Breast cancer screening offers a delicate balance between modest benefit and modest harm. The relative reduction in breast cancer mortality due to the mammography screening is typically estimated to be 20 percent; recent research suggests that it could be as low as 2 percent (Reference Kalager, Zelen, Langmark and Adami8). One problem is a large number of false positive screening mammograms leading to unnecessary, even invasive examinations.
In Finland, national breast cancer screening was launched in 1987 for women aged 50 to 59 years. The screening was expanded to include women of 60–69 years after publishing of a health technology assessment (HTA) by the Finnish office for HTA (Finohta) in 2006 (Reference Mäkelä, Saalasti-Koskinen, Saarenmaa and Autti-Rämö13). As part of the HTA, the content and quality of the information delivered to Finnish women invited to breast cancer screening was examined. The information was insufficient, presenting mainly the benefits of screening. In a re-evaluation 3 years later, the situation had not improved (Reference Saalasti-Koskinen, Mäkelä, Saarenmaa and Autti-Rämö19). The municipalities are legally responsible for monitoring the quality of screening services in Finland, including the quality of patient information. By commission of the Finnish Screening Board at the Ministry of Welfare and Health, an expert team was appointed to prepare sufficient information material for women invited for mammography screening. Authors of this article were members of the expert team.
In public screening programs, healthy individuals are invited for screening. The contact between them and screening test provider may offer little possibility for discussion. Information given by the service provider to women attending breast cancer screening has been scant, presenting predominantly the pros of screening (Reference Hoffman, Lewis and Pignone7;Reference Saalasti Koskinen, Pasternack and Leipälä20). Patient Decision Aids are paper-based or electronic tools that provide evidence based and balanced information about the advantages and disadvantages of options, and help in clarifying personal preferences and values regarding the intervention (6). They increase people's involvement and are more likely to lead to informed, value-based decisions (Reference O'Connor, Bennett and Stacey15). Several agencies produce patient decision aids, for example, NHS (14), Ottawa Hospital Research Institute (OHRI) (16), and University of Sidney (23). The role of decision aids is particularly important for interventions where there is a need to balance potential benefits and harms. Randomized trials have shown that decision aids may increase knowledge and informed consent (which is a composite of knowledge, values, and intention) to participate mammography screening, without increasing anxiety (Reference Mathieu, Barratt and McGeechan11;Reference Mathieu, Barratt and Davey12). On the other hand, increased information may lead into lower satisfaction with the decision (Reference Politi, Clark, Ombao, Dizon and Elwyn18) or reduce the willingness to participate in screening (Reference Adab, Marshall and Rouse1;Reference Mathieu, Barratt and McGeechan11). An important issue to consider when providing such information is that the concepts of risk and probability are poorly understood by both public and professionals.
Understanding Numbers
Women easily overestimate the effectiveness of mammography screening (Reference Domenighetti, D'Avanzo and Egger3), and those who overestimate benefits are known to participate more actively (Reference Chamot and Perneger2). Even interest and confidence in ability to use numerical information are not associated with actual comprehension or ability to use numbers (Reference Woloshin, Schwartz and Welch25). A recent review examined how numerical formats affect interpretation (Reference Visschers, Meertens, Passchier and de Vries24). When the probability of the test outcome is presented either in percentage (Reference Elwyn, O'Connor and Stacey5 percent) or as single event probability (.05), the vast majority of experts and lay people strongly overestimate the value of the test. When the same information is presented in frequencies (5 of 100), the risk estimates are much more realistic. Bigger numbers are easily interpreted as higher probability; 1200 deaths of 10,000 (probability 0.1) is perceived a greater risk than 24 deaths out of 100 (probability 0.2).
Reduction in mortality due to screening is usually expressed as relative risk reduction (RRR). When the probability of the event (cancer) is low, even small changes in event rates result in a large RRR, although the absolute risk reduction (ARR) is small. For example, the figures for risk reduction by mammography presented in the Finnish health technology assessment report (Reference Mäkelä, Saalasti-Koskinen, Saarenmaa and Autti-Rämö13) are of different magnitude: RRR 20 percent and ARR 0.1 percent. Therefore, RRR should be presented together with ARR to give a more balanced view. The inverse of ARR, number needed to treat (NNT), could be an intuitively clear presentation of risk, but frequently people misinterpret it as well (Reference Visschers, Meertens, Passchier and de Vries24).
Words seem to be more understandable, but they tend to carry imprecise meaning. People have different probability lexicons; they interpret, e.g., the word likely from a probability of 0.5 to 0.95. Framing affects perception; positive framing, for example, 9 of 10 are healthy instead of 1 of 10 has cancer is generally recommended in patient information. Visual representations of probability are more easily understood. Pictograms are a recommended and popular way to present risk information (Reference Visschers, Meertens, Passchier and de Vries24).
Providing accurate information in an understandable format does not necessarily improve communication of risk. Much of the literature on risk perception is concerned with how well people understand and can recall the numbers or the probabilities associated with the risks. Few studies have addressed how people weigh or order the information, and how decision-making processes work (Reference Lloyd10).
This study describes the process of producing a set of information material as decision aids for the Finnish public mammography screening program, analyzes the challenges in production and implementation, and presents the initial feedback received.
METHODS
The team used the Finnish breast cancer screening HTA from 2006 (Reference Mäkelä, Saalasti-Koskinen, Saarenmaa and Autti-Rämö13) as their base document. Additional information was retrieved from recent published randomized trials, systematic reviews and HTAs, and two doctoral theses (Reference Sarkeala21;Reference Seppänen22). For identifying relevant content and format for the decision aid, the team used the European guidelines for quality assurance in breast cancer screening and diagnosis (Reference Perry, Broeders and Wolf17), and additional studies on risk communication referenced in the background section of this article. Content and format were further elaborated after piloting the documents among users. A Web-based questionnaire was sent to service providers 6 months after opening the Web site and publishing the information package, including the letter templates with decision aid.
RESULTS
Our intention was to engage all relevant stakeholders in the expert team. Joint design of decision aid was especially important in Finland where HTA has no normative power. The expert team consisted of HTA professionals, representatives from a municipal breast cancer screening unit and the largest private sector provider, and experts from the Finnish Cancer Registry; it included multiple expertises from radiology, radiography, oncology, registers, service provision, clinical epidemiology, ethics and science communication. Four face-to-face meetings were held from May to December 2009. Because of lack of tradition and procedures for early patient or citizen involvement, user representation was not invited among women invited for screening or from patient organisations. The team acknowledged this as an apparent shortcoming which they intended to partly redress by user testing.
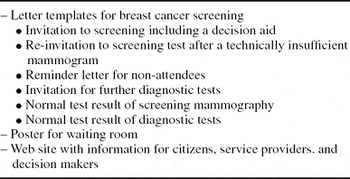
The expert team agreed that the decision aid should be attached to the back of the invitation letter to reach everyone. Women's concerns and information needs differ at first invitation and later, at successive screening rounds or when receiving invitation to diagnostic tests due to mammogram findings. Therefore, tailored content for six different letter types used in different phases of screening was prepared (Table 1). The first invitation letter includes the full decision aid. The other letters contain selected sections of the decision aid incorporated in the text.
Table 1. Project Outputs: Information Materials Mroduced for the Finnish Breast Cancer Screening Program
Sixty-two possible content items to be included in the decision aid were identified from breast cancer screening research publications and the European quality assurance guidelines (Reference Perry, Broeders and Wolf17). Each team member scored the importance of each item (1 = not important, 2 = somewhat important, 3 = important), and a mean was calculated for each. Key items (mean > 2) were considered more carefully when preparing letter templates. The list of content items selected for the first invitation letter is presented in Supplementary Table 1, which can be viewed online at www.journals.cambridge.org/thc2011027. Format requirements (Table 2) were also identified and used as guidance in editing the decision aid. Deviations from these requirements occurred mostly in letter templates with strict space constraints, for example, expressions using outcome frequencies (8 of 10) were replaced by shorter but still understandable percentages (80 percent).
Table 2. Format of Information for Breast Cancer Screening
Most mammography screening providers in Finland use electronic mass posting when sending invitations. The electronic letter templates have limited space for content; additional pages mean extra cost, which decreases willingness to implement. However, a separate leaflet in an envelope would be even more expensive. Some information in the decision aid (aim of screening, pain during examination, and voluntary participation), are presented on the front page of the invitation letter together with practical and process related information, such as preselected appointment and guidance on how to change the appointment, time required for examination, and how results will be given. The remaining information of the decision aid was placed on the back of the letter (Supplementary Figure 1, which can be viewed online at www.journals.cambridge.org/thc2011027). The letter templates are available in Finnish, Swedish, and English (Reference Saalasti Koskinen, Pasternack and Leipälä20).
Because all necessary information could not fit in the letter templates, a need for additional information site became apparent and a new web site was created. The information on the Web site is primarily targeted to women who consider attending breast cancer screening. For service providers and municipal decision makers, information and tools for the implementation of the national screening program are available. These include legislation, norms and administrative guidance on quality assurance of national screening programs, advice for tender invitations, the new letter templates, and a poster designed for waiting rooms. The poster illustrates a woman's chances of having a suspicious finding in screening mammogram or actually having a cancer (Supplementary Figure 2, which can be viewed online at www.journals.cambridge.org/thc2011027). The Web site opened in May 2010 and will be available in English in 2011. Between August and December 2010 there were 2040 visits to the pages, averaging 7 page views each.
User Feedback
The service provider members of the expert team selected three screening units, one municipal and two private, for readability testing of the new letter templates. A convenience sample (N = 160) of women attending breast cancer screening in these units were asked to read and comment on the new letter templates. The expert team also approached their acquaintances who were not medically trained for feedback from women under 50 years old (N = 15). The views of younger women, who are presumably more naïve to the breast cancer screening issues, were assumed to differ from older groups. Of 175 delivered questionnaires, 70 were returned (response rate 40 percent). Most respondents (73 percent) were 50–59 years of age, and nearly all (85 percent) had experienced mammography.
Forty-eight of seventy women (69 percent) thought that the text in the invitation letter (the decision aid) is important for them; the percentage was a little higher in the older age groups (Table 3). The majority (86 percent) found that the invitation letter gave enough information for an informed decision; this percentage was a little higher in the youngest age group. The sentence “Despite a normal screening result, a woman might develop cancer before next screening” caused most confusion, and was called “a harsh statement”. Softer expressions were suggested, some including advice on breast self examination. In general, women wanted to have a more personal tone, while some stated that there was a sense of respect of individual in the way the possible harms were presented in the letters. “It is difficult to make a personal judgement of an issue that requires expertise. Still, I feel that the way you have presented the information, is right.” The team agreed to implement suggestions from the users in the final letter templates.
Table 3. User Feedback on Letter Template
Six months after the letter templates were made available we asked all the 11 service providers in Finland about their use. Two of them had taken the letters into use by then, and three intended to do that soon. Two providers were not yet aware of them and one preferred letter templates they had developed themselves. As decision making in municipalities is slow, three were still in the process of changing letters.
DISCUSSION
The attendance rate of the public breast cancer screening program in Finland is exceptionally high. Therefore, the aim for producing the decision aids for Finnish women was not to increase participation, but to provide knowledge to empower women against perceived professional paternalism. Decision aids can also have unwanted consequences; they may increase anxiety and confusion, and reduce satisfaction in decisions or participation in screening. The aims of producing this decision aid and its possible unwanted consequences were discussed and balanced in the expert team. Professionals and service providers would have been more careful in including information of the harms of screening in the decision aid. The team had repeated, detailed discussions of the terms used and the overall tone in the text. HTA professionals stressed the priority of citizens' ethical and legal rights to receive balanced and understandable information when considering participation in healthcare interventions. Researchers cannot guarantee that decision aids will be read and understood as intended, but they must aim at correct, clear, and balanced information. This principle was in the end shared by the whole team.
The team did not follow a strict protocol; instead, the extent and quality of the outputs were developed during the project. The original plan was to produce a single template for national invitation letter. The final outputs instead included a set of tailored information materials for each step of the screening process, both electronically and on paper. These were presented in numbers, words and visual images, and targeted to women, decision makers, and service providers. Prolonged discussions toward consensus slowed the process but made the outputs more relevant and sustainable. The use of the website for providing more detailed information probably means some women have difficulty in accessing this information; however, the core information needed for informed decision is presented in the paper invitation letter.
We tested the readability and perceptions of the letter templates with a small number of women and the response rate was relatively low. Therefore the information was used only for providing corrections to the letter templates. Optimally, suggestions from user feedback would have been analyzed more carefully, and motivation for selection or omission registered clearly.
Planning decision aids requires expertise and procedures that were not fully established at Finohta, despite earlier experience in preparing information for patients and citizens on foetal screening (Reference Leipälä, Ignatius and Autti-Rämö9). The process was not optimal; in particular, the public input could have been stronger. Women's views were collected afterward by user testing, clearly an insufficient approach. Three healthcare professionals who had earlier been involved in preparing patient information for breast cancer screening commented the letter templates and their general view was that the text is too top-down and that the word “harmful effects” should be replaced by “limitations.” Finland lacks the tradition of involving public or patients in HTA. Updating the policies and processes for public involvement, and considering explicit use of theories to guide the design of decision aid as proposed by Elwyn et al. (Reference Elwyn, Stiel, Durand and Boivin4), is needed.
The decision aid created in this project has not been validated against established criteria; such as the International Patient Decision Aid Standards Collaboration Checklist (Reference Elwyn, O'Connor and Stacey5) or the CREDIBLE criteria (16). This shortcoming can be partly explained by the fact that the expert team was not aware that they were producing decision aids. The original task was to produce templates for cancer screening invitation letters. Only at the end of the project it became apparent that decision aid is the closest definition for the work that had been done.
The implementation of the decision aid and other information material is still incomplete. This is partly due to slow decision processes in municipalities, partly due to deficient dissemination. In a couple of years, re-evaluating the use of letter templates and perhaps the effect of the materials on service use would be interesting.
CONCLUSION
Working with expert team with multiple skills and views is challenging but fruitful when producing a set of materials to aid screening decisions. The wide range of HTA evidence used required careful thinking, selection and formatting. The decision aid attached to breast cancer screening invitation letter has gained acceptance but also created confusion among Finnish women and professionals. The uptake and use of the new information tools into the national screening is still incomplete.
CONTACT INFORMATION
Iris Pasternack, MD ([email protected]), Medical Researcher, Ulla Saalasti-Koskinen, MSc (health care) ([email protected]), Research Officer, Marjukka Mäkelä, MD, PhD, MSc ([email protected]), Research Professor, FINOHTA (Finnish Office for Health Technology Assessment), at THL (National Institute for Health and Welfare), P.O. Box 30, 00271 Helsinki, Finland
CONFLICT OF INTEREST
All authors report they have no potential conflicts of interest. Outside this work, the authors' institute receives a grant from the European Commission.

Breast cancer screening offers a delicate balance between modest benefit and modest harm. The relative reduction in breast cancer mortality due to the mammography screening is typically estimated to be 20 percent; recent research suggests that it could be as low as 2 percent (Reference Kalager, Zelen, Langmark and Adami8). One problem is a large number of false positive screening mammograms leading to unnecessary, even invasive examinations.
In Finland, national breast cancer screening was launched in 1987 for women aged 50 to 59 years. The screening was expanded to include women of 60–69 years after publishing of a health technology assessment (HTA) by the Finnish office for HTA (Finohta) in 2006 (Reference Mäkelä, Saalasti-Koskinen, Saarenmaa and Autti-Rämö13). As part of the HTA, the content and quality of the information delivered to Finnish women invited to breast cancer screening was examined. The information was insufficient, presenting mainly the benefits of screening. In a re-evaluation 3 years later, the situation had not improved (Reference Saalasti-Koskinen, Mäkelä, Saarenmaa and Autti-Rämö19). The municipalities are legally responsible for monitoring the quality of screening services in Finland, including the quality of patient information. By commission of the Finnish Screening Board at the Ministry of Welfare and Health, an expert team was appointed to prepare sufficient information material for women invited for mammography screening. Authors of this article were members of the expert team.
In public screening programs, healthy individuals are invited for screening. The contact between them and screening test provider may offer little possibility for discussion. Information given by the service provider to women attending breast cancer screening has been scant, presenting predominantly the pros of screening (Reference Hoffman, Lewis and Pignone7;Reference Saalasti Koskinen, Pasternack and Leipälä20). Patient Decision Aids are paper-based or electronic tools that provide evidence based and balanced information about the advantages and disadvantages of options, and help in clarifying personal preferences and values regarding the intervention (6). They increase people's involvement and are more likely to lead to informed, value-based decisions (Reference O'Connor, Bennett and Stacey15). Several agencies produce patient decision aids, for example, NHS (14), Ottawa Hospital Research Institute (OHRI) (16), and University of Sidney (23). The role of decision aids is particularly important for interventions where there is a need to balance potential benefits and harms. Randomized trials have shown that decision aids may increase knowledge and informed consent (which is a composite of knowledge, values, and intention) to participate mammography screening, without increasing anxiety (Reference Mathieu, Barratt and McGeechan11;Reference Mathieu, Barratt and Davey12). On the other hand, increased information may lead into lower satisfaction with the decision (Reference Politi, Clark, Ombao, Dizon and Elwyn18) or reduce the willingness to participate in screening (Reference Adab, Marshall and Rouse1;Reference Mathieu, Barratt and McGeechan11). An important issue to consider when providing such information is that the concepts of risk and probability are poorly understood by both public and professionals.
Understanding Numbers
Women easily overestimate the effectiveness of mammography screening (Reference Domenighetti, D'Avanzo and Egger3), and those who overestimate benefits are known to participate more actively (Reference Chamot and Perneger2). Even interest and confidence in ability to use numerical information are not associated with actual comprehension or ability to use numbers (Reference Woloshin, Schwartz and Welch25). A recent review examined how numerical formats affect interpretation (Reference Visschers, Meertens, Passchier and de Vries24). When the probability of the test outcome is presented either in percentage (Reference Elwyn, O'Connor and Stacey5 percent) or as single event probability (.05), the vast majority of experts and lay people strongly overestimate the value of the test. When the same information is presented in frequencies (5 of 100), the risk estimates are much more realistic. Bigger numbers are easily interpreted as higher probability; 1200 deaths of 10,000 (probability 0.1) is perceived a greater risk than 24 deaths out of 100 (probability 0.2).
Reduction in mortality due to screening is usually expressed as relative risk reduction (RRR). When the probability of the event (cancer) is low, even small changes in event rates result in a large RRR, although the absolute risk reduction (ARR) is small. For example, the figures for risk reduction by mammography presented in the Finnish health technology assessment report (Reference Mäkelä, Saalasti-Koskinen, Saarenmaa and Autti-Rämö13) are of different magnitude: RRR 20 percent and ARR 0.1 percent. Therefore, RRR should be presented together with ARR to give a more balanced view. The inverse of ARR, number needed to treat (NNT), could be an intuitively clear presentation of risk, but frequently people misinterpret it as well (Reference Visschers, Meertens, Passchier and de Vries24).
Words seem to be more understandable, but they tend to carry imprecise meaning. People have different probability lexicons; they interpret, e.g., the word likely from a probability of 0.5 to 0.95. Framing affects perception; positive framing, for example, 9 of 10 are healthy instead of 1 of 10 has cancer is generally recommended in patient information. Visual representations of probability are more easily understood. Pictograms are a recommended and popular way to present risk information (Reference Visschers, Meertens, Passchier and de Vries24).
Providing accurate information in an understandable format does not necessarily improve communication of risk. Much of the literature on risk perception is concerned with how well people understand and can recall the numbers or the probabilities associated with the risks. Few studies have addressed how people weigh or order the information, and how decision-making processes work (Reference Lloyd10).
This study describes the process of producing a set of information material as decision aids for the Finnish public mammography screening program, analyzes the challenges in production and implementation, and presents the initial feedback received.
METHODS
The team used the Finnish breast cancer screening HTA from 2006 (Reference Mäkelä, Saalasti-Koskinen, Saarenmaa and Autti-Rämö13) as their base document. Additional information was retrieved from recent published randomized trials, systematic reviews and HTAs, and two doctoral theses (Reference Sarkeala21;Reference Seppänen22). For identifying relevant content and format for the decision aid, the team used the European guidelines for quality assurance in breast cancer screening and diagnosis (Reference Perry, Broeders and Wolf17), and additional studies on risk communication referenced in the background section of this article. Content and format were further elaborated after piloting the documents among users. A Web-based questionnaire was sent to service providers 6 months after opening the Web site and publishing the information package, including the letter templates with decision aid.
RESULTS
Our intention was to engage all relevant stakeholders in the expert team. Joint design of decision aid was especially important in Finland where HTA has no normative power. The expert team consisted of HTA professionals, representatives from a municipal breast cancer screening unit and the largest private sector provider, and experts from the Finnish Cancer Registry; it included multiple expertises from radiology, radiography, oncology, registers, service provision, clinical epidemiology, ethics and science communication. Four face-to-face meetings were held from May to December 2009. Because of lack of tradition and procedures for early patient or citizen involvement, user representation was not invited among women invited for screening or from patient organisations. The team acknowledged this as an apparent shortcoming which they intended to partly redress by user testing.
The expert team agreed that the decision aid should be attached to the back of the invitation letter to reach everyone. Women's concerns and information needs differ at first invitation and later, at successive screening rounds or when receiving invitation to diagnostic tests due to mammogram findings. Therefore, tailored content for six different letter types used in different phases of screening was prepared (Table 1). The first invitation letter includes the full decision aid. The other letters contain selected sections of the decision aid incorporated in the text.
Table 1. Project Outputs: Information Materials Mroduced for the Finnish Breast Cancer Screening Program
Sixty-two possible content items to be included in the decision aid were identified from breast cancer screening research publications and the European quality assurance guidelines (Reference Perry, Broeders and Wolf17). Each team member scored the importance of each item (1 = not important, 2 = somewhat important, 3 = important), and a mean was calculated for each. Key items (mean > 2) were considered more carefully when preparing letter templates. The list of content items selected for the first invitation letter is presented in Supplementary Table 1, which can be viewed online at www.journals.cambridge.org/thc2011027. Format requirements (Table 2) were also identified and used as guidance in editing the decision aid. Deviations from these requirements occurred mostly in letter templates with strict space constraints, for example, expressions using outcome frequencies (8 of 10) were replaced by shorter but still understandable percentages (80 percent).
Table 2. Format of Information for Breast Cancer Screening
Most mammography screening providers in Finland use electronic mass posting when sending invitations. The electronic letter templates have limited space for content; additional pages mean extra cost, which decreases willingness to implement. However, a separate leaflet in an envelope would be even more expensive. Some information in the decision aid (aim of screening, pain during examination, and voluntary participation), are presented on the front page of the invitation letter together with practical and process related information, such as preselected appointment and guidance on how to change the appointment, time required for examination, and how results will be given. The remaining information of the decision aid was placed on the back of the letter (Supplementary Figure 1, which can be viewed online at www.journals.cambridge.org/thc2011027). The letter templates are available in Finnish, Swedish, and English (Reference Saalasti Koskinen, Pasternack and Leipälä20).
Because all necessary information could not fit in the letter templates, a need for additional information site became apparent and a new web site was created. The information on the Web site is primarily targeted to women who consider attending breast cancer screening. For service providers and municipal decision makers, information and tools for the implementation of the national screening program are available. These include legislation, norms and administrative guidance on quality assurance of national screening programs, advice for tender invitations, the new letter templates, and a poster designed for waiting rooms. The poster illustrates a woman's chances of having a suspicious finding in screening mammogram or actually having a cancer (Supplementary Figure 2, which can be viewed online at www.journals.cambridge.org/thc2011027). The Web site opened in May 2010 and will be available in English in 2011. Between August and December 2010 there were 2040 visits to the pages, averaging 7 page views each.
User Feedback
The service provider members of the expert team selected three screening units, one municipal and two private, for readability testing of the new letter templates. A convenience sample (N = 160) of women attending breast cancer screening in these units were asked to read and comment on the new letter templates. The expert team also approached their acquaintances who were not medically trained for feedback from women under 50 years old (N = 15). The views of younger women, who are presumably more naïve to the breast cancer screening issues, were assumed to differ from older groups. Of 175 delivered questionnaires, 70 were returned (response rate 40 percent). Most respondents (73 percent) were 50–59 years of age, and nearly all (85 percent) had experienced mammography.
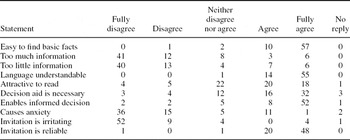
Forty-eight of seventy women (69 percent) thought that the text in the invitation letter (the decision aid) is important for them; the percentage was a little higher in the older age groups (Table 3). The majority (86 percent) found that the invitation letter gave enough information for an informed decision; this percentage was a little higher in the youngest age group. The sentence “Despite a normal screening result, a woman might develop cancer before next screening” caused most confusion, and was called “a harsh statement”. Softer expressions were suggested, some including advice on breast self examination. In general, women wanted to have a more personal tone, while some stated that there was a sense of respect of individual in the way the possible harms were presented in the letters. “It is difficult to make a personal judgement of an issue that requires expertise. Still, I feel that the way you have presented the information, is right.” The team agreed to implement suggestions from the users in the final letter templates.
Table 3. User Feedback on Letter Template
Note. Evaluation of invitation letter template with decision aid (N = 70, number of respondents in each category)
Six months after the letter templates were made available we asked all the 11 service providers in Finland about their use. Two of them had taken the letters into use by then, and three intended to do that soon. Two providers were not yet aware of them and one preferred letter templates they had developed themselves. As decision making in municipalities is slow, three were still in the process of changing letters.
DISCUSSION
The attendance rate of the public breast cancer screening program in Finland is exceptionally high. Therefore, the aim for producing the decision aids for Finnish women was not to increase participation, but to provide knowledge to empower women against perceived professional paternalism. Decision aids can also have unwanted consequences; they may increase anxiety and confusion, and reduce satisfaction in decisions or participation in screening. The aims of producing this decision aid and its possible unwanted consequences were discussed and balanced in the expert team. Professionals and service providers would have been more careful in including information of the harms of screening in the decision aid. The team had repeated, detailed discussions of the terms used and the overall tone in the text. HTA professionals stressed the priority of citizens' ethical and legal rights to receive balanced and understandable information when considering participation in healthcare interventions. Researchers cannot guarantee that decision aids will be read and understood as intended, but they must aim at correct, clear, and balanced information. This principle was in the end shared by the whole team.
The team did not follow a strict protocol; instead, the extent and quality of the outputs were developed during the project. The original plan was to produce a single template for national invitation letter. The final outputs instead included a set of tailored information materials for each step of the screening process, both electronically and on paper. These were presented in numbers, words and visual images, and targeted to women, decision makers, and service providers. Prolonged discussions toward consensus slowed the process but made the outputs more relevant and sustainable. The use of the website for providing more detailed information probably means some women have difficulty in accessing this information; however, the core information needed for informed decision is presented in the paper invitation letter.
We tested the readability and perceptions of the letter templates with a small number of women and the response rate was relatively low. Therefore the information was used only for providing corrections to the letter templates. Optimally, suggestions from user feedback would have been analyzed more carefully, and motivation for selection or omission registered clearly.
Planning decision aids requires expertise and procedures that were not fully established at Finohta, despite earlier experience in preparing information for patients and citizens on foetal screening (Reference Leipälä, Ignatius and Autti-Rämö9). The process was not optimal; in particular, the public input could have been stronger. Women's views were collected afterward by user testing, clearly an insufficient approach. Three healthcare professionals who had earlier been involved in preparing patient information for breast cancer screening commented the letter templates and their general view was that the text is too top-down and that the word “harmful effects” should be replaced by “limitations.” Finland lacks the tradition of involving public or patients in HTA. Updating the policies and processes for public involvement, and considering explicit use of theories to guide the design of decision aid as proposed by Elwyn et al. (Reference Elwyn, Stiel, Durand and Boivin4), is needed.
The decision aid created in this project has not been validated against established criteria; such as the International Patient Decision Aid Standards Collaboration Checklist (Reference Elwyn, O'Connor and Stacey5) or the CREDIBLE criteria (16). This shortcoming can be partly explained by the fact that the expert team was not aware that they were producing decision aids. The original task was to produce templates for cancer screening invitation letters. Only at the end of the project it became apparent that decision aid is the closest definition for the work that had been done.
The implementation of the decision aid and other information material is still incomplete. This is partly due to slow decision processes in municipalities, partly due to deficient dissemination. In a couple of years, re-evaluating the use of letter templates and perhaps the effect of the materials on service use would be interesting.
CONCLUSION
Working with expert team with multiple skills and views is challenging but fruitful when producing a set of materials to aid screening decisions. The wide range of HTA evidence used required careful thinking, selection and formatting. The decision aid attached to breast cancer screening invitation letter has gained acceptance but also created confusion among Finnish women and professionals. The uptake and use of the new information tools into the national screening is still incomplete.
SUPPLEMENTARY MATERIAL
Supplementary Table 1
Supplementary Figure 1
Supplementary Figure 2
www.journals.cambridge.org/thc2011027
CONTACT INFORMATION
Iris Pasternack, MD ([email protected]), Medical Researcher, Ulla Saalasti-Koskinen, MSc (health care) ([email protected]), Research Officer, Marjukka Mäkelä, MD, PhD, MSc ([email protected]), Research Professor, FINOHTA (Finnish Office for Health Technology Assessment), at THL (National Institute for Health and Welfare), P.O. Box 30, 00271 Helsinki, Finland
CONFLICT OF INTEREST
All authors report they have no potential conflicts of interest. Outside this work, the authors' institute receives a grant from the European Commission.