Ventriculoperitoneal shunt (VPS) insertion is a common neurosurgical procedure and firstline treatment for chronic hydrocephalus. Incidence of VPS infections varies from <1% to 20%, Reference Kulkarni, Drake and Lamberti-Pasculli1–Reference Yang, Zhang, Gu and Song19 with reported mortality rates of 1%–22%. Reference Sarmey, Kshettry and Shriver8,Reference Yang, Zhang, Gu and Song19–Reference Malm, Kristensen and Stegmayr21 Many VPS infections are due to skin flora, particularly Staphylococcus epidermidis and S. aureus. Reference Kulkarni, Drake and Lamberti-Pasculli1,Reference Tulipan and Cleves2,Reference Prusseit, Simon and von der Brelie4,Reference Rehman, Rehman, Bashir and Gupta5,Reference James, Hartley, Morgan and Ternier7,Reference Omrani, O’Connor, Hartley and James10–Reference Kalangu, Esene and Dzowa15,Reference Raygor, Oh and Hwang17,Reference Al-Shudifat, Alsabbagh and Al-Matour18 VPS infection risk has been associated with male sex, low socioeconomic status, young age (≤18 years old), diabetes, previous VPS revision or infection, type of hydrocephalus, myelomeningocele, use of intraoperative single-glove technique, intraoperative duration of VPS insertion, number of surgeons and experience of surgeons performing VPS insertion, and postoperative CSF leak. Reference Kulkarni, Drake and Lamberti-Pasculli1–Reference Pirotte, Lubansu and Bruneau3,Reference James, Hartley, Morgan and Ternier7–Reference Mallucci, Jenkinson and Conroy14,Reference Al-Shudifat, Alsabbagh and Al-Matour18,Reference Yang, Zhang, Gu and Song19
Management of VPS infection entails shunt removal and antibiotic treatment, and such infections may lead to shunt failure, prolonged hospitalization, neurological disability, increased hospital cost, and higher mortality. Reference Kulkarni, Drake and Lamberti-Pasculli1,Reference Prusseit, Simon and von der Brelie4–Reference Kestle, Riva-Cambrin and Wellons6,Reference Sarmey, Kshettry and Shriver8,Reference Erps, Roth and Constantini11,Reference Yakut, Soysal and Kepenekli-Kadayifci12,Reference Kalangu, Esene and Dzowa15,Reference Al-Shudifat, Alsabbagh and Al-Matour18–Reference Shannon, Simon and Reed22 Strategies used to prevent VPS infection include preoperative use of chlorhexidine shampoo, cutaneous antisepsis (eg, use of chlorhexidine or povidone-iodine), rigorous aseptic technique, limiting shunt contact with the patient’s skin during insertion, using instruments to handle the shunt intraoperatively, a no-shave policy, hematoma prevention, and laparoscopic VPS placement. Reference Pirotte, Lubansu and Bruneau3–Reference Sarmey, Kshettry and Shriver8,Reference Omrani, O’Connor, Hartley and James10,Reference Okamura, Maruyama and Fukuda13,Reference Arts, Boogaarts and van Lindert16,Reference Gruber, Riemer and Rozzelle23–Reference Lee, Kwon and Cho25
VPS infection risk can be significantly reduced by double gloving for shunt insertion and manipulation. Reference Kulkarni, Drake and Lamberti-Pasculli1,Reference Tulipan and Cleves2,Reference Kestle, Riva-Cambrin and Wellons6,Reference Sarmey, Kshettry and Shriver8,Reference Gruber, Riemer and Rozzelle23,Reference Kanev and Sheehan24 Moreover, removing or changing the outer glove immediately prior to handling the VPS may further reduce infection risk, but the significance and magnitude of this effect has thus far been variable. Reference Pirotte, Lubansu and Bruneau3,Reference Rehman, Rehman, Bashir and Gupta5,Reference Bashir and Sørensen9,Reference Okamura, Maruyama and Fukuda13 In this literature review, we have summarized available evidence for surgical personnel to remove or change their outer gloves prior to handling shunt instrumentation.
Methods
We performed a systematic search of the PubMed database for studies of institutions documenting VPS infection rates before and after standardizing double gloving with removal or change of the outer glove immediately before shunt insertion using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Reference Moher, Liberati, Tetzlaff and Altman26 PubMed was used to search for articles in any language published January 1, 1970, through August 31, 2021 using the following search terms: surgical infection glove change (261 articles), shunt glove change (9 articles), and shunt infection glove (17 articles). Bibliographies of articles investigating outer-glove removal or change during CSF shunt placement were also searched, producing an additional 6 articles not previously identified, leading to a total of 272 identified articles after 21 duplicates were removed. The methods sections of all full-text articles were assessed for eligibility. Studies were included if a control group was used to compare the CSF shunt infection rates following placement with or without intraoperative removal or change of the outer pair of double gloves immediately prior to handling the shunt before insertion. Studies were excluded if there was no comparison of outer-glove removal or change to no glove removal or change, or if only qualitative comparative results were provided. The primary outcome measure for all included articles was a VPS infection. Of the 245 full-text articles identified among the 272 identified records, 18 addressed glove protocol in VPS placement. Of these 18 records, 14 were ineligible for not specifically documenting intraoperative outer glove removal or change immediately before VPS manipulation as a process measure. Ultimately, 4 studies met the inclusion criteria Reference Pirotte, Lubansu and Bruneau3,Reference Rehman, Rehman, Bashir and Gupta5,Reference Bashir and Sørensen9,Reference Okamura, Maruyama and Fukuda13 (Fig. 1). Meta-analysis was not performed given the low number of studies and heterogeneity between the studies.
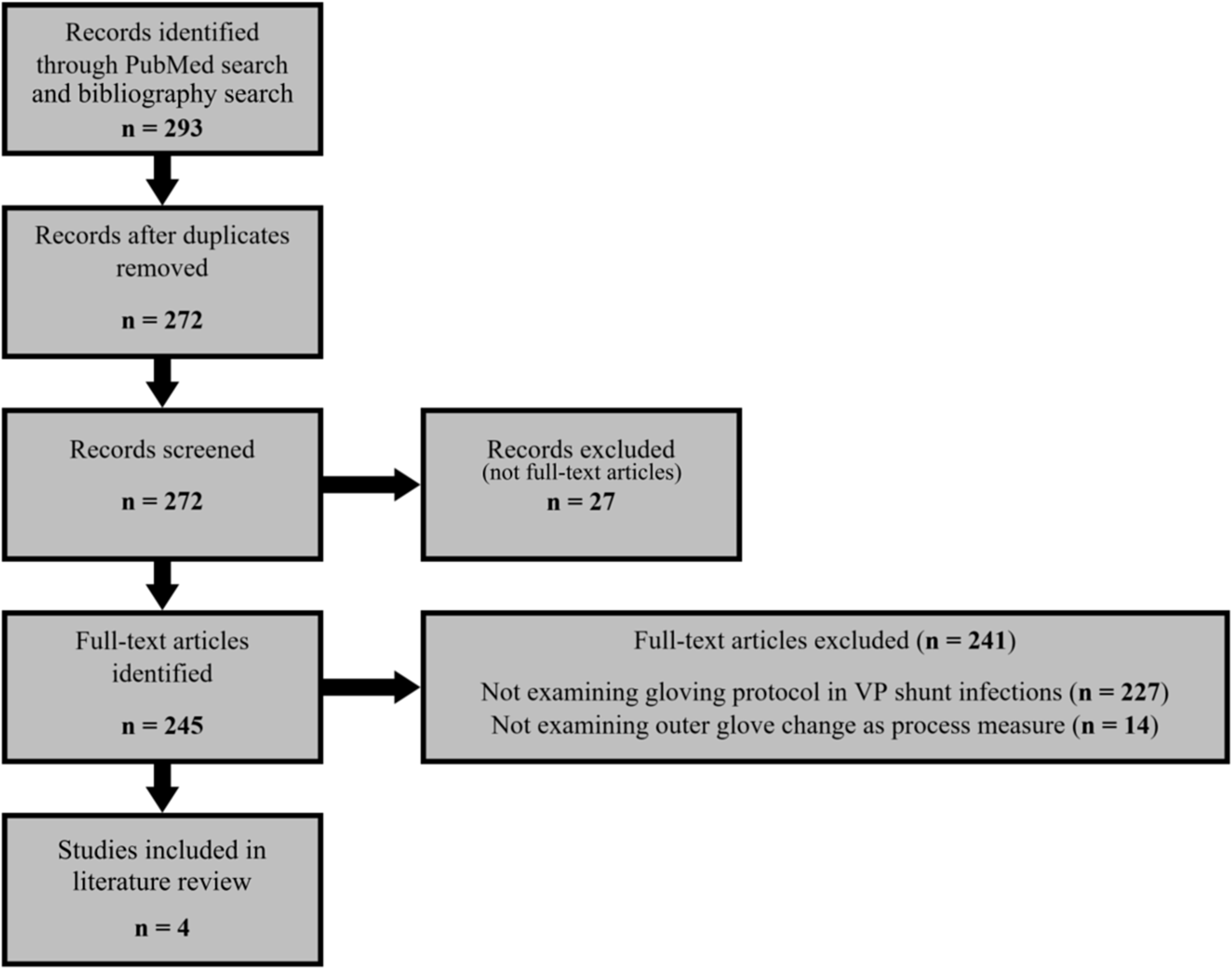
Fig. 1 PRISMA flow diagram. VP, ventriculoperitoneal.
Results
We identified 4 quasi-experimental, single-center studies involving 934 patients of all ages. Reference Pirotte, Lubansu and Bruneau3,Reference Rehman, Rehman, Bashir and Gupta5,Reference Bashir and Sørensen9,Reference Okamura, Maruyama and Fukuda13
One study prospectively assessed VPS infection rates in a pediatric population (aged <18 years) undergoing shunt insertion surgery after implementation of an intraoperative infection control protocol that included removal of the outer gloves prior to VPS insertion, completing the procedure with a single glove layer. Reference Pirotte, Lubansu and Bruneau3 The second study retrospectively assessed VPS infection rates in 2 consecutive cohorts of a neonatal population (aged <1 month) undergoing shunt insertion surgery. Reference Rehman, Rehman, Bashir and Gupta5 The authors used an intraoperative infection prevention bundle in for the second cohort with the addition of an adhesive sheet to the skin after draping and double gloving with the removal of the outer-glove layer before handling the shunt. The procedure was completed with a single glove layer. The third study retrospectively assessed VPS infection rates in 2 consecutive cohorts of an adult population (aged >18 years) undergoing shunt insertion surgery before and after implementation of an outer-glove change protocol. Reference Bashir and Sørensen9 These investigators used an intraoperative infection prevention bundle for both cohorts; however, in the second cohort, the investigators added a change of the second glove layer before handling the shunt materials. The final study retrospectively assessed VPS infection rates in 2 consecutive cohorts adult patients undergoing shunt insertion surgery before and after implementation of an intraoperative infection prevention bundle that included replacement of the outer glove layer prior to handling shunt materials. Reference Okamura, Maruyama and Fukuda13
Three studies included outer-glove change or removal as part of an infection control bundle; 1 study investigated outer-glove change without other concomitant infection control interventions. Across the 4 studies, the aggregate incidence of VPS infection was 11.8% before the change in intraoperative protocol (55 infections among 465 patients) and 4.9% after the protocol change (23 infections among 469 patients). Of the 4 studies, 2 reported a significant difference in the incidence of VPS infections with and without intraoperative outer-glove removal prior to VPS insertion (Table 1), regardless of duration of surgery, combined surgery (eg, with cranioplasty), or immunosuppressed status (Table 1). Reference Rehman, Rehman, Bashir and Gupta5,Reference Bashir and Sørensen9,Reference Okamura, Maruyama and Fukuda13 Outer-glove change was also associated with shorter hospital length of stay. Reference Rehman, Rehman, Bashir and Gupta5 A significant decrease in the likelihood of VPS infection was also observed with outer-glove change at 6-month follow-up in a subgroup of patients who underwent first-time VPS insertion after adjusting for the cause of hydrocephalus Reference Bashir and Sørensen9 (Table 1).
Table 1. Incidence of Ventriculoperitoneal Shunt (VPS) Infections Before and After Standardized Intraoperative Outer-Glove Change or Removal

a Glove change/removal was part of an infection control bundle.
b OR, 0.04; 95% CI, 0.01–0.33; P = .002.
c OR, 0.24; 95% CI, 0.06–0.94; P = 0.046.
d OR, 0.90; 95% CI, 0.4.0–2.06; P = 0.807; at 6 month follow-up OR 0.10: 95% CI, 0.01–1.01; P = .050.
e OR, 0.12; 95% CI, 0.01–2.03; P = 0.140.
Discussion
A significant reduction in risk of VPS infection was achieved with a change from single gloves to double gloves during VPS insertion. Reference Kulkarni, Drake and Lamberti-Pasculli1,Reference Tulipan and Cleves2,Reference Kanev and Sheehan24 In our review of the literature, intraoperative outer-glove change or removal prior VPS insertion, particularly as part of an infection control bundle, appears to further reduce risk of VPS infections. Other strategies for preventing VPS infection have been proposed, Reference Tulipan and Cleves2–Reference James, Hartley, Morgan and Ternier7,Reference Bashir and Sørensen9,Reference Omrani, O’Connor, Hartley and James10,Reference Okamura, Maruyama and Fukuda13–Reference Kalangu, Esene and Dzowa15,Reference Raygor, Oh and Hwang17,Reference Al-Shudifat, Alsabbagh and Al-Matour18 including use of intraventricular and topical vancomycin, Reference Raygor, Oh and Hwang17 antibiotic-containing sutures, Reference Sarmey, Kshettry and Shriver8,Reference Gruber, Riemer and Rozzelle23 and antimicrobial-impregnated shunts. Reference James, Hartley, Morgan and Ternier7,Reference Mallucci, Jenkinson and Conroy14 Some of these strategies have demonstrated feasibility of systematic implementation with consistent and significant reductions in VPS infections. Reference Sarmey, Kshettry and Shriver8,Reference Arts, Boogaarts and van Lindert16,Reference Liu, Dumville and Norman27 However, given the varying quality of evidence underlying these strategies, as well as provider preference, implementation is not standardized across US hospitals. Reference Gruber, Riemer and Rozzelle23,Reference Kanev and Sheehan24,Reference Kraemer, Sandoval-Garcia, Bragg and Iskandar28 Use of antimicrobial-impregnated catheters has been shown to reduce VPS infections, Reference Prusseit, Simon and von der Brelie4,Reference James, Hartley, Morgan and Ternier7,Reference Sarmey, Kshettry and Shriver8,Reference Erps, Roth and Constantini11,Reference Mallucci, Jenkinson and Conroy14,Reference Arts, Boogaarts and van Lindert16 but cost of implementation has been cited as a barrier to implementation, particularly in resource-limited environments. Reference Erps, Roth and Constantini11,Reference Arts, Boogaarts and van Lindert16,Reference Kraemer, Sandoval-Garcia, Bragg and Iskandar28
Glove change before handling vascular grafts and cardiac implantable electronic devices has been demonstrated to reduce glove contamination. Reference Ward, Cooper and Lippert29,Reference Kozon, Riahi and Lundbye-Christensen30 Systematic intraoperative glove changes during orthopedic surgery reduces the frequency of occult perforations and bacterial loading of glove surfaces. Reference Sørensen, Ejlertsen, Aaen and Poulsen31–Reference Al-Maiyah, Bajwa and Finn34 Substantial glove contamination has also been demonstrated intraoperatively before handling a VPS for insertion. Reference Kulkarni, Drake and Lamberti-Pasculli1,Reference Makama, Okeme, Makama and Ameh35,Reference Dawson-Bowling, Smith and Butt36 Some authors have suggested frequent glove changes throughout surgical procedures, at least every 20–90 minutes and at critical points (eg, after draping, before handling of instrumentation, and prior to wound closure). Reference Harnoss, Partecke, Heidecke, Hübner, Kramer and Assadian37,Reference Beldame, Lagrave and Lievain38 In 1 study, standardizing glove change 1 hour after initiating surgery resulted in a 10% absolute reduction in glove contamination. Reference Makama, Okeme, Makama and Ameh35 During VPS insertion, one group of investigators found that contamination of sterile gloves occurred within 15 minutes of donning and recommended that ‘a simple measure would be to change the outer pairs of gloves before handling of the shunt material during surgery.’ Reference Dawson-Bowling, Smith and Butt36 Unsurprisingly, outer-glove change prior to handling instrumentation during lumbar fusion led to an 86% reduction in postoperative infections. Reference Rehman, Rehman, Rehman and Freeman39 Nevertheless, more data are needed with glove change alone to prove its utility as a standard of care for all procedures involving implantation of permanent devices.
Our review has a number of limitations. Publication bias is possible due to the small number of studies that met our inclusion criteria. Heterogeneity of the studies was high, and the studies were underpowered. One study assessed only intraoperative glove removal or change, Reference Bashir and Sørensen9 whereas the 3 others assessed intraoperative glove change as part of an infection prevention bundle. Reference Pirotte, Lubansu and Bruneau3,Reference Rehman, Rehman, Bashir and Gupta5,Reference Okamura, Maruyama and Fukuda13 In the lone study that assessed glove change alone, Reference Bashir and Sørensen9 the reduction in VPS infection rate only reached statistical significance at a 6-month follow-up. As such, the attributable effect of outer-glove removal alone is likely contributory to lower infection rates, but further research is needed to unequivocally confirm the impact of this preventative strategy.
In conclusion, reducing risk of a VPS infection may be achieved through a low-cost protocol of standardizing outer-glove removal or change as part of an infection control bundle prior to handling of the shunt during intraoperative insertion. Replacing the outer glove may be preferable in the event that the inner glove was contaminated due to unsuspected outer-glove perforation during a surgical procedure or during outer-glove removal. This intervention does not require purchase of new equipment, is easy to implement, and can be utilized in resource limited and well-resourced settings.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





