Published online by Cambridge University Press: 06 May 2022
South Africa has embarked on major health policy reform to deliver universal health coverage through the establishment of National Health Insurance (NHI). The aim is to improve access, remove financial barriers to care, and enhance care quality. Health technology assessment (HTA) is explicitly identified in the proposed NHI legislation and will have a prominent role in informing decisions about adoption and access to health interventions and technologies. The specific arrangements and approach to HTA in support of this legislation are yet to be determined. Although there is currently no formal national HTA institution in South Africa, there are several processes in both the public and private healthcare sectors that use elements of HTA to varying extents to inform access and resource allocation decisions. Institutions performing HTAs or related activities in South Africa include the National and Provincial Departments of Health, National Treasury, National Health Laboratory Service, Council for Medical Schemes, medical scheme administrators, managed care organizations, academic or research institutions, clinical societies and associations, pharmaceutical and devices companies, private consultancies, and private sector hospital groups. Existing fragmented HTA processes should coordinate and conform to a standardized, fit-for-purpose process and structure that can usefully inform priority setting under NHI and for other decision makers. This transformation will require comprehensive and inclusive planning with dedicated funding and regulation, and provision of strong oversight mechanisms and leadership.
T.K. and M.W. are partly supported by the Research, Evidence and Development Initiative (READ-It) project. READ-It (project number 300342-104) is funded by UK aid from the UK Government; however, the views expressed do not necessarily reflect the UK Government’s official policies.

Table 1. National Health Insurance Policy Documents
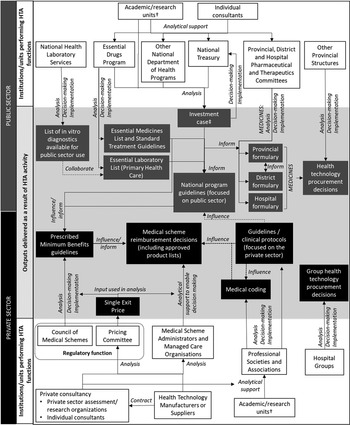
Figure 1. Health Technology Assessment (HTA) activities in South Africa which incorporate elements of HTA. Overview of the type of institutions with direct links to prioritization, pricing and procurement, or coverage decisions. These are the main bodies and institutional types conducting HTA-like activities. †Only analytical contributions to HTA processes are presented here, but many individuals working at academic/research units contribute to various stages in the HTA process, including decision making (e.g., individuals serving on committees), implementation (e.g., through teaching at universities), or conducting policy relevant research. ‡ An investment case describes a proposed set of budgeted interventions in a specific program area and is used to request additional funding for budget line items.
South Africa has pursued universal health coverage (UHC) for its population since the first democratic elections in 1994. Addressing historical disparities and taking reasonable measures, within available resources, to progressively realize South Africans’ right to access good quality, essential healthcare is enshrined in the country’s constitution (1). Fulfilling this requirement remains challenging in a highly resource-constrained environment amidst growing healthcare demands, extreme disparities in healthcare access, quality and spending between public and private sectors (Reference Gray, Vawda, Moeti and Padarath2;Reference Blecher, Pillay and Patcharanarumol3), as well as variable quality of public sector healthcare between and within provinces. Approximately 80 percent of South Africa’s population utilize healthcare provided by tax-financed public sector institutions. Less than 15 percent of the population are enrolled in private voluntary health insurance enabling access to private sector providers (4), and a proportion of uninsured patients also access private sector healthcare paying out of pocket (Reference Girdwood, Govender, Long, Miot and Meyer-Rath5).
The planned National Health Insurance (NHI) Fund will provide UHC for all South Africans, using a single payer and a common health benefits package (HBP) to purchase health services from both public and private sector providers. The design of the NHI HBP will require South Africa to transition from implicit to explicit decision-making processes to ensure consistent, equitable, and fair access to health technologies at all levels of care. The draft NHI Bill and earlier policy papers (Table 1) highlight the central role that health technology assessment (HTA) will play in decision making relating to NHI benefits and the financial sustainability of the NHI Fund.
Table 1. National Health Insurance Policy Documents
HTA uses scientific evidence, interpreted through the lens of social and scientific value judgements, to inform an accountable approach to making health technology access and resource allocation decisions. A health technology is any intervention used to prevent, diagnose, or treat a condition or disease and can be a medicine, vaccine, test, procedure, device, program, or system of care (Reference O’Rourke, Oortwijn and Schuller6). HTA is a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle. The institutional governance of HTA is largely driven by local policy intent and health system environments (7). The importance of HTA for UHC goals has been highlighted in multiple initiatives, including the World Health Assembly resolution on UHC and the Lancet Commission on Essential Medicines Policies (Reference Wirtz, Hogerzeil and Gray8).
A 2017 review of the policy and legal landscape of HTA in South Africa found that, even though much work had been done, implementation efforts were fragmented (Reference Siegfried, Wilkinson, Hofman, Padarath and Barron9). The aim of this paper was to consolidate the experience and opinions of specialists in HTA in their particular institutions and disciplines to describe the HTA activities underway in South Africa, why these might be useful in supporting NHI planning, and further important considerations for HTA implementation.
Current HTA activities in South Africa
Despite policy intent to create HTA processes since 1996, there is still no formal national HTA institution in South Africa (Reference Siegfried, Wilkinson, Hofman, Padarath and Barron9). There is currently little coordination of HTA activities and limited capacity for analysis or the appraisal thereof (Reference Wilkinson, Hofman, Young, Schmidt and Kredo10). In addition, there are no formalized and agreed HTA methods consistently used in South Africa; hence, outputs are likely to differ considerably, especially considering the varying motivations and decision-making perspectives of different healthcare providers and funders.
There are however many processes that use elements of HTA to inform resource allocation and/or access decisions. Figure 1 identifies the type of institutions with direct links to prioritization, pricing and procurement, or coverage decisions. Although Figure 1 is not an exhaustive representation of the extensive network performing analytical work to support health policy making in South Africa, it identifies the main bodies and institutional types conducting HTA-like activities and that are central to ongoing HTA development. Patient and health professional interest groups (e.g., nongovernmental organizations [NGOs]) play a vital role in promoting patient and provider needs and demands, and contribute to the HTA activities described in this paper to varying degrees across institution types. HTA activities performed by commercial organizations (e.g., medical device or pharmaceutical industry) and professional associations are largely limited to informing private sector healthcare decision making but may impact public sector healthcare indirectly (see Figure 1).
Figure 1. Health Technology Assessment (HTA) activities in South Africa which incorporate elements of HTA. Overview of the type of institutions with direct links to prioritization, pricing and procurement, or coverage decisions. These are the main bodies and institutional types conducting HTA-like activities. †Only analytical contributions to HTA processes are presented here, but many individuals working at academic/research units contribute to various stages in the HTA process, including decision making (e.g., individuals serving on committees), implementation (e.g., through teaching at universities), or conducting policy relevant research. ‡ An investment case describes a proposed set of budgeted interventions in a specific program area and is used to request additional funding for budget line items.
Examples of HTA activities performed by some of these institutions are described below.
National Department of Health
Under South Africa’s decentralized system, the National Department of Health (NDoH) and nine Provincial Departments of Health share responsibility for health services. The NDoH is responsible for overall normative guidance, and provinces take specific budget and implementation decisions given their substantial financial autonomy.
Program streams within the NDoH are responsible for governance and policy development in their respective areas (e.g., the NHI program; tertiary services allocations). National programs may issue disease-specific guidelines; however, the NDoH has little control over provincial implementation except in instances where programs are funded by conditional grants from the national budget (e.g., Antiretroviral Treatment Program). Conditional grants represent additional funding to provincial health departments for a specific package of services.
National level regulation and coordination of public sector procurement and maintenance strategies for medical devices and equipment has evolved over time but is still characterized by inequities and inefficiencies.
Essential Drugs Programme
The NDoH Essential Drugs Programme (EDP) coordinates the development of the national Essential Medicines List (EML) and Standard Treatment Guidelines (STGs). The EML is an explicit list of medicines that should be available in the public health system. The EML is delineated according to level of care (primary, secondary, and tertiary/quaternary) with associated STGs for primary and secondary levels of care. STGs provide guidance to healthcare professionals on the rational use of medicines.
The selection of essential medicines and development of STGs is based on assessment of evidence for efficacy, safety, cost-effectiveness, and affordability, generally compared to the current standard of care. Global normative guidance such as the World Health Organization Model List of Essential Medicines may also influence listing decisions. The EDP depends on NDoH staff, who serve as the secretariat for a series of ministerially appointed advisory committees. There are currently three Expert Review Committees (ERCs) covering different levels of care, each representing multiple disciplines including clinicians, pharmacists, health economists, public health specialists, and epidemiologists. The ERCs make recommendations to the National Essential Medicines List Committee (NEMLC). Evidence reviews and analyses are produced by EDP staff, ERC members or co-opted ERC members (usually conducted without compensation), or by external consultants/research units (with costs borne by the research units or funded by NGOs). Collaboration with postgraduate departments in Schools of Public Health, Pharmacy, and so on are also utilized to support evidence reviews/economic analysis to encourage upskilling and increase capacity. Stakeholders are invited to comment on most NEMLC decisions, and technology assessment documents are published on the NDoH website. Final NEMLC decisions are published and disseminated and can be appealed if stakeholders consider that NEMLC failed to act fairly or the decision taken was unreasonable in light of the evidence presented (11). More recently, the EML review process has been strengthened in response to the COVID-19 pandemic. COVID-19 rapid reviews have provided up-to-date, critical appraisals of evidence to inform decisions for therapeutics in the management of COVID-19 (Reference Leong, McGee and Gray12).
The current EML review process is largely focused on medicines. The approach to reviewing evidence and implementing national guidance on nonmedicine technologies (e.g., medical devices) is less well established. An Essential Laboratory List (ELL) for the primary healthcare setting has been extracted from the Primary Health Care STGs.
The EDP methods and processes are continually updated to incorporate elements of HTA, such as evidence of public health relevance, cost-effectiveness, affordability, feasibility, and equity implications, in addition to clinical evidence. A recently developed draft HTA Methods Guide for the assessment of medicines was issued for public consultation in July 2021. However, monitoring and evaluation of guideline implementation, procurement practices, and rational use of essential medicines is severely lacking; thus, the impact of NEMLC decisions is unknown.
National Treasury
The National Treasury of South Africa has repeatedly called for improved HTA capacity in the health sector, as it frequently receives large budget bids from the NDoH for additional funding for new technologies or programs, without robust assessment of effectiveness, affordability, and cost-effectiveness.
Due to these deficiencies, the Treasury has undertaken substantial modeling in areas such as childhood vaccination and HIV/AIDS, relying on internal capacity with external expert inputs where required, and using various analytical approaches adapted to the particular intervention or program. Initial work has also been done on benefit design for NHI.
National Health Laboratory Service
The National Health Laboratory Service (NHLS) provides diagnostic pathology services across all public sector healthcare facilities. NHLS investment decisions can have substantial impact on public sector spending. This influences the availability of diagnostic, prognostic, and predictive tests and therefore the clinical care delivered. The NHLS has been engaging with the EDP with the updating of the PHC ELL.
The NHLS HTA process is in nascent stages and could potentially be strengthened and coordinated with other decision-making bodies in the health system (Reference Moodliar and Basu13).
Provincial Departments of Health
Provincial Departments of Health have multiple structures responsible for procurement and management of health technologies, including Central Medical Depots, Pharmacy Services, Supply Chain Management, and Health Technology Directorates.
Assessment of medicines: Pharmaceutical and Therapeutics Committees
Pharmaceutical and Therapeutics Committees (PTCs) are responsible for regional medicine selection and rational use decisions in the public health sector and should exist at provincial, district, subdistrict, and hospital levels. The PTCs provide feedback mechanisms between the national and operational levels of care and are designed to enable close collaboration with the EML process (14). The provincial NEMLC representative is usually a member of the provincial PTC. Local guidance on the use of medicines is aligned to the national EML and STGs, while medicine use recommendations for limited non-EML medicines are developed by the PTCs. PTCs play a vital role in ensuring local medicine access decisions are informed by evidence and not individual clinician preferences.
The performance and complexity of the HTA processes employed by the PTCs across South Africa vary considerably, with no standardized approach in place. This situation has been exacerbated by the COVID-19 pandemic with some provincial and hospital PTCs no longer functioning.
Assessment of non-medicine technologies
Evaluation of nonmedicine technologies is limited and usually conducted on an ad hoc basis to support procurement processes. The focus of assessment is primarily on safety and functional performance, with evidence assessed generally limited to compliance with the applicable international and/or local standards and technical specifications reflecting institutional and clinical needs.
As for many other health technologies, purchase price is the dominant criterion in the procurement process. However, lack of proper specification and evaluation can result in substandard products being procured, thus negating the supposed benefits of the process while potentially impacting on patient access and/or safety. For this and other reasons, it is increasingly accepted that quality-related considerations must play a larger role not only in procurement decisions but also in evidence-based life-cycle management of technologies.
NDoH Pricing Committee
The NDoH Pricing Committee (PC) is an independent statutory committee that makes recommendations to the Minister of Health regarding the pricing of medicines in South Africa’s private sector. As part of a legislated transparent pricing system, pharmaceutical companies may only charge a single exit price (SEP) for medicines in the private sector, regardless of the volume procured. The SEP is the factory gate price (in South African Rand [ZAR]) and includes the logistics fee paid to wholesalers and distributors. The Minister of Health sets a maximum annual increase as well as maximum dispensing fees to be charged by pharmacists and other licensed dispensing practitioners. In addition, the Medicines and Related Substances Act (Act 101 of 1965) prohibits any form of bonusing, sampling, or discounting in the supply of medicines.
Although pharmaceutical companies may provide HTAs to support these private sector prices, use of the guidelines for pharmacoeconomic submissions published in 2012 (15) remains optional. Importantly, the launch SEP for a newly marketed medicine is not subjected to any pharmacoeconomic evaluation and is at the sole discretion of the manufacturer/importer. Under current regulations, the Director-General of Health may request a pharmacoeconomic analysis to justify the launch SEP, but this mechanism has not been used to date.
In contrast to the process used to set the SEP for medicines in the private sector, the price of EML medicines/consumables available in the public sector is the result of the national tender process, reference pricing, and price negotiations with manufacturers/suppliers. National pharmaceutical tenders are managed by the NDoH Affordable Medicines Directorate, with input from provincial representatives, academic leads and NEMLC members, and employing set criteria.
Council for Medical Schemes
There are currently seventy-six medical insurance schemes registered in South Africa, serving 8.89 million beneficiaries (4). A medical scheme is a form of voluntary health insurance which is a mutual, not-for-profit entity, governed by a board of trustees. Medical schemes are frequently provided partially or wholly within employment packages, with a dedicated scheme for government employees and dependents. Members contribute monthly premiums (in ZAR) toward a pooled fund in exchange for the scheme taking financial liability for costs associated with relevant private healthcare services, in accordance with a predefined set of scheme rules (16).
Medical schemes are regulated by the Council for Medical Schemes (CMS), a statutory body established by the Medical Schemes Act (Act 131 of 1998). The CMS maintains the Prescribed Minimum Benefits (PMBs), which is a set of defined benefits all medical insurers are mandated to cover (i.e., costs relating to diagnosis, treatment, and care) for all members regardless of the benefit option they selected (17). For twenty-five diagnoses on the Chronic Disease List (CDL), the CMS also provides treatment algorithms defining which medicines must be covered as PMBs. In relation to HIV care, medical schemes are required to offer at least the treatment and prevention options provided in the public sector. To some extent, albeit without clear legislative backing, the public sector EML/STGs establish a standard of care that is applied by the CMS in PMB definition, especially when there is no specific CDL algorithm for a specific condition. This link between the EML/STGs and the PMBs has been highlighted as a point of concern in that it might perversely incentivize manufacturers of technologies to seek the listing of technologies on the EML/ELL (at acquisition prices achieved through the national tendering process) with the aim of increasing private sector sales (at legislated SEPs that are usually higher than public sector prices).
Existing legislation explicitly requires that medical schemes apply evidence-based medicine principles in the development of healthcare programs and funding policies, taking into consideration cost-effectiveness and affordability (16). In the past, there was no formal HTA process applied to the determination of PMB conditions, but the CMS is incorporating some HTA principles in the current PMB review process (18). Multiple stakeholders provide input to the development of benefit definition guidelines.
Medical Scheme Administrators and Managed Care Organizations
Medical scheme administrators and managed care organizations (MCOs) are for-profit entities that are subject to registration and accreditation by the CMS under the Medical Schemes Act (1998). While some medical schemes have in-house administration functions, others contract administrators for functions including benefit design and data management. MCOs, which can either be independent business entities or subsidiaries/divisions of medical scheme administrators, are tasked to perform the functions of “clinical and financial risk assessment and management of healthcare, with a view to facilitating appropriateness and cost-effectiveness of relevant health services within the constraints of what is affordable, through the use of rules-based and clinical management-based programmes” (16). In so doing, medical schemes use HTA and forms of stakeholder engagement to inform benefit definitions and risk management strategies.
Manufacturers or suppliers usually initiate the HTA process by submitting clinical and economic evidence dossiers using the templates provided by the assessing organization. These submissions are subject to reviews of the published clinical data, followed by assessments of the financial implications of introducing the technologies. Clinical effectiveness, affordability, and the impact of introducing an intervention on medical scheme premiums are important decision-making criteria, with training requirements, organizational impact, and acceptability also considered. End-user preferences and input can be submitted as part of the evidence submissions and applicants may appeal negative decisions under certain circumstances.
The approach to HTA within the medical scheme environment is fragmented. Several HTA processes are in existence within the various administrators/MCOs, and many are reported to be well developed. However, these forums are convened independently of each other. This is in part due to concerns surrounding the potential for collusion amongst MCOs, as well as the inclusion of large amounts of “commercial in confidence” information which limits the opportunity for external peer review. Given the different member profiles and differences in financial status of the medical schemes, the decisions made are not applied consistently across the privately insured population (between schemes as well as within schemes for different scheme options). The performance of managed care interventions, in particular, forms the basis for competition between administrators and is therefore not openly reported.
Academic Units
Academic units in South Africa routinely publish cost-effectiveness analyses and provide technical and/or clinical expertise that directly inform decisions on health technologies. Many academics also hold formal positions on decision-making bodies, such as NEMLC and the PC. Despite their core role in supporting HTA in South Africa, there is no central coordination, prioritization, or funding of HTA-related research, meaning that research is likely to be driven by individual research interests and funding priorities from predominantly international funders.
There are many institutions in South Africa offering courses or degree programs that support HTA capacity, frequently housed in Schools of Public Health, Science Councils, or Health Sciences Faculties/Colleges. Research for the award of postgraduate degrees has been aligned to HTA policy questions to provide additional analytical capacity for existing processes.
Clinical Societies and Associations
Many clinical societies and/or associations produce clinical guidelines (Reference Wilkinson, Wilkinson and Kredo19), but the methodological rigor and impact of the guidelines varies significantly (Reference Kredo, Wiseman and Gray20). Health economic evidence is usually not considered in the production of these guidelines. These guidelines often guide clinical practice in the private sector but also impact on the provision of clinical care in the public sector, albeit indirectly.
Clinical societies also develop and introduce clinical coding guidance and norms which facilitates the introduction and reimbursement of new technologies.
Industry
The pharmaceutical, medical device, and in vitro diagnostic industries utilize in-house teams as well as private consultancies to produce clinical and costing evidence required for reimbursement consideration. In addition, pharmaceutical companies can also provide HTAs to support private sector price applications to the PC. As many of these analyses contain proprietary information, there are limited examples in the public domain, which increases risk of duplication and unwarranted methodological variation.
Private Consultancies
There are multiple consulting organizations and individuals providing analytical services to inform health technology funding decisions. These services are mainly focused on the private sector but could potentially be utilized for public sector HTA activities under coordinated governance and commissioning mechanisms.
Hospital Groups
Private hospital groups are conducting HTAs to inform equipment and other medical device procurement decisions. Hospital-based HTA processes have been evolving over the last few years but tend to operate in isolation from the other institutions involved in HTA activities.
HTA in Support of National Health Insurance
The proposed NHI reforms have multiple components including the establishment of a NHI Fund to contract with accredited providers in public and private sectors to deliver a comprehensive package of care for all South Africans. The NHI HBP is intended to be provided universally and consistently, guaranteeing South Africans vertical and horizontal equity in healthcare access and provision.
The 2017 NHI White Paper (Table 1) explicitly identifies a prominent role for HTA, noting its role informing prioritization, selection, distribution, and management of health interventions and technologies. The NHI Bill (Table 1) specifies that the NHI Fund will establish an internal unit focused on benefits design (Section 20(3)) and that a Benefits Advisory Committee will advise the NHI Fund on the NHI HBP and determine and review cost-effective treatment guidelines (Section 25). As part of the Transitional Arrangements under the NHI Bill, a “Ministerial Advisory Committee on Health Technology Assessment for National Health Insurance” will be established within Phase 1 (theoretically scheduled for 2017–22) and will form a precursor to an HTA Agency (Section 57). However, the HTA Agency is not formally provided for in the NHI Bill. Thus, while the NHI Bill and preceding White Papers specified an explicit role for HTA in support of NHI, the detail of how the HTA processes or Agency would operate and be funded is yet to be determined.
Existing HTA approaches for determining packages of care in both the public and private healthcare sectors are highly relevant to discussions about the structure, functions, and process of a central HTA entity that can efficiently inform NHI decision makers, and potentially other organizations that will utilize HTA for services not covered under NHI. Additional important considerations when designing an HTA process to inform NHI benefit package design are presented below.
Principles for HTA under NHI in South Africa
HTA in support of NHI is anchored in ideals of UHC and contributes to defined policy objectives such as improving health outcomes, addressing inequalities in access to effective health technologies, and achieving value for money. Equity is a central theme and motivating factor for NHI implementation—to address persistent historical inequities in access to and financing of healthcare in South Africa.
Governance is a critical requirement of a successful HTA system. HTA processes in South Africa will need to incorporate strong leadership, management, transparency, accountability, and sustainable funding.
Barriers and Risks to the use of HTA in the Current Environment
The opportunities, challenges, and potential solutions to elements of HTA and NHI policy reform have been reported extensively (Reference Siegfried, Wilkinson, Hofman, Padarath and Barron9;Reference Blecher, Daven, Harrison, Moeti and Padarath21) and agencies like the National Institute of Health and Care Excellence in the United Kingdom and the Health Intervention Technology Assessment Program in Thailand have provided a potential vision for local policy makers. However, delays in implementation of various aspects of NHI have frustrated progress. Weak coordination and harnessing of valuable HTA skills risk South Africa falling behind middle-income country peers in developing HTA institutions, well-considered benefit packages, and a robust and consistent approach to economic evaluation.
The lack of consolidation and coordination of HTA and a dedicated funding plan at the national level remains the central barrier to HTA in South Africa. NHI will require integration of many of the processes detailed in this paper, notably the established EML/STG process with other guideline-producing units. Although there are international examples of clinical guideline production operating separately to individual technology funding decisions, this may not be an efficient use of current limited capacity. Institutional notions of ownership of particular HTA functions will need to be relaxed to enable a common, open, and multi-institutional approach to the delivery of the HTA process.
There is a very small pool of HTA analytical, procedural, and project management capacity in South Africa. This creates a risk that individual interests, piecemeal funding, or poor-quality analysis can drive HTA decisions. Major initiatives such as dedicated HTA commissioning budgets and mandated requirements for HTA from the industry will increase demand. A coordinated and long-term capacity strengthening approach involving NHI planning stakeholders, academic organizations, public sector units, and the private industry will be needed to proactively address supply.
The limited inclusion of patient and public stakeholders in existing HTA processes in South Africa creates a risk that the voices of those that the HTA process is intended to serve are not incorporated (Reference Cannon22). The mechanism for wider stakeholder engagement in HTA will need to be tailored to South Africa’s unique social and cultural context.
Conclusion
A credible, successful, and sustainable HTA process will require ongoing investment in personnel with a range of competencies. While South Africa has this expertise across private and public sectors, an HTA process in support of NHI will require adaptation, expansion, and coordination of these competencies. Approaches to all stages in the HTA process need strengthening, not least the efficient implementation and monitoring of decisions. A dedicated funding plan and model to enable HTA in support of NHI and provision of strong oversight mechanisms and leadership to facilitate this transformation will be critical success factors.
NHI represents an opportunity for all South Africans to realize their constitutional right of progressive access to quality healthcare under a UHC system. The experiences of other countries which have applied HTA systems and processes in support of UHC are instructive. However, the practical structures of the South African HTA process will need to be “home grown,” tailored to local context, and built on existing approaches.
Conflicts of Interest
The views expressed by authors do not necessarily reflect the view of respective organizations. M.W., T.W., and K.M. have received funding from the Better Health Program South Africa (United Kingdom Foreign and Commonwealth Development Office) for technical assistance to the South African National Department of Health Essential Drugs Programme (EDP) on Health Technology Assessment. J.M. and T.W. have received funding from the US Agency for International Development (USAID) to conduct health economic evaluations for the EDP. T.S. is employed by VI Research which has received funding from several pharmaceutical companies. T.K. is the co-director of the South African GRADE Network; a member of the National Essential Medicines List Committee (NEMLC) and the National Essential Medicine List Ministerial Advisory Committee on COVID-19 Therapeutics (NEML MAC on COVID-19 Therapeutics); a methodologist for WHO guidelines; and a Trustee on Board of Cochrane. A.L.G. has served as a member of the NEMLC; chairs NEMLC’s Paediatric Expert Review Committee (PERC); and is a member of the NEML MAC on COVID-19 Therapeutics. K.C. has served as a member of the NEMLC; chairs NEMLC’s Primary Health Care and Adult Hospital Level Expert Review Committee; and is a member of the NEML MAC on COVID-19 Therapeutics. R.W. is employed by Liberty Health (Pty) Ltd, a private health insurer operating in South Africa and across the broader African continent; has served as a member of the NEMLC; chairs NEMLC’s Tertiary and Quaternary Level Expert Review Committee; and has acted as a reviewer for the NEML MAC on COVID-19 Therapeutics. P.R. serves as a member of the NEMLC; has chaired NEMLC’s Tertiary and Quaternary Expert Review Committee; and is currently the Chair of the South African Health Products Regulatory Authority (SAHPRA), Clinical Trial Committee (CTC), and COVID-19 Working Group. S.M. is a member of the NEMLC’s Primary Health Care and Adult Hospital Level Expert Review committee; and the National Operations Manager of the Ophthalmological Society of South Africa (OSSA). M.P. is a member of the International Federation of Medical and Biological Engineering (IFMBE) Clinical Engineering Division Board, Global Clinical Engineering Alliance (GCEA) Founders Council and the South African Health Technology Assessment Society Executive Committee. Y.J. is a member of the NEMLC; and secretariat of the Western Cape Government Health Pharmacy and Therapeutics Committee. At the time of writing, E.M. was employed by the Council for Medical Schemes (CMS), a regulator for medical schemes in South Africa; and was a member of the NEMLC and NEMLC’s Tertiary and Quaternary Level Expert Review Committee. None of the other authors have any interests to declare.