Published online by Cambridge University Press: 13 February 2020
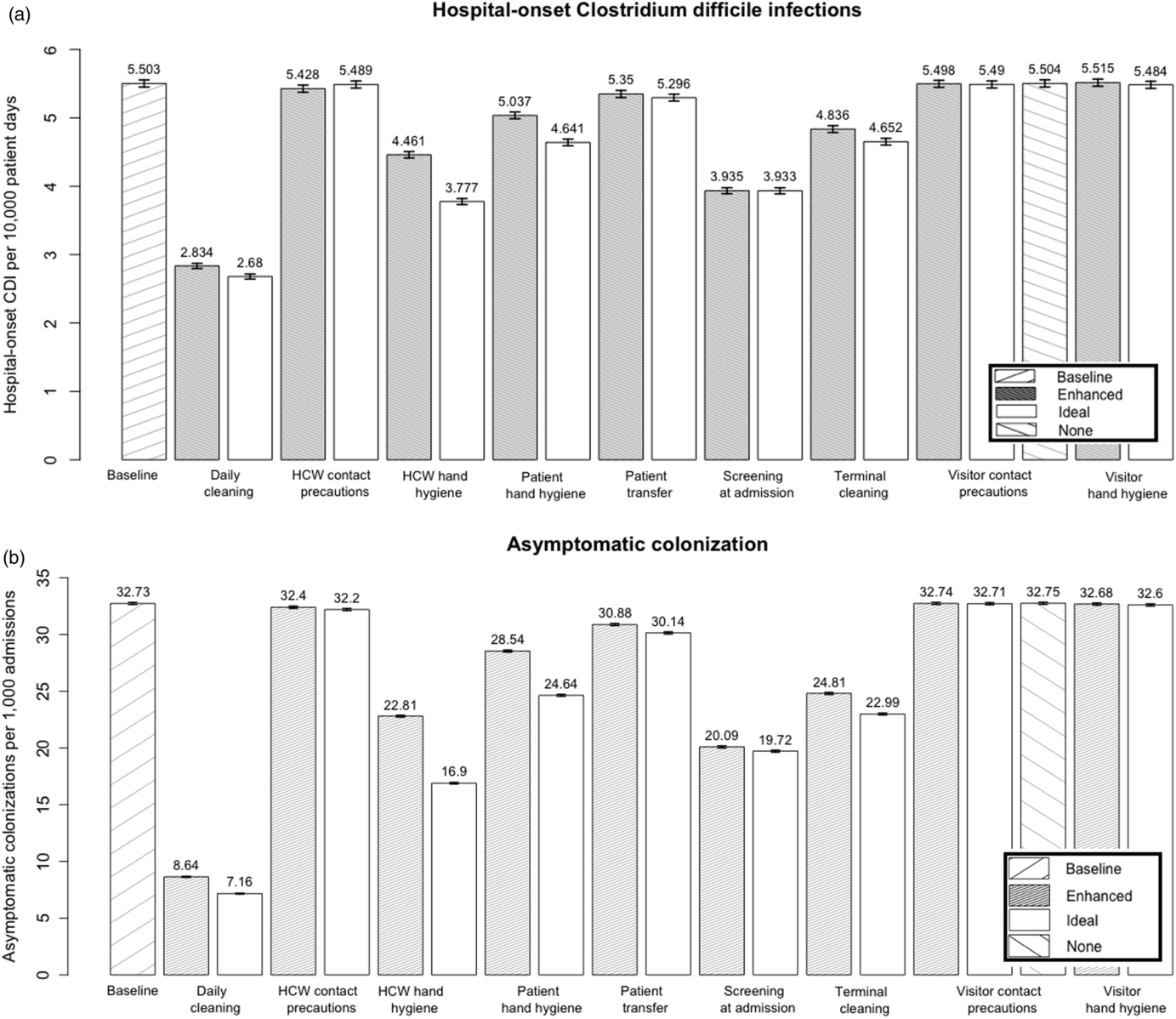
Clostridioides difficile infection (CDI) is rapidly increasing in children’s hospitals nationwide. Thus, we aimed to compare the effectiveness of 9 infection prevention interventions and 6 multiple-intervention bundles at reducing hospital-onset CDI and asymptomatic C. difficile colonization.
Agent-based simulation model of C. difficile transmission.
Computer-simulated, 80-bed freestanding, tertiary-care pediatric hospital, including 8 identical wards with 10 single-bed patient rooms each.
The model includes 5 distinct agent types: patients, visitors, caregivers, nurses, and physicians.
Daily and terminal environmental disinfection, screening at admission, reduced intrahospital patient transfers, healthcare worker (HCW), visitor, and patient hand hygiene, and HCW and visitor contact precautions.
The model predicted that daily environmental disinfection with sporicidal product, combined with screening for asymptomatic C. difficile at admission, was the most effective 2-pronged infection prevention bundle, reducing hospital-onset CDI by 62.0% and asymptomatic colonization by 88.4%. Single-intervention strategies, including daily disinfection, terminal disinfection, asymptomatic screening at admission, HCW hand hygiene, and patient hand hygiene, as well as decreasing intrahospital patient transfers, all also reduced both hospital-onset CDI and asymptomatic colonization in the model. Visitor hand hygiene and visitor and HCW contact precautions were not effective at reducing either measure.
Hospitals can achieve substantial reduction in hospital-onset CDIs by implementing a small number of highly effective interventions.
PREVIOUS PRESENTATION. An abstract of this study was presented as a poster at IDWeek 2018 on October 4, 2018, in San Francisco, California, and was included with the published conference proceedings: Barker, A, Scaria E, Alagoz, O, Sethi, A, Safdar, N. Clostridium difficile reduction: an agent-based simulation modeling approach to evaluating intervention comparative effectiveness at pediatric hospitals. Open Forum Infect Dis 2018;5:S197–S198.