shown that exercise adherence is enhanced through the use of social support. At a time when we are being encouraged to meet physical activity guidelines, a large proportion of the public fail to do so. Better quality evidence may encourage government policy to promote walking in groups organized by the groups themselves. Adequately powered multi-center RCTs along with qualitative process evaluation should be undertaken to test the efficacy of walking group encouragement interventions.
SUPPLEMENTARY MATERIAL
Supplementary Table 1: https://doi.org/10.1017/S0266462317001088
Supplementary Figure 2: https://doi.org/10.1017/S0266462317001088
Supplementary Table 2: https://doi.org/10.1017/S0266462317001088
Supplementary Figure 2: https://doi.org/10.1017/S0266462317001088
Supplementary Table 3: https://doi.org/10.1017/S0266462317001088
Supplementary Table 4: https://doi.org/10.1017/S0266462317001088
Supplementary Figure 3: https://doi.org/10.1017/S0266462317001088
CONFLICTS OF INTEREST
No conflicts of interest for C. Meads or J. Exley.






