Acute-care hospital antibiotic stewardship programs (ASPs) promote appropriate durations of therapy for patients cared for during their hospital stay. The Centers for Disease Control and Prevention (CDC) Core Elements advise hospitals to develop and implement facility-specific treatment recommendations to optimize antibiotic selection and duration as well as track the impact of their ASP.Reference Pollack and Srinivasan1 In-hospital antimicrobial durations, however, only capture a portion of the total antimicrobial exposure attributable to hospitalization. In fact, the majority of the antibiotic course may be completed postdischarge for many common infections.Reference Jenkins, Knepper and Sabel2, Reference Jenkins, Stella and Cervantes3
Promoting shorter durations has become a goal for many ASPs, as data are emerging on successful short course therapy for common infectious syndromes, such as community-acquired pneumonia, urinary tract infection, intra-abdominal infections, and skin and soft-tissue infections.Reference Spellberg4, Reference Hayashi and Paterson5 Hospitals that only assess inpatient days of antibiotics are not able to demonstrate the full impact of duration-focused interventions that affect both inpatient and postdischarge days of antibiotics. Assessing total duration of therapy could allow better understanding of duration-focused stewardship interventions and identify opportunities to optimize antibiotic use at transitions of care.
This study was part of a larger project entitled, “Developing Patient Safety Outcome Measures and Measurement Tools for Antibiotic Stewardship Programs,” that aimed to identify metrics that reflect the impact of the ASP on patient safety and quality of care.6 A metric of total duration was deemed useful in demonstrating ASP impact by the Structured Task Force of Experts Working at Reliable Standards for Stewardship (STEWARDS) panel, but the panel was uncertain of feasibility of data collection and analysis in hospitals utilizing electronic health records (EHRs). To our knowledge, most prior studies reporting total duration have used chart review and manual extraction to quantify the intended postdischarge days of antibiotic therapy.Reference Jenkins, Knepper and Sabel2, Reference Jenkins, Stella and Cervantes3, Reference Tamma, Avdic and Keenan7–Reference Moehring, Anderson and Cochran9 Historically, total duration has been difficult to capture electronically because inpatient and outpatient data were housed in different electronic systems or discharge prescriptions were hand written. Electronic capture could allow stewards to analyze prescribing durations without performing labor-intensive chart reviews.
In this study, we aimed to determine the feasibility of capturing electronic discharge prescription information in addition to inpatient antimicrobial use to calculate total duration of antimicrobials. The secondary aim was to quantify total antimicrobial durations attributable to inpatient hospital stays and the proportion contributed by postdischarge days.
Methods
We performed a retrospective cohort study of inpatient and discharge antimicrobial use data from April to September 2016 at 1 large, academic medical center and 2 community teaching hospitals (each with 300+ beds) located in the southeastern United States. The electronic health systems in use were Epic (2015 Upgrade, Verona, WI; www.epic.com) for 2 hospitals and McKesson (version 10.1.4.3, build 5, San Francisco, CA; www.mckesson.com) for the third. Electronic medication administration records were used to calculate inpatient antimicrobial days. Electronic discharge prescriptions (“e-scripts”) data were accessed to capture intended postdischarge antimicrobial days.
E-script data files were requested from the 3 pilot sites for the 6-month period. Two hospitals had a previously prepared data extraction file used by their outpatient pharmacy for administrative purposes. The third hospital employed a local analyst to create a new extract file for the purpose of the study. Fields in the data file included patient and admission/encounter identifiers, drug name, instructions for medication administration (sig), frequency, dispense number (quantity), duration (in days), and order date. Data files for electronic discharge prescriptions from the 2 sites using an existing data report did not include a discrete field for days duration, so we calculated duration using sig and quantity fields. E-scripts were linked to admission identifiers using dates of discharge prescription orders matched to hospitalization dates.
A 200-patient validation was performed at 1 site (hospital B) to ensure that electronic data were being captured appropriately. The validation included 2 random samples of patient admissions that were pulled from the primary EHR and compared to the e-script data extract: 100 patients discharged with e-scripts captured in the extract file and 100 patients with no e-script in the extract file. Reviewers manually analyzed the data captured in data extract files compared to documentation in the EHR.
For the analysis of total duration, adult or pediatric patients who received at least 1 day of antimicrobial therapy on an inpatient unit were included. Antimicrobial therapy received in outpatient units (eg, emergency departments) and procedural areas (eg, cardiac catheterization lab, operating room) were excluded. Antimicrobial agents were limited to those in the National Healthcare Safety Network Antimicrobial Use module, since most other agents would not be used for acute illnesses (eg, HIV medications).10 Agents administered systemically (eg, intravenous, oral, and intramuscular) were included; topical (eg, creams, drops) and inhaled agents were excluded. Definitions of days of therapy and length of therapy (LOT) were taken from Polk et al.Reference Pollack and Srinivasan1, Reference Polk, Hohmann, Medvedev and Ibrahim11, Reference Ibrahim and Polk12 The primary metric of total duration was defined as the inpatient LOT plus postdischarge LOT, the count of calendar days when antimicrobials were received irrespective of the number of agents or doses on each calendar day. Postdischarge prescription durations were calculated based on orders and not administrations because patient adherence with ordered durations could not be measured. Descriptive statistics were used to describe (1) admissions with inpatient antimicrobials only versus admissions with both inpatient and postdischarge antimicrobials, (2) the distributions of total and postdischarge durations, and (3) the total duration among admissions with syndrome diagnoses. Syndrome diagnoses were defined using the Agency for Healthcare Research and Quality Clinical classifications software categories for pneumonia (122), urinary tract infection (159), skin and soft-tissue infection (197), and intra-abdominal infections (142, 146, 148, 149) based on International Classification of Disease, Tenth Revision (ICD-10) diagnosis codes associated with each admission.13
Data were analyzed using SAS version 9.4 software (SAS Institute, Cary, NC). The study was deemed exempt research by the institutional review boards at Duke University and Southeastern Regional Medical Center.
Results
Feasibility and validation
For 2 pilot sites, analysts calculated days duration for the electronic prescriptions from text in the sig and quantity fields. Due to inconsistencies in the text of sig fields, 437 e-scripts (5%) had durations that could not be calculated. This occurred most commonly for oral solutions and intravenous antibiotic orders. A metrics guide is available on our website that describes the structure of the data extracts from the 2 EHR systems in detail.6 This guide also includes detailed description of data cleaning steps required to match discharge prescriptions to inpatient admissions and to quantify days duration from sig and quantity fields.6
The manual validation steps taken at hospital B included 200 patients: 100 patients discharged with e-scripts captured in the extract file and 100 patients with no e-script in the extract file. Patients from the first sample had 100% accurate capture of e-scripts data, including dose and days duration, in extract files. In the other sample, validators found inconsistencies in clinicians’ use of e-scripts. Clinicians used written prescriptions or documented intent to prescribe antimicrobials without inputting e-scripts in 23 patients (23%). Clinical scenarios in which written or verbal order prescriptions were utilized instead of e-scripts included patients discharged to and receiving antimicrobials in long-term care facilities or dialysis centers.
Analysis of total duration
A total of 45,693 inpatient admissions were analyzed during the 6-month study period: 23,447 admissions (51%) received inpatient antimicrobials and 7,442 admissions (16%) received e-scripts at discharge. E-scripts were prescribed for 348 (5%) admissions who had not received antimicrobials as an inpatient. Patients most commonly received e-scripts for 1 antimicrobial agent on discharge (82%) or 2 antimicrobial agents on discharge (16%). The most common e-scripts were for fluoroquinolones, cephalosporins, and β-lactam/β-lactamase inhibitor combination antibiotics (Table 1). For all 3 hospitals, fluoroquinolones were the most common class; the most common agent was ciprofloxacin. Approximately 75% of discharge e-scripts were written on medical, surgical, and hematology/oncology wards. Durations of e-scripts were longer for surgical and hematology/oncology wards.
Table 1. Number of Electronic Discharge Prescriptions by Antimicrobial Class, Agent, and Discharge Unit Type
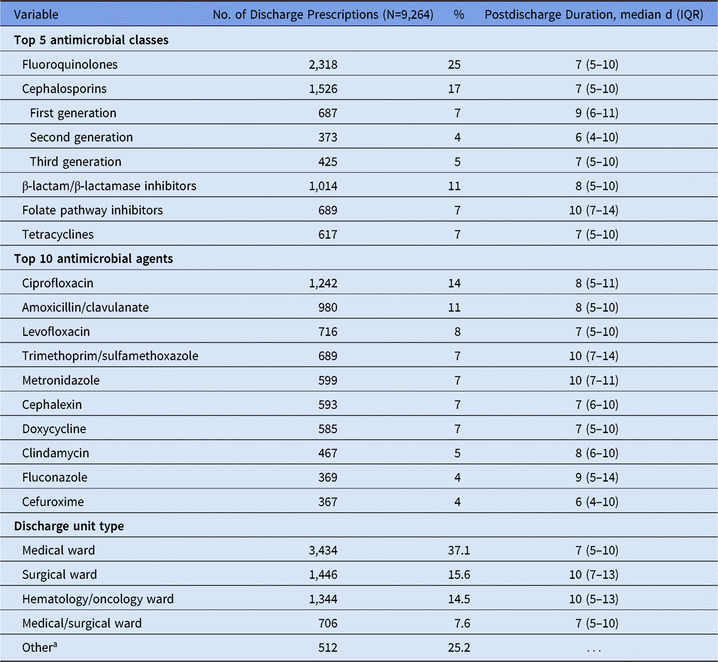
Note. IQR, interquartile range.
a Includes specialty wards (eg, pulmonary or orthopedic wards), labor and delivery wards, pediatric wards, and critical care units.
E-scripts accounted for 38% of the overall duration of therapy for patients (Table 2). Postdischarge duration among all discharge e-scripts was a median of 8 days (range, 1–360). The most common postdischarge durations were 3, 5, 7, and 10 days (Fig. 1B). There were also increased frequencies of e-scripts with 14- and 30-day durations. Also, 14 patients had discharge durations that exceeded 100 days of therapy. Drugs with median LOTs of ≥30 days included drugs used in outpatient parenteral antimicrobial therapy (OPAT) (eg, cefazolin, penicillin V), oral anti-fungals for mold treatment and prophylaxis (eg, posaconazole, voriconazole), and agents for treatment of nontuberculous mycobacteria (eg, amikacin, tedizolid, cefoxitin). Patients with inpatient plus discharge antimicrobial e-scripts were older, more likely to be male, and had higher Elixhauser comorbidity scores, but these patients had a shorter mean length of stay compared to admissions with inpatient antimicrobials only (Table 3).

Fig. 1. Distribution of total duration and postdischarge durations for all hospitals. The graphs were limited to durations of ≤30 days. Total durations ranged from 1 to 367 days, and 4.2% of durations were >30 days. Postdischarge durations ranged from 1 to 360 days, and 3% of these were >30 days.
Table 2. Percentage of Postdischarge and Total Durations of Therapy
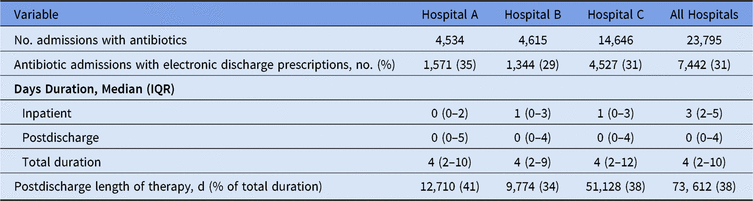
Table 3. Characteristics of Patient Admissions by Receipt of Inpatient Only or Inpatient Plus Postdischarge Antimicrobials

Note. For all hospitals, 349 (1%) patients had an electronic discharge prescription for an antimicrobial without receiving a prescription for inpatient antimicrobials. Missing data were not included in above percent calculations and represented <1% for any single characteristic. Pediatric admission was defined as age at admission 0–17 years.
Patients with an ICD-10 diagnosis for pneumonia, urinary tract infections, skin and soft-tissue infection, or intra-abdominal infection were often discharged on antibiotic therapy (Table 4). Using a 7-day duration as a recommended typical course for most uncomplicated infections, most patients (78%) who received e-scripts exceeded this duration compared to 16% of patients who had inpatient antimicrobials only. Among patients with a diagnosis of these infectious syndromes, total durations for patients who received discharge e-scripts exceeded those for patients without discharge prescriptions despite having shorter lengths of stay. The difference in total duration was most pronounced among those with a diagnosis of intra-abdominal infection.
Table 4. Length of Stay and Total Duration by Syndrome Diagnosis
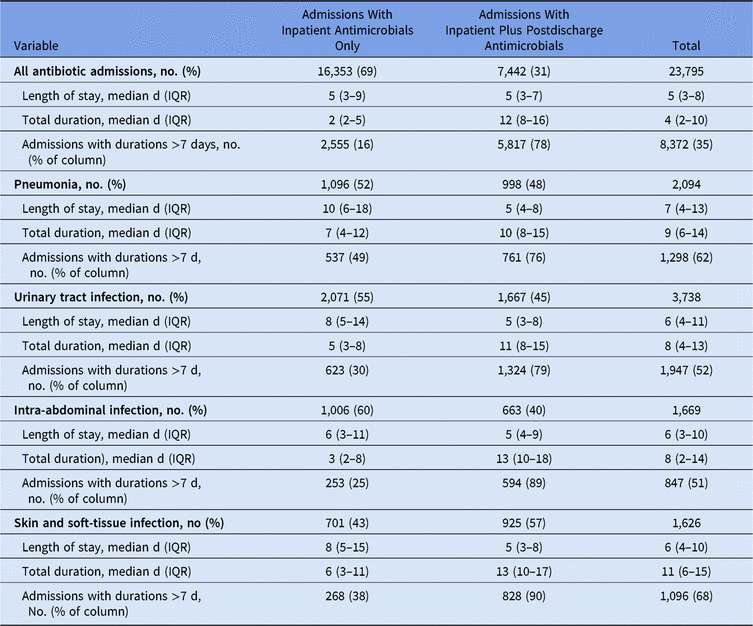
Note. IQR, interquartile range.
Discussion
ASPs must target decisions occurring at transitions of care and processes for discharge antimicrobial prescriptions to improve patient safety. Our study indicates that more than one-third of hospital-related antimicrobial exposure occurred after the patient was discharged. More than three-quarters of patients given e-scripts received a total antibiotic course that exceeded 7 days. In addition, our study highlights the complexity of the task of electronic data collection and analysis from EHRs. Our estimates of postdischarge antimicrobial days were subject to missing data based on clinician work flow and avoidance of the electronic system in certain clinical scenarios. Thus, the estimates of total antibiotic exposure attributed to postdischarge prescriptions in this study were likely underestimates of the intended antibiotic duration.
Several potential causes of excessive antimicrobial durations at hospital discharge are possible. Errors in the ordering process or electronic system “defaults” for outpatient prescriptions may result in longer durations than intended. Extended durations may be prescribed due to lack of knowledge, uncertainty about the patient’s diagnosis or readiness for discharge, or inadequate attention to the task of defining the start and stop dates to meet the intended total duration of treatment. Growing administrative pressure to shorten hospital lengths of stay and perform discharges earlier in the day might lead clinical teams to rush the discharge process. Antimicrobial stewardship and appropriate antimicrobial durations should be included in efforts to improve patient safety and to prevent negative consequences that could be attributed to hospital discharges. Discharge processes are a key area in which ASPs need to engage other healthcare professionals, such as hospitalists, nurses, and clinical pharmacists to improve patient safety.
Previous investigation of discharge prescriptions has indicated that when discharge durations are factored into prescribing, 55% of patients receive a course of antibiotics that is too long and, on average, patients receive 3.8 days of unnecessary antibiotic therapy.Reference Scarpato, Timko and Cluzet15 For many common infections, short-course therapy is equivalent to longer durations of therapy.Reference Spellberg4 In controlled clinical trials, uncomplicated community acquired pneumonia can be treated in as few as 5 days; hospital-acquired or ventilator-associated pneumonia, ≤7–8 days; pyelonephritis, 5–7 days; cellulitis, 5 days; and intra-abdominal infection, 4–7 days.Reference Uranga, Espana and Bilbao16–Reference Hepburn, Dooley, Skidmore, Ellis, Starnes and Hasewinkle23 The most common postdischarge durations in our study were 5, 7, and 10 days, with additional spikes at 14 and 30 days. Since each of these are common durations for full antimicrobial treatment courses, this finding may indicate that clinicians did not factor in the patient’s inpatient LOT when selecting outpatient antibiotic duration or may have relied on electronic defaults within the prescribing system. Frequency of discharge prescribing by agent and drug class revealed that 25% of all discharge antimicrobial prescriptions were fluoroquinolones. Our data are consistent with those of Scarpato et al,Reference Scarpato, Timko and Cluzet15 who noted a similar fluoroquinolone prescribing rate of 23.5% when evaluating discharge prescriptions from the Hospital of the University of Pennsylvania. Our fluoroquinolone prescribing rate was lower than the 40% rate described by Yogo et alReference Yogo, Haas, Knepper, Burman, Mehler and Jenkins8 for discharge prescriptions filled within 7 days posthospitalization, lower than the 47%–49% rate of outpatient prescribing noted in a study of community acquired pneumonia by Yi et al,Reference Yi, Hatfield and Baggs24 and lower than the 30.5% rate cited by Vaughn et alReference Vaughn, Gandhi, Conlon, Chopra, Malani and Flanders25 from patients diagnosed with urinary tract infection or community-acquired pneumonia in 48 hospitals in the Michigan Hospital Medicine Safety Consortium.Reference Pollack and Srinivasan1, Reference Yogo, Haas, Knepper, Burman, Mehler and Jenkins8, Reference Yi, Hatfield and Baggs24, Reference Vaughn, Gandhi, Conlon, Chopra, Malani and Flanders25 The inpatient stewardship programs at all 3 study hospitals were actively engaged in initiatives to decrease inpatient fluoroquinolone prescribing due to resistance of common pathogens to these agents and the increased risk Clostridioides difficile infections.Reference Barlam, Cosgrove and Abbo26, Reference Pepin, Saheb and Coulombe27 Based on this growing body of literature incorporating postdischarge antibiotics, ASPs should focus on discharge processes as a key area to promote avoidance of fluoroquinolones and shorter, syndrome-based durations.Reference Vaughn, Gandhi, Conlon, Chopra, Malani and Flanders25
Syndrome-focused antimicrobial stewardship has been identified as a strategy to better engage with clinicians. Institution-specific syndromic guidelines and order sets, however, often focus on empiric therapy and do not address decisions after the time of antibiotic initiation. Thus, antibiotic de-escalation and duration may not be included in syndrome-specific antimicrobial stewardship initiatives. We did not specifically evaluate indication with in-depth chart review, but we attempted to understand indication by looking at ICD-10 diagnosis codes for the major infectious clinical syndromes. Although not specifically investigated in this study, we suspect that use of indications documented by prescribers at the time of order entry may be more accurate than ICD-10 codes assigned at discharge, as large proportions of antibiotic-associated encounters have no associated infection diagnosis codes.Reference Chua, Fischer and Linder29 We observed that in pneumonia, patients received a median of 10 days of treatment when they received a e-scripts and a median of 7 days when they completed therapy as an inpatient. This finding is consistent with other estimates of median of 10 days duration for community-acquired pneumonia.Reference Yi, Hatfield and Baggs24 In a single-center study, direct prescriber feedback, coupled with education, decreased the median total antimicrobial durations for community-acquired pneumonia from 10 days to 7 days and led to a 61% reduction in overall antibiotic durations.Reference Avdic, Cushinotto and Hughes28 These data indicate that stewardship strategies targeting durations for specific indications can have an impact.
Moreover, 78% of patients with discharge e-scripts received a total duration of therapy in excess of 7 days. We observed that patients with diagnoses for infectious syndromes who completed their course of antibiotics while they were inpatients had longer mean hospital stays but received shorter total durations of antibiotic therapy. Presumably, patients who remain hospitalized have more complex clinical management for infections than those discharged sooner. Thus, the finding of longer durations in those discharged earlier is not consistent with the expected clinical status and requires further exploration to better elucidate the clinical rationales for long durations. Notably, few patients in the inpatient only group suffered in-hospital death (n = 689, 4%); thus, death is unlikely to explain the difference in antimicrobial durations between the 2 groups.
Our study had both strengths and limitations. We evaluated >45,000 patients and reviewed data from both community hospitals and an academic medical center. We assessed the feasibility of e-script data capture and its validity from the EHR. Our electronic data extraction method had feasibility barriers that required extra analysis time before proceeding with analyses of total duration. In addition, we discovered there was a missing data bias in the electronic prescriptions data due to lack of use of the electronic system in certain clinical scenarios. Based on the estimate of 23% of the sampled patients with no e-script data who did have postdischarge antibiotic exposures, we expect that this bias reduced our estimate of postdischarge days and, in particular, missing courses of intravenous antibiotics. Notably, missing e-scripts were common for patients going to skilled nursing facilities and patients receiving antibiotics with hemodialysis. This population would be useful to target both for measurement of postdischarge antibiotic exposure and to capitalize on opportunities to avoid outpatient parenteral antimicrobial therapies.Reference Shrestha, Bhaskaran and Scalera30 Despite the e-scripts data being incomplete, it is still highly useful for hospital ASPs to better target interventions focused at transitions of care to optimize the e-script process. We did not evaluate for appropriateness of antibiotic choice or intended duration; however, prior studies of outpatient prescribing at the time of hospital discharge have found that 53% of discharge prescriptions were inappropriate.Reference Yogo, Haas, Knepper, Burman, Mehler and Jenkins8 Thus, we believe that there is ample opportunity to investigate the rationales for long durations and promote more appropriate antibiotic durations upon discharge in our study hospitals. Interventions targeted to these rationales could then be tracked using similar electronic data collection methods. Finally, we captured intended discharge durations but did not assess prescription fills or patient adherence with prescribed antimicrobials. The study results raised additional questions for future investigation. We did not specifically compare e-script durations for children to that of adults, and we did not evaluate discharge e-script prescribing patterns for individual prescribers. Outpatient practices and some hospital ASPs use individual prescriber data feedback with peer comparisons of antibiotic use to encourage behavior change.Reference Meeker, Linder and Fox31, Reference Bork, Morgan, Heil, Pineles and Kleinberg32 The addition of postdischarge data to inpatient prescriber feedback reports would provide a more comprehensive look at antibiotic use by prescriber, especially in assessments of antibiotic duration attributed to the inpatient stay and specific syndromes. ASPs that do not attempt to measure postdischarge days may underestimate the effects of inpatient stewardship initiatives on antibiotic use, especially for interventions that intend to shorten durations of therapy. Finally, this study only partially touched on the significant challenges faced by inpatient ASPs in accessing electronic, patient-level datasets, developing standard ways to measure outcomes important and meaningful to ASPs, and implementing these measures on a large scale.
Antimicrobial prescriptions written at the time of hospital discharge led to more than one-third of total antimicrobial days attributable to an inpatient hospital stay. Durations in excess of 7 days were common for the major infectious syndromes among those receiving e-scripts. Our findings suggest that prolonged courses of antibiotic therapy are common at discharge and may contribute to unnecessary antimicrobial exposure in patients. ASPs that target discharge prescription duration and appropriateness have an opportunity to reduce unnecessary antimicrobial use and its resultant harms. These activities should be incorporated to hospital quality improvement initiatives focused at improving safety at transitions of care.
Author ORCIDs
April Dyer, 0000-0002-0360-8172
Acknowledgments
We thank the antibiotic stewards and clinicians at Duke University Hospital, Duke Regional Hospital, and Southeastern Regional Medical Center for their input into this study. This work is a result of the project entitled, “Developing Patient Safety Outcome Measures and Measurement Tools for Antibiotic Stewardship Programs,” a joint initiative made possible by a partnership between the CDC Foundation and Merck & Co (Kenilworth, NJ).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support
Funding for this work was provided by the CDC Foundation. R.W.M. was supported by the Agency for Healthcare Research and Quality (AHRQ grant no. K08 HS023866).
Conflict of interest
All authors report no conflict of interest relevant to this article. Dr. Dodds Ashley reports grants from CDC, personal fees from SHEA that included a Merck funded workshop, personal fees from University of Maryland supporting the AHRQ funded grant, outside the submitted work. Dr. Anderson reports grants from AHRQ, grants and other from CDC, personal fees from UpToDate Online, grants from NIH/NIAID, outside the submitted work. Dr. Moehring reports grants from AHRQ, grants from CDC, outside the submitted work.







