Older adults living in long-term, residential aged-care services (RACS) are among the most vulnerable to coronavirus disease 2019 (COVID-19). Increased frailty increases their risks of severe complications, hospitalization, and death compared with their counterparts not living in RACS. As of February 2021, residents of RACS were estimated to account for an average of 41% of deaths due to COVID-19, and up to 5% of all care-home residents in Belgium, France, the Netherlands, Slovenia, Spain, Sweden, the United Kingdom, and the United States had been infected. Reference Comas-Herrera, Zalakaín and Lemmon1 In Victoria, Australia, residents of RACS accounted for 10% of cases in 2020 and 80% of COVID-19 deaths. 2
Close living arrangements enable transmission of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) among residents, which is enhanced by staff and visitors moving from room to room. Staff training in infection prevention and control practices, including hand hygiene, may be suboptimal in RACS. Reference Lee, Lee, Lee and Park3 Infection prevention and control interventions, such as confining residents to their rooms, can have negative mental health consequences and can be challenging to enforce, particularly for residents with dementia. Reference Chu, Donato-Woodger and Dainton4 In jurisdictions or organizations where the aged-care workforce is increasingly casualized, the absence of sick-leave benefits may perversely incentivize working while ill. In addition, the high proportion of asymptomatic infections among COVID-19 cases, Reference Mizumoto, Kagaya, Zarebski and Chowell5 and the delay between infectiousness and disease onset Reference Johansson, Quandelacy and Kada6 means that staff and residents may unwittingly spread the virus prior to developing symptoms. Reference Arons, Hatfield and Reddy7,Reference Kimball, Hatfield and Arons8
The most common source of introduction of SARS-CoV-2 into a RACS has been via staff, possibly because of limiting visitors as a transmission prevention measure. Once an infection occurs, high interconnectedness of RACS enables transmission to other facilities and into the wider healthcare system and community. In addition to personal-care attendants, general practitioners, allied health professionals, and even grounds and/or maintenance staff may be required to work across facilities and healthcare settings, especially within provider networks. For example, in the first 8 long-term care facilities reporting COVID-19 cases in King County, Washington, at least 4 were epidemiologically linked, either through shared staff or resident transfers. Reference McMichael, Currie and Clark9
In July 2020, to mitigate the risk of staff transmitting the virus from one facility to another, the Australian government provided economic support packages to RACS and staff to enable single-site work. Reference Hunt10 The policy ensured that workers were supported, paid their usual income, not disadvantaged, and had choice over their place of employment. Workers required to isolate were also offered alternative accommodation and a one-time payment of AU$1,500 (∼US$971). However, several occupations were exempt from the single-site policy, including contractors, the emergency workforce and agency staff, as well as personal-care attendants in settings with critical staff shortages. Moreover, household relationships between aged-care staff provided an additional transmission pathway for COVID-19 to spread between RACS.
By examining contact-tracing data from a series of outbreaks in RACS in Victoria, Australia, we reconstructed the social networks to demonstrate the possible pathways of disease transmission through both work and household connections that might have compromised the single-site policy. These findings may guide public policy decisions about preventing RACS outbreaks in the future.
Methods
Between May and October 2020, the state of Victoria, Australia, experienced a COVID-19 epidemic involving >20,000 cases and a large number of outbreaks in RACS. 2 Due to the high vulnerability of RACS residents, a COVID-19 “outbreak” was declared in a RACS if a single case was onsite during their infectious period. For this study, we differentiate any exposure (ie, exposure site or exposure event) from those that resulted in probable on-site transmission (ie, 2 or more staff and/or resident cases within 14 days), which were defined as outbreaks. At the facility level, all exposures triggered the initiation of lock-down procedures for at least 2 weeks, including regular testing of staff and residents, use of personal protective wear (masks, visors, gloves, and gowns), and staff furloughs for any potentially exposed staff. This protocol imposed a huge burden on these facilities.
Cases were epidemiologically linked to exposure sites and close contacts based on information provided in case reviews by public health case, contacts, and outbreaks management teams. Data for all cases linked to RACS exposure sites from May to October 2022 (inclusive) were extracted from the state’s case and contacts management system and included clinical and demographic information about cases as well as epidemiological links of cases and contacts to exposure sites.
Social network analysis
Social network analysis (SNA) Reference Wasserman and Faust11,Reference Moreno and Jennings12 was used to visualize the extent to which RACS were linked through both workplace and household networks. Visualizations were created using Pajek software. Reference Mrvar and Batagelj13 Two networks were constructed: (1) a work network in which edges (ie, lines) represent direct links associated with staff working for multiple facilities (Supplementary Fig. 1a) and (2) a household network in which edges represent indirect links between RACS originating from staff sharing a household (Supplementary Fig. 1b). Other indirect links were not considered because pandemic mitigation measures in place at the time severely limited social interactions. When there was both a work connection and a household connection between RACS, we included both ties in the network visualization. Thus, the work and household networks were not mutually exclusive, which enabled the examination of the overlap and independent contribution of work (direct) and social (indirect) connections. The quadratic assignment procedure (QAP) was used to assess the correlation between the 2 networks. Reference Borgatti, Everett and Johnson14
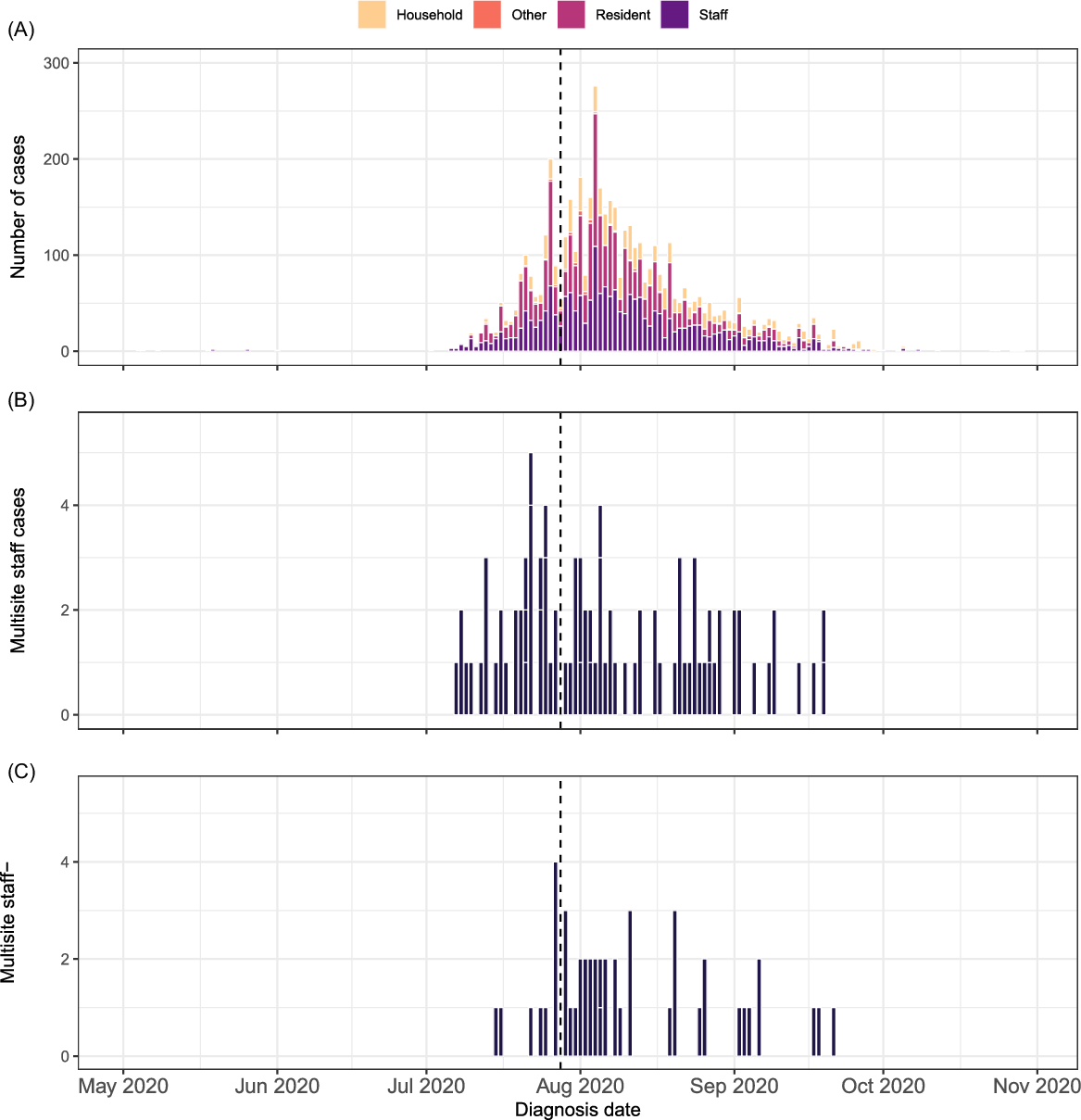
Fig. 1. Timeline of (A) cases linked to RACS exposure sites, (B) staff infections associated with multisite work, and (C) staff–household transmission, May–October 2020. The upper panel shows the epidemic curve by how the case was linked to the exposure site. The middle panel shows the number of staff linked to another facility by date. The lower panel shows the number of facilities to which a staff member was linked. The dashed vertical line indicates the date by which providers were required to implement the single-site policy. Although the single-site policy was introduced in July, the identification of multisite staff cases continued into September 2020.
Exposure events attributable to multisite work
A case was identified as a multisite staff case if it was epidemiologically linked as staff to 2 or more RACS exposure sites (Supplementary Fig. 1). The demographic characteristics of multisite staff cases were compared with other staff cases. To estimate the proportion of RACS exposure events potentially attributable to multisite work, the case notes for the subset of these staff cases, who were the index case for a RACS exposure site, were reviewed. Index cases were defined as the case(s) with the earliest diagnosis date in each event. Multisite work was determined to have plausibly initiated the event when either (1) an index staff case who acquired infection in the community worked at 2 or more RACSs during their presumed infectious period or (2) the staff index case was probably infected at one RACS and then worked at another RACS during their presumed infectious period. The exposure period was defined as the 2 weeks prior to symptom onset. Reference Backer, Klinkenberg and Wallinga15 The infectious period was defined as the 2 days prior to symptom onset or diagnosis if asymptomatic until cleared by the Department of Health. Reference He, Guo, Mao and Zhang16
The number of exposure sites, cases, and deaths that might have been avoided had staff worked at a single site was estimated from the number of cases and deaths linked to each exposure site. When the index case introduced infection into >1 facility, the number of potentially avoidable cases and deaths was calculated as a lower and upper range because information was not available to identify the preferred workplace.
Exposures events linked via households
Following a similar process, cases were identified as a multisite staff-household case if they were linked to 1 or more RACSs as staff and 1 or more RACSs as a household contact (Supplementary Fig. 1). In case not all household contacts were epidemiologically linked to an exposure site, the home addresses for all cases were reviewed to identify households. The characteristics of multisite staff–household cases were compared with other staff cases. To estimate the proportion of RACS exposure events potentially attributable to transmission in households, the notes of all multisite staff-household index cases were reviewed. These were compared with events caused by multisite staff index cases.
Ethical statement
Data were collected in accordance with the Victorian Public Health and Wellbeing Act 2008, 17 and formal ethical approval was not required because this work was conducted to inform the COVID-19 response in aged care.
Results
Overall, 166 exposure sites involving staff were declared in 164 RACSs between May and October 2020 (Fig. 1 and Supplementary Fig. 2). Of these, 166 were outbreaks involving onsite transmission (ie, 2 or more staff and/or resident cases within 14 days). The index case was a staff member in 89% of these outbreaks. There were 5,234 cases linked to these exposure sites, including 2,147 staff links, 1,954 resident links, and 1,076 household-contact links (Table 1). Because a case could have been linked to >1 site, the number of individuals was 5,049 with 2,034 individuals linked as staff to at least 1 site.
Table 1. Summary of RACS Exposure Events Involving at Least 1 Staff Case in Victoria, May–October 2020
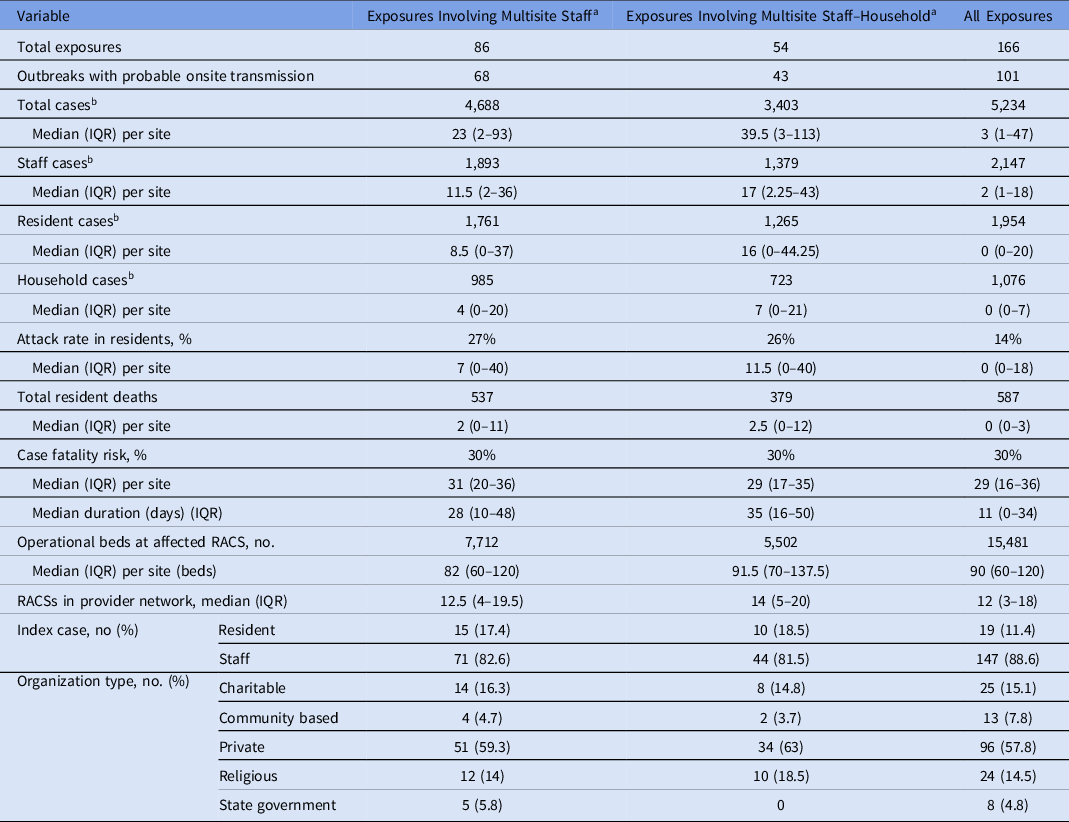
Note. IQR, interquartile range; RACS, residential aged-care services.
a Exposures involving multisite staff and multisite staff-household cases are not mutually exclusive.
b Case counts represent the total number of cases linked to RACS exposure sites and exceed the total number of cases (5,049) because a case can be linked to >1 site. Counts for cases linked as something other than staff, resident, or household are not shown.
Multisite staff cases
Among the 2,034 staff cases, 91 (4.5%) were considered multisite staff cases (Table 2). Multisite staff worked at up to 6 additional RACSs (n = 1), though most only worked at 1 additional RACS (n = 76). Cases were identified from July through October 2020 (Fig. 1b). Compared with all staff cases, a higher proportion of multisite staff cases were male (31% vs 21%), were younger (60% vs 51% <35 years) and spoke a language other than English at home (49% vs 43%). Multisite staff were also more likely to be allied health professionals (6.6% vs 1.6%).
Table 2. Summary of Cases With Links to Multiple Exposure Sites as Staff or as Staff and Household
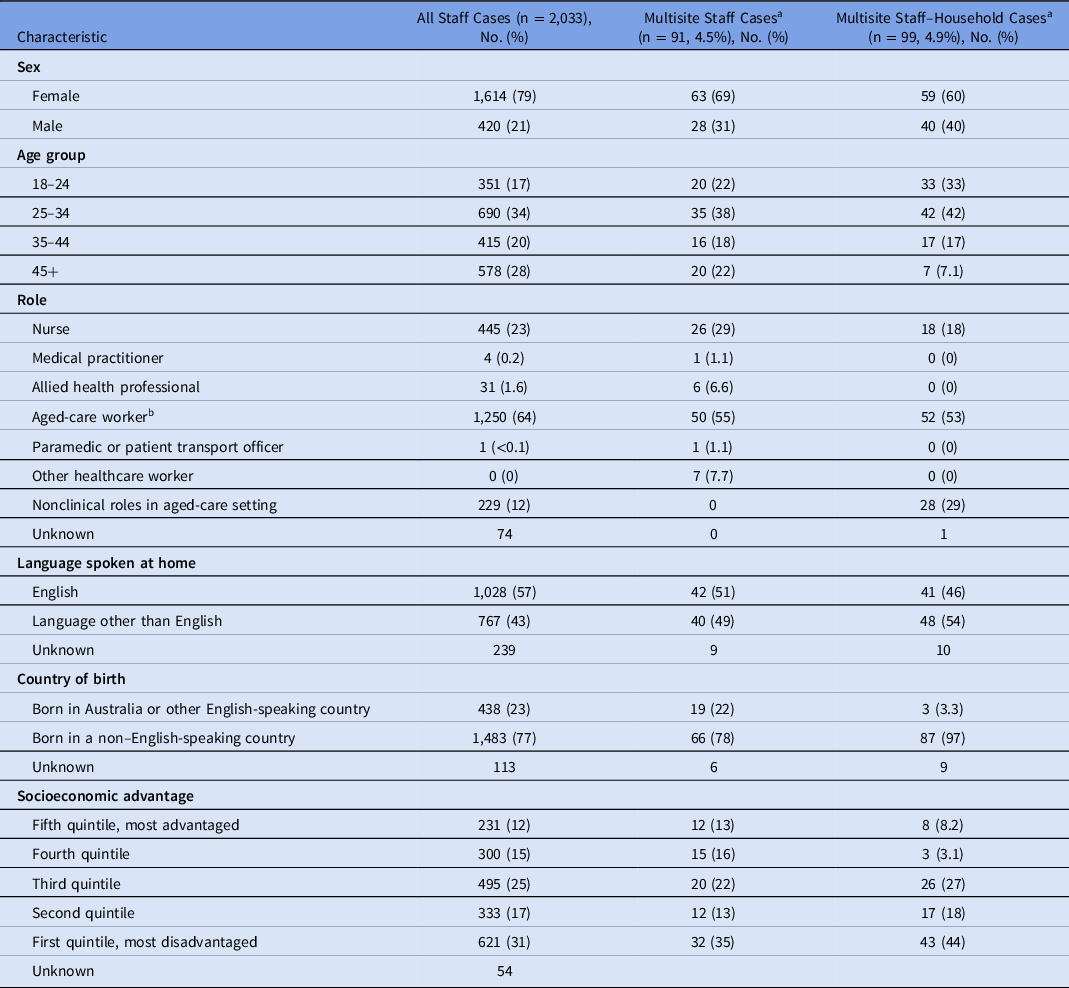
a Cases counted as multisite staff and multisite staff-household are not mutually exclusive.
b Aged-care workers are predominantly personal care assistants; allied health professionals include physiotherapists, optometrists, occupational therapists, etc. Socioeconomic disadvantage is estimated from the home address using statistical area 1 estimates of socioeconomic advantage and disadvantage. 39
Multisite staff-household cases
In total, 99 staff cases (4.9%) were identified as coresident, multisite, staff-household cases (Table 2 and Fig. 1c). These cases were more likely to be male (40% vs 20%), to be aged <35 years (75% vs 49%), to have been born in a non–English-speaking country (97% vs 76%), and to speak a language other than English at home (54% vs 42%) (Table 2).
Social network analysis
The work network considered 156 relationships (network density, 0.006), while the household network considered 36 relationships (network density, 0.001) (Fig. 2a and 2b). As seen in Figure 2c, the work and household networks overlapped. The correlation between the work and household networks was small yet significant (r = 0.091; P < .001), indicating the co-occurrence of work and household ties between pairs of RACS sites (ie, 7 overlapping work and household connections). However, if a one-site policy had been strictly imposed with no exemptions and all the direct work ties illustrated in Figure 2a had been eradicated, there still would have been substantial connectivity via indirect household ties between RACSs (ie, 29 connections between RACS) (Fig. 2b). Second, outbreaks involving a higher number of staff infections (larger nodes) often occurred within the connected region of the network. Similarly, a high proportion of exposure sites involving residents (red nodes) also occurred in the connected region. Fourth, RACSs with no resident cases (white nodes) and few staff cases (small nodes) were connected to RACSs with high numbers of staff infections. These cases often represented staff who had worked at an exposure site and then worked while infectious at a second site but did not cause an outbreak (exposures with no on-site transmission).
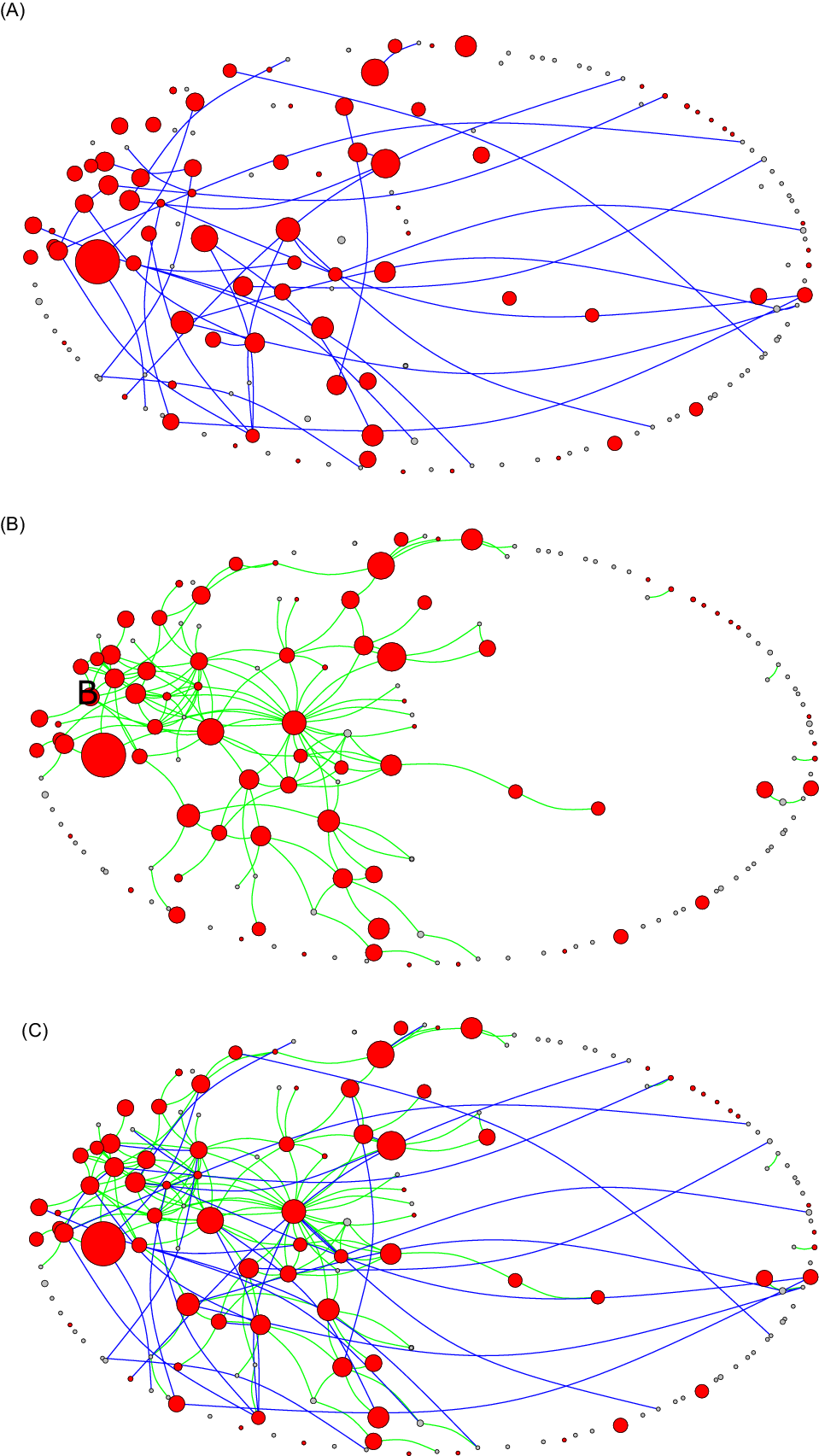
Fig. 2. Network diagram showing (A) direct work links (green lines), (B) indirect household links (light blue lines), and (C) the overlapping work and household networks among 164* RACS in Victoria, May–October 2020. Red nodes indicate RACS outbreaks involving resident cases, while white nodes represent RACS with no reported resident infections; the size of the nodes is relative to the number of positive staff cases. The extracted network data are a 1-mode projection (ie, RACS-RACS) of 2-mode data (ie, RACS–staff), which essentially collapses down the close-contact relationships between people and aggregates these to relationships between RACS. Although there may be many relationships between people between 2 RACSs, for the sake of parsimony these are dichotomized (any tie between 2 RACS = 1, otherwise = 0), because even a single relationship can enable transmission. *Notably, 2 RACS had 2 exposure events.
Exposures initiated by multisite work
In total, 35 multisite staff cases were the likely index case for 43 exposure sites. Of these, 35 (20% of all exposure sites) were considered potentially avoidable if a single-site policy had been in place. This analysis did not consider when the exposure occurred with respect to the single-site policy because of the exemptions. Also, 27 staff were likely infected at one RACS and initiated an exposure site at another RACS; thus, all were considered potentially avoidable under a strict single-site policy. Furthermore, 8 staff were infected outside work and worked at 2 separate RACS (16 exposure sites in total). Thus, only 1 of the 2 sites was considered potentially avoidable, but it was not possible to determine which, and therefore, only a range could be estimated. The potentially avoidable exposure events involved 277–446 staff cases, 181–370 resident cases, and 54–121 resident deaths (Table 1 and Supplementary Fig. 2).
Exposures initiated by household transmission
Among 99 multisite staff-household cases, 21 (12% of exposure sites) were index cases for exposure events involving 590 cases (Table 1). Of these, 10 involved no onward transmission. However, the remaining 11 ranged in size from 2 to 132 cases, totaling 579 cases, including 247 staff cases, 218 resident cases and 65 resident deaths (Table 1 and Supplementary Fig. 2).
Discussion
In this study we have shown that extensive worker and household networks contributed to the transmission of SARS-CoV-2 among RACS in Victoria in 2020. We identified 35 (21%) exposure events initiated by multisite staff cases and 21 (12%) initiated by multisite staff-household cases. The social network analysis of staff cases revealed an extensive inter-RACS network, which extended beyond the workplace to enable transmission. Thus, although it is highly probable that many exposures were averted at the time by the single-site policy in place, spread of infection from one RACS to another would not have been entirely avoided through a strict single-site policy (ie, one which did not permit exemptions) because of these household links. Such a strict policy may have in fact resulted in more harm because staffing shortages across the sector at the time were prevalent, raising concerns for resident well-being. For example, an independent inquiry into the 2020 RACS outbreaks reported that staff shortages negatively impacted resident health, Reference Gilbert and Lilly18 and a French study from 2020 speculated that some resident deaths were due to neglect associated with staff shortages. Reference Tarteret, Strazzulla and Rouyer19 Agency staff have been integral to outbreak management and were exempt from the single-site policy. Although modeling studies have demonstrated that agency nurses are a key risk for the spread of SARS-CoV-2 across long-term care homes, this risk can be mitigated with better training, routine testing, and the formation of workplace “bubbles” that limit the number of care homes where staff work. Reference Nguyen, Megiddo and Howick20,Reference Nguyen, Howick and McLafferty21
In addition to limiting worker mobility, initiatives that reduce the chance of onward transmission within the households of RACS staff members should be considered as part of the public health response to COVID-19 outbreaks in the aged-care sector. Other strategies could include readily accessible supported accommodation programs, which provide hotel or other accommodation for front-line staff who are working during an outbreak, 22 as well as broad close-contact definitions for RACS staff, provided there were adequate staff replacements available.
Although we considered only 1 index case for each site, it is likely that in some outbreaks there were multiple, separate introductions of SARS-CoV-2, some of which may have been due to transmission through workplace and household networks. Victoria’s 2020 COVID-19 epidemic was a point-source epidemic with extremely limited virus diversity, Reference Lane, Sherry and Porter23 making it difficult to differentiate new introductions of virus during an outbreak involving many cases. Genomic analysis of samples collected from residents of care homes in the United Kingdom, where virus diversity was higher, identified some monoclonal phylogenies suggestive of point-source outbreaks and some polyphyletic outbreaks suggesting multiple introductions with limited onward transmission. Reference Hamilton, Tonkin-Hill and Smith24 It is therefore likely that not all cases linked to these outbreaks were directly attributable to the multisite worker.
We identified most multisite staff cases after the single-site policy was introduced in July 2020. Some workers were exempt from single-site work, such as general practitioners, allied health professionals, agency staff, and other consultants and contractors for whom it may have been unfeasible to expect a provider to regulate. However, based on our review of case interviews, some staff were in breach of the policy, which relied on recording staff attestations. It is possible that such staff were ineligible or perceived that they were ineligible for government support payments or supported accommodation and that they may not have had access to other forms of income. Reference Reid, Ronda-Perez and Schenker25 A large proportion of workers were from culturally and linguistically diverse backgrounds and may have had trouble understanding the guidelines, which were not translated into languages other than English until late in 2020. 26 It is important these staff not be vilified and that government agencies identify appropriate supports to enable staff to follow new policies.
It is not possible to quantify the reduction in potential outbreaks that occurred as a result of the single-site policy. We observed that 4.5% of staff worked at >1 RACS, but we did not have information from the prepandemic period to estimate the degree to which this was a reduction in multisite work. Data from other nations suggests that it may have been a substantial reduction, suggesting high compliance by the aged-care workforce. For example, a 2016 study from the Netherlands reported that 12% of nursing home staff worked at other healthcare institutions, predominantly residential care facilities. An earlier Norwegian study reported that 18% of staff worked at >1 type of healthcare setting. Reference Sie, Thorstad and Andersen27 Those studies used surveys to estimate the extent of multisite work. In contrast, we used contact-tracing data, which were only collected when there was an outbreak and only collected detailed data from cases. As such, there may have been greater connectivity among the nonoutbreak RACSs than we were able to assess.
We did not consider SARS-CoV-2 transmission associated with RACS staff working in other kinds of healthcare settings, such as hospitals and other residential care settings, where staff networks may also have been important sources of infection. Reference van Gaalen, Hopman and Haenen28 RACSs are recognized as a key source of interfacility transmission within large health networks. Reference Lee, McGlone and Song29–Reference Wiener, Quinn and Bradford31 Many multidrug-resistant organisms are endemic in nursing homes, Reference Mody, Foxman and Bradley32,Reference Kahvecioglu, Ramiah and McMaughan33 and residents have been implicated in the importation and dissemination of multidrug-resistant Klebsiella pneumonia Reference Snitkin, Won and Pirani34 and methicillin-resistant Staphylococcus aureus (MRSA). Reference Lee, Bartsch and Wong35,Reference Chow, Lim and Khan36 Moreover, RACS staff have been implicated in the importation and spread of norovirus Reference Nguyen and Middaugh37 and MRSA. Reference Schwaber, Masarwa and Navon-Venezia38 Therefore, other healthcare settings should be considered in public health strategies to mitigate RACS outbreaks.
In conclusion, the high interconnectedness of RACS via staff mobility, as well as household networks, highlights multiple pathways of transmission between RACSs. Where pharmaceutical interventions are unavailable, extensive contact tracing of primary and secondary contacts can help identify these potential chains of transmission and enable the use of interventions which encourage, where practical, single-site work to limit transmission within staff households. Vaccines and antiviral prophylaxis may limit the need for such disruptive interventions. Nevertheless, our study highlights the importance of rapid contact-tracing systems to understand the possible multiplicity of social connections that can spread infectious diseases in vulnerable settings.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2022.243.
Financial support
This research was supported by the Victorian Department of Health.
Conflicts of interest
All authors declare no financial conflicts of interest relevant to this work.







