Injectable disease-modifying treatments (DMTs) are the standard therapy for multiple sclerosis (MS), and their efficacy and safety profiles are well-established (Reference Cross and Naismith1). Dosing mode and frequency varies across injectable DMTs: Interferon beta-1b is administered subcutaneously (SC) every other day, interferon beta-1a is administered as a once-weekly intramuscular (IM) injection or a three-times-weekly SC injection, peginterferon beta-1a is a SC injection administered once every 2 weeks, and glatiramer acetate is an SC injection administered three times a week or once per day, depending on the dose. Numerous studies have demonstrated that adherence to DMT is suboptimal (Reference Treadaway, Cutter and Salter2;Reference Treadaway, Cutter and Salter3). Injection fatigue, injection fear, and injection-related side effects are common reasons for non-adherence (Reference Devonshire, Lapierre and Macdonell2–Reference Giovannoni, Southam and Waubant4), and adherence has also been demonstrated to be lower when dosing frequency is higher (Reference Devonshire, Lapierre and Macdonell2;Reference Treadaway, Cutter and Salter3;Reference Fox, Salter and Tyry5–Reference Chen, Baraban and Stuchiner8).
The most commonly reported adverse events (AEs) associated with injectable DMTs include injection-site reactions (ISRs; particularly with SC formulations) (Reference Kleinschnitz, Niemczyk, Rehberg-Weber and Wernsdörfer9), and flu-like symptoms (FLS; associated with interferon therapies in particular) (Reference Giovannoni, Southam and Waubant4;Reference Patti10;Reference Wingerchuk and Carter11). Perceptions of and experiences with treatment efficacy, side effects, dosing frequency, and dosing complexity influence patient adherence to injectable DMTs, which impacts clinical outcomes (Reference Fox, Salter and Tyry5–Reference Fernández, Aguera and Izquierdo7). The literature on adherence to injectable DMTs for patients with MS suggests that improvements in administration, including reduced dosing frequency and/or increased ease of administration by means of the use of auto-injectors, could improve adherence to treatment and, hence, outcomes (Reference Devonshire, Lapierre and Macdonell2;Reference Tan, Qian and Agarwal12;Reference Johnson, Van Houtven and Ozdemir13). Examination of patients' preferences for features of injectable DMTs can provide quantitative measures of the tradeoffs patients are willing to make between the benefits of treatment, the AEs associated with treatment, and other dosing features (Reference Hauber, Fairchild and Reed Johnson14). Minimum acceptable efficacy (MAE) is one of several ways to quantify preferences (Reference Hauber, Fairchild and Reed Johnson14).
Several recent papers have reported preferences for treatment attributes of DMTs. One quantified preferences for features or attributes of quality of life but not for features of treatment (Reference Rosato, Testa and Oggero15). Others quantified preferences for features of injection devices (Reference Shingler, Swinburn and Ali16) or outcomes (Reference Johnson, Van Houtven and Ozdemir13) for DMTs, but did not include treatment side effects or dosing attributes. Wicks et al. (Reference Wicks, Brandes and Park17) quantified preferences for oral DMTs only including efficacy, side effects, and dosing frequency, and found that side effects were weighted more heavily than efficacy.
Conversely, Utz et al. (Reference Utz, Berg and Lämmer18) quantified preferences for oral and injectable DMTs and found that route of administration and treatment frequency are important to patients and can be more important than mild side effects. However, in this study, the mild side effects were not specified, so preference weights for specific side effects could not be estimated. One study from a single university-based center in the United States (U.S.) (Reference Wilson, Loucks and Bui19;Reference Wilson, Loucks and Gipson20) quantified preferences for features of oral, injectable, and intravenously administered DMTs including efficacy, side effects, and dosing characteristics among U.S. patients to estimate risk preferences. These studies found that severe side effects, MS symptoms, and progression were most important over the ranges examined. However, the study could not dissociate effects of dosing frequency from mode of administration on patient preference.
Poulos et al. (2016) quantified preferences for features of injectable DMTs in a sample of patients from across the United States (Reference Poulos, Kinter, Yang, Bridges, Posner and Reder21) and replicated the study in Germany (Reference Riñon, Buch, Holley and Verden22). In both the U.S. and German studies (Reference Poulos, Kinter, Yang, Bridges, Posner and Reder21;Reference Riñon, Buch, Holley and Verden22), delaying disease progression and preventing FLS were key drivers of preferences, and some changes in dosing frequency were as important as changes in efficacy and FLS. These studies used a discrete choice experiment, a stated preference method, to examine preferences for treatment features characterizing treatments that were both currently available and possibly available in future treatments. Riñon et al. (Reference Riñon, Buch, Holley and Verden22) show that the percentage of patients initiating DMTs or interrupting or discontinuing treatment vary considerably across different countries, suggesting that preferences vary across countries.
To further explore preferences in different countries, a study similar to the Poulos et al. (2016) study (Reference Poulos, Kinter, Yang, Bridges, Posner and Reder21) was carried out among individuals living with MS in the United Kingdom (U.K.) and France to quantify preferences for attributes of injectable DMTs and assess the relative importance of changes in these attributes needed to offset changes in other treatment attributes. We are aware of no previous studies that have been carried out in these regions.
METHODS
The study replicated a study design previously administered in the United States and Germany (Reference Poulos, Kinter, Yang, Bridges, Posner and Reder21;Reference Poulos, Kinter and Yang23) The study was approved by the Institutional Review Board at the Office of Research Protection at RTI International. The study involved minimal risk; no personally identifiable information was collected from respondents. It was determined that local ethics review was not required in the United Kingdom due to the fact that respondents were not recruited through the National Health Service. Local ethics review in France was determined to be unnecessary due to the fact that the study was noninterventional.
Discrete-Choice Experiment
The study used a discrete-choice experiment (DCE) (Reference Bridges, Hauber and Marshall24;Reference Marshall, Bridges and Hauber25) to elicit preferences using a series of paired comparison questions in which respondents were asked to choose which of two hypothetical treatments they preferred. Each hypothetical treatment had six attributes (years until disability progression, number of relapses in the next 4 years, injection time, injection frequency, FLS, and ISRs) and each attribute had various levels (Supplementary Table 1). DCE studies postulate that the benefit or utility of a treatment is a weighted sum of its attributes, where the estimated weights reflect respondents' perceptions about the strength of preference for each attribute level and can be used to calculate patients' willingness to trade-off among attribute levels. The weights for each attribute level, indicating strength of preference for each attribute level, were estimated by means of statistical analysis of the series of treatment choices.
The draft survey instrument was pretested before the U.S. study using in-depth, in-person interviews with fifteen adult patients with a self-reported physician diagnosis of MS. The interviews were used to evaluate and improve the understandability of the survey instrument, appropriateness of descriptive information, level of difficulty of the choice questions, and ranges of attribute levels. The pretests indicated that the information in the survey and the survey questions were understood and accepted by participants and the survey was neither too long nor too complex. The survey was adapted for the United Kingdom and France, translated (for France), and then refined based on five semi-structured in-person interviews in each country.
Experimental Design
The treatments and treatment pairs were determined using a best-practice experimental design with statistical properties that optimize the statistical information generated from a given sample size (Reference Johnson, Lancsar and Marshall26). The U.K. and French studies used the experimental design developed for the U.S. and German studies (Reference Poulos, Kinter, Yang, Bridges, Posner and Reder21;Reference Poulos, Kinter and Yang23).
Hypothetical treatments were constructed using the information in Supplementary Table 1. The treatment attributes and levels of the hypothetical treatments were informed by consultation with experts and characteristics of currently available injectable DMTs as well as levels that may characterize treatments that are currently unavailable, but may be possible in the future. DCE (and other stated preference methods) permit the exploration of preferences for treatment features that lie outside the range of currently available treatments. This can help us understand the importance of treatment features over a broader range than presently observed, but one that could be realized in the future.
Each respondent was asked nine choice questions, including one repeated choice question. Each choice question included two hypothetical injectable MS treatments and respondents were asked to indicate which treatment they would choose if these were the only two available. Example treatment-choice questions from the U.K. and France surveys are shown in Figure 1. Patients were provided with a hypothetical reference scenario (progressing from mild to moderate symptoms, as described using the Hohol, or Disease Steps, scale) (Reference Hohol, Orav and Weiner27) to ensure that each patient was considering the same initial disability level and the same change in disability when answering questions. The Hohol scale was selected because of the simplicity of the scale compared with the Expanded Disability Status Scale.
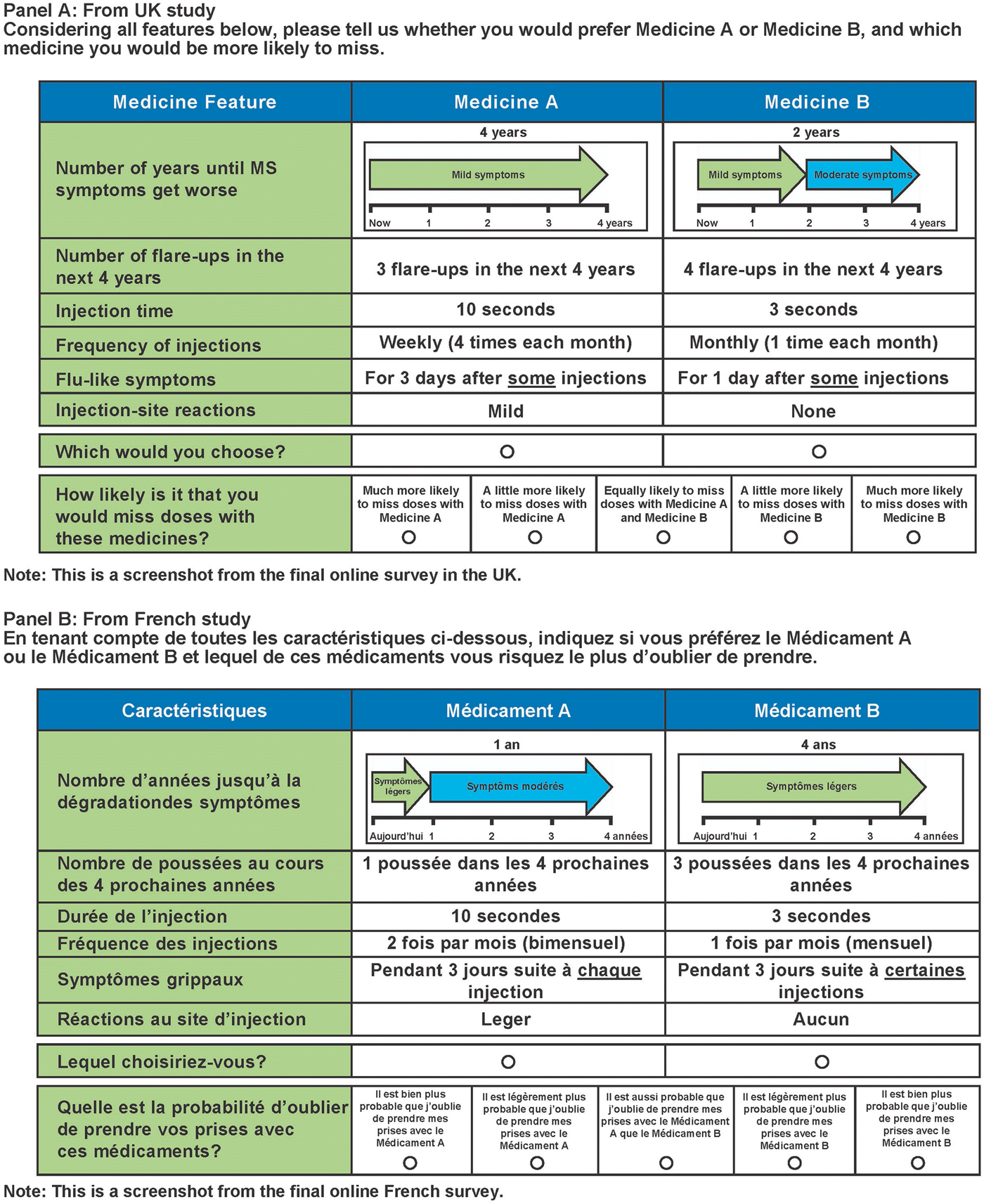
Figure 1. Example treatment-choice questions.
Study Sample
Nielsen Healthcare (formerly Harris Interactive) invited Web panelists with MS in the United Kingdom and France to participate in an online survey by means of email invitations with a link to the survey. All respondents were aged 18 years or older and had a self-reported physician diagnosis of MS. Data collection was terminated when the target sample of 100 respondents was achieved for each country. The 25-min online survey was administered in the United Kingdom in April 2014 and in France in July and August of 2014. Patients were given Harris Interactive Loyalty Points if they completed at least one treatment-choice question in the survey. These points may be accumulated by panel members and exchanged for cash or gifts. The study was approved by one of RTI International's Institutional Review Boards (Federalwide Assurance #3331). All patients provided informed consent before their inclusion in the study.
Model and Analysis
These studies replicated the statistical analysis plan completed in the U.S. and German studies (Reference Poulos, Kinter, Yang, Bridges, Posner and Reder21;Reference Poulos, Kinter and Yang23).
Treatment-choice data were analyzed using a mixed logit (or random parameter logit [RPL]) model, which controls for heterogeneity in preferences and the panel nature of data (Reference Train, Sonnier, Scarpa and Alberini28;Reference Train29). The dependent variable was the treatment (Medicine A or Medicine B) selected in each DCE question for each respondent. Independent variables included all attribute levels in Supplementary Table 1. All independent variables were effect coded with normally distributed random parameters.
RPL regression parameters are preference weights, or utilities, of attribute levels. The vertical distance, or difference, between preference weights associated with different levels of an attribute measures the relative importance, or utility differences, of the corresponding change in each attribute (Reference Hauber, González and Groothuis-Oudshoorn30).
The estimated preference weights were used to calculate MAE estimates for various changes in treatment-related features and outcomes. MAE is a measure of the relative importance of efficacy and is higher when changes have a greater perceived importance. It is calculated as the delay in disability progression (in years) or the reduction in the number of relapses that would exactly offset the perceived importance of a given worsening in dosing frequency or a given worsening in FLS. The MAE is the mean minimum level of change in efficacy that respondents consider to be equivalent to a given change in treatment-related attributes or outcomes. The mean MAE is calculated as the ratio of the relative importance of a given change in a treatment attribute divided by the relative importance of a unit change in either the delay in disability or the number of relapses.
For example, the MAE in terms of delay in disability progression for an increase in dosing frequency from 1 to 12 times per month is calculated as the ratio of the relative importance of the change in dosing frequency (i.e., the vertical distance between the preference weights corresponding to 12 and 1 doses per month) to the relative importance of a 1-year delay in disability progression. To calculate the denominator, the relative importance of a 1-unit change in efficacy was calculated using linear interpolation between the effect coded parameter estimates over the utility range defined in the ratio's numerator.
RESULTS
Sample
In both the United Kingdom and France, 100 respondents completed the survey and provided sufficient data for analysis. Nielsen Healthcare invited 2,111 individuals in France to be screened for participation in the survey. Of those invited, 521 (25 percent) responded to the invitation. Of those who responded, 127 (24 percent) were eligible to participate. Of those who were eligible to participate, 123 (97 percent) consented to participate. Of those who were eligible and consented to participate, 100 (81 percent) completed the survey. In the United Kingdom, Nielsen Healthcare invited 1,246 patients in the United Kingdom to participate in the survey. Of those invited, 455 individuals (37 percent) responded to the invitation. Of those who responded, 141 (31 percent) were eligible to participate. Of those who were eligible to participate, 129 (91 percent) consented to participate. Of those who were eligible and consented to participate, 100 (78 percent) completed the survey.
Sample characteristics are reported in Table 1. In both samples, the mean age was 46 years and the mean time since receiving a diagnosis of MS was 10 years. Both samples were weighted toward female respondents (59 percent and 71 percent in the United Kingdom and France, respectively) with relapsing-remitting MS (45 percent and 51 percent, respectively). Twenty-four percent of respondents in the United Kingdom were treatment-naïve and 11 percent of respondents in France were treatment-naïve. A total of 50 percent of the respondents in the U.K. sample were injection-naïve, while only 24 percent were injection-naïve in the French sample.
Table 1. Characteristics of survey respondents
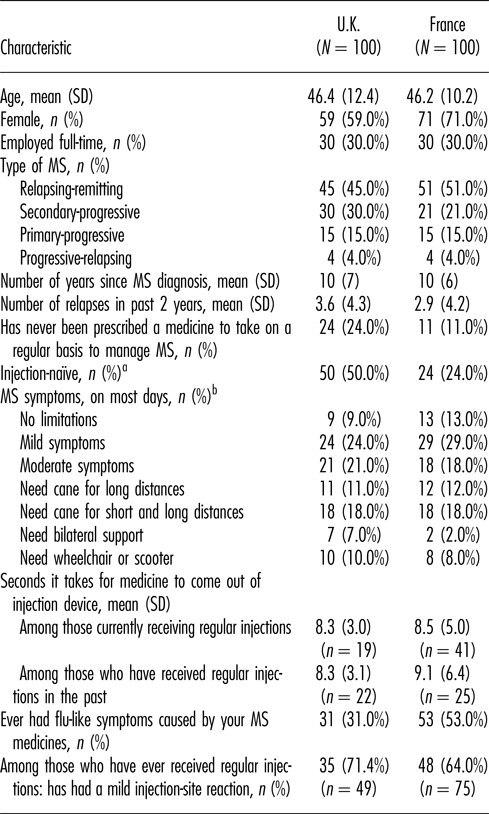
MS, multiple sclerosis; SD, standard deviation; U.K., United Kingdom.
a Has never received injections on a regular basis to treat MS.
b As described in the Hohol scale.26
Preference Weights
The results of the RPL estimation (Figure 2) show that respondent preferences were generally consistent with the expectation that better outcomes (e.g., better efficacy, fewer side effects, less frequent administration) would be preferred to worse outcomes in the United Kingdom and France. The U.K. results suggest that four relapses are preferred to three relapses and a longer injection (10 sec) is preferred to a shorter injection (3 sec), but in both cases the differences between the pairs of preference weights are not statistically significantly different, suggesting that respondents did not distinguish between these levels when making choices in the survey. Regression results are shown in Supplementary Tables 2 and 3.
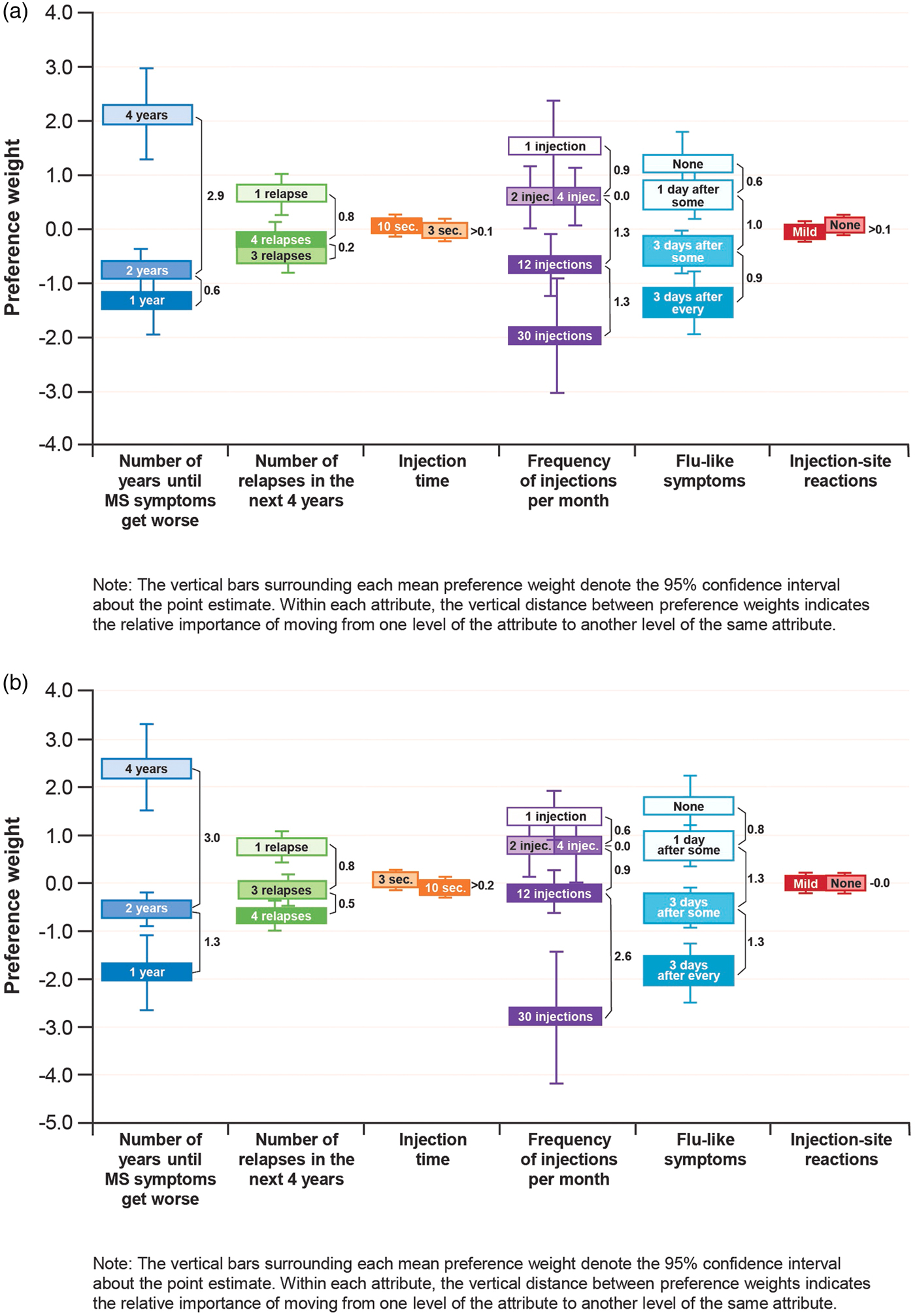
Figure 2. Preference weights for patients surveyed in the United Kingdom (a) and France (b); (N = 100 each).
In the United Kingdom, improving the time until disability progression from 2 to 4 years and reducing injection frequency from “daily” to “every 2 weeks” had a relative importance (vertical distance between preference weights for attribute levels) of 2.9 and 2.6, respectively (Figure 2a). The importance of these changes was similar to the importance of reducing the frequency of FLS from 3 days after every dose to none (2.5), and between 3.5 and 3 times more important, respectively, than reducing the number of relapses in the next year from 4 to 1 (0.8).
In France, improving the time until disability progression from 2 to 4 years and reducing injection frequency from “daily” to “every 2 weeks” had a relative importance of 3.0 and 3.5, respectively (Figure 2b). The importance of this change in dosing frequency was similar to the importance of reducing FLS from 3 days after every dose to none (3.4), and more than 2.5 times as important as reducing the number of relapses in the next year from 4 to 1 (1.3). In both countries, these changes in disability progression, dosing frequency, and FLS were more important than changes in the other attributes assessed, and the relative importance of improvements in injection time and ISRs was much smaller than changes among the other attributes.
Minimum Acceptable Efficacy
The MAEs (in terms of delay in disability progression and decrease in relapses over 5 years) are shown for different changes in dosing frequency and FLS in Table 2 for both countries. The MAEs were higher for the U.K. sample than for the French sample, although the differences are not statistically significant. On average, respondents in the United Kingdom and France, respectively, would trade an increase in injection frequency from 2 to 30 times per month for 9.1 and 8.2 fewer relapses in the next 5 years or for 2.4 and 2.5 years of delay in disability progression. As measured by MAE, these changes in dosing frequency from 2 to 30 times per month are similar to the changes in FLS from none to 3 days after each injection.
Table 2. MAE estimates (95% CI)
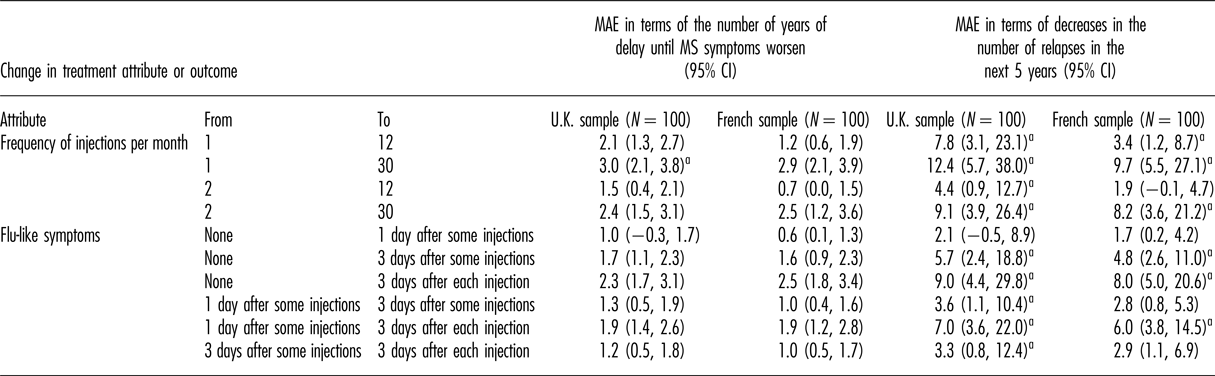
CI, confidence interval; MAE, minimum acceptable efficacy; MS, multiple sclerosis; U.K., United Kingdom.
a For this change, the MAE was calculated by extrapolating preferences beyond the range for the levels included in the study.
DISCUSSION
In this study, both in the United Kingdom and France, lengthening the time until disability progression, reducing injection frequency, and reducing FLS were key drivers of patient preferences. These results suggest that people with MS might be willing to trade off efficacy for improvements in dosing frequency and improvements in FLS, and the MAE estimates indicate the tradeoffs that might, on average, be acceptable. Most of the mean MAE estimates were statistically similar for the United Kingdom and France.
These results are similar to the study findings in the United States and Germany. Among 192 respondents from the United States, we found that similar levels of importance were placed on reduction in injection frequency or FLS as were placed on treatment efficacy (Reference Poulos, Kinter, Yang, Bridges, Posner and Reder21). The German study, in 189 patients, also showed similar levels of importance placed on improvements in the frequency of dosing and delay in disability progression (Reference Poulos, Kinter and Yang23).
The present study was the first study examining patient preferences for DMT attributes in the United Kingdom and France, and is the only study to specify the mild side effects separately and evaluate tradeoffs among efficacy and different side effects. This study is focused only on injectable treatments. Wilson (Reference Wilson, Loucks and Bui19;Reference Wilson, Loucks and Gipson20) is a comprehensive study, but carried out only in the United States with a geographically limited sample. These results, combined with results from previous studies on patient preferences for MS therapies, suggest that some changes in injection frequency of MS injectable treatments can be as important to patients as some changes in efficacy or side effect attributes of those treatments. These studies highlight the perceived benefits of therapies with lower dosing frequencies, as a reduction in dosing complexity and injection-related side effects may lead to improvements in patient adherence.
One potential limitation of this study is that a substantial number of patients in both samples were injection-naïve, which might be expected to limit their preferences for DMT treatment attributes. However, we found no evidence that preferences among patients with and without injection experience were statistically different from one another (data available upon request).
General limitations of this type of assessment include the evaluation of hypothetical treatment profiles as opposed to actual treatments, and the omission of possible important contextual factors, such as cost, which may influence actual treatment choices. In particular, because hypothetical treatment profiles were constructed, the selection of attributes and levels used in this study may have influenced the results observed. However, the study design was informed by the reviews of medical experts to ensure that the attributes selected were clinically meaningful and reflective of the treatment attributes that, based on their experience, influence treatment choice.
The survey was also pretested with patients to explore whether there were salient treatment attributes that were not included in the survey. The pretests and consultations with experts suggested that the selection of attributes and levels was clinically appropriate and did not noticeably influence survey responses. In addition, there is always the possibility that small samples in surveys may not be representative of the overall patient populations in the areas studied. Furthermore, the lack of data on the populations of MS patients limit our ability to evaluate the representativeness of the sample. However, the majority of study respondents in the United Kingdom and France were women (59 percent and 71 percent, respectively), which is consistent with the results of epidemiological studies showing that MS occurs more often in women than men (Reference Bainbridge, Corboy, Dipiro, Talbert and Yee31–Reference Orton, Herrera and Yee34).
The physician diagnoses of MS were self-reported, which is challenging because not all patients will know, but the design attempted to minimize the possibility of misidentification by screening respondents for study eligibility without revealing the subject of the survey. The screening question involved selecting health conditions that had been diagnosed by a health care provider from a prespecified list that included MS. While this will not eliminate the chance of misidentification, it is likely to reduce the risk of strategic bias in self-reporting. There are also acknowledged limitations of the use of an online panel to recruit patients, including potential sample bias and mode effects. However, research has shown that results from online DCEs are, in general, not statistically different from those elicited through face-to-face or telephone interviews (Reference Nielsen35;Reference Marta-Pedrosa, Freitas and Domingos36).
Strengths of this study include the design of the survey in collaboration with clinical experts and pretesting using in-depth interviews with MS patients. The treatment-choice data were analyzed using RPL methods that avoid estimation bias from unobserved variation across sample and within-sample correlation for each patient. In this study, unlike previous studies, the importance of changes in selected attributes are calculated in terms of MAE, which allows more direct comparison between two countries. To our knowledge, these measures have not been used previously to explore the relative importance of DMT treatment features.
There are no direct implications of this research for public policy. However, the results may be of importance among physicians and payers who influence the selection of treatments to add to formulary, reimburse, or offer to patients.
In conclusion, the results of these surveys suggest that some changes in the injection frequency and side effects (FLS) of injectable treatments for MS can be as important to some patients as improvements in treatment efficacy. This information may be useful to providers when discussing treatment choices with their patients.
ACKNOWLEDGMENTS
The authors express their gratitude to A. Brett Hauber (RTI Health Solutions) and Jui-Chen Yang and Juan Marcos Gonzalez (formerly of RTI Health Solutions) for contributions to the statistical analysis plan in the U.S. study, and the data analysis in this study. The authors would also like to acknowledge Jenna Steere (CircleScience) for support in preparation of this manuscript, which was funded by Biogen.
SUPPLEMENTARY MATERIAL
Supplementary Table 1: https://doi.org/10.1017/S0266462318000491
Supplementary Table 2: https://doi.org/10.1017/S0266462318000491 Supplementary Table 3: https://doi.org/10.1017/S0266462318000491
CONFLICTS OF INTEREST
C. Poulos is an employee of RTI Health Solutions, an independent scientific research organization. J. Posner was an employee of RTI Health Solutions at the time of the study. The study that is the subject of this manuscript was conducted by RTI Health Solutions and funded by Biogen. E. Kinter, J. van Beek and K. Christensen are employees of Biogen. The authors have full control of all primary data and agree to allow the journal to review the data if requested. Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






