Data are conflicting regarding whether healthcare personnel (HCP) are at increased risk for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection compared to non-HCP peers and whether work in dedicated coronavirus disease 2019 (COVID-19) units increases this risk. Reference Iversen, Bundgaard and Hasselbalch1,Reference Steensels, Oris and Coninx2 Previous studies have suggested that increased intensity or duration of patient contact and performing aerosol generating procedures (AGPs) increase an HCP’s risk of SARS-CoV-2 infection. Reference Baker, Rhee and Fiumara3,Reference Heinzerling, Stuckey and Scheuer4 Understanding the determinants of risk for SARS-COV-2 infection is critical for implementing effective infection prevention (IP) measures and maintaining the safety of HCP.
The COVID-19 Prevention in Emory Healthcare Personnel (COPE) study is a longitudinal serosurveillance cohort at four hospitals monitoring the incidence of SARS-CoV-2 infection in HCP over 12 months. We assessed SARS-CoV-2 seroprevalence among HCP at enrollment and the risk of certain occupational characteristics.
Methods
Recruitment and enrollment
We recruited a convenience sample of HCP by e-mailing employee listservs and posting flyers. Eligible participants were >18 years old, were employed at a study hospital, had worked a shift in the previous 2 weeks, and reported no current COVID-19 symptoms. Informed consent was obtained, and the date of phlebotomy (May 6, 2020–June 12, 2020) was used as the enrollment date. The Emory University Institutional Review Board approved this study.
Study setting and infection prevention practices
Study hospitals included a 751-bed academic, tertiary referral hospital (“referral hospital”), a 529-bed hybrid academic-community tertiary-care hospital (“academic-community hospital”), a 410-bed community hospital (“community hospital”), and a 961-bed urban safety-net hospital (“safety-net hospital”) in Atlanta, Georgia, with ∼17,000 total HCP. In the 2 months prior to enrollment, each hospital sequentially implemented visitor restrictions, universal masking, symptom and temperature screening, and universal testing of admitted patients. No PPE shortages were reported.
Serologic testing
Indirect enzyme-linked immunosorbent assay detected IgG antibodies against the receptor-binding domain of the SARS-CoV-2. Reference Suthar, Zimmerman and Kauffman5 Each signal was normalized to an internal control, and receiver operating characteristic curve analysis was used to determine the cutoff (sensitivity, 86.7%; specificity, 99.3%). Positive samples were confirmed on a second run.
Data on exposures and variable definitions
Participants completed an online survey on demographics, medical history, prior testing for SARS-CoV-2, community and occupational activities, PPE use and adherence to IP recommendations in the previous 2 weeks. We focused on the previous 2 weeks to maximize accuracy of recall, assuming that this would be representative of typical occupational activities. We classified HCP into the following occupations: (1) nurses, (2) physicians or advanced practice providers (APPs), (3) other HCP, (4) respiratory therapists, (5) radiology technicians, or (6) healthcare administrators (“administrators”). Categories 1–5 were considered direct patient care roles. The Supplemental Material (online) contains a complete list of the other occupations and key study variable definitions.
Statistical analysis
Univariable logistic regression identified characteristics associated with SARS-CoV-2 seropositivity. Multivariable logistic regression assessed the association between SARS-CoV-2 seropositivity and occupational exposure variables including proportion of shift spent at bedside, shifts in COVID-19 units, and performing or being present during ≥1 AGP in a COVID-19 unit. The final model also included covariates based on published literature and those identified in the univariable analysis (P ≤ .1). There were no significant interactions among proportion of shift spent at the bedside, race, and shifts worked in COVID-19 units. For the main exposure variables, participants in the administrator group were included in the lowest risk category.
Results
Among 353 HCP, most participants were female (76%) and white (69%). The median age was 37 years (interquartile range [IQR], 30–49). Most (56%) worked at least some shifts in COVID-19 units, and almost one-third (27%) performed or were present during ≥1 AGP in these units. More than half (54%) reported typically spending >50% of their shift directly at the bedside; this was frequent for nurses (80%) and respiratory therapists (71%) but less common for physicians and APPs (35%) and other HCP (9%). Also, 36% consistently socially distanced from coworkers (Table 1). Self-reported PPE use during patient care activities in COVID-19 units was high for gloves (100%) and for either respirators (powered air purifying respirators [PAPR] or N95 respirators) or face masks (99%) and was lower for gowns (91%) and eye protection (88%). In non–COVID-19 units, 99% of HCP reported wearing respirators or face masks during patient care.
Table 1. Description of Demographics and Healthcare Occupational Activities Stratified by Job Title in Healthcare Personnel in Four Hospitals in Atlanta, Georgia
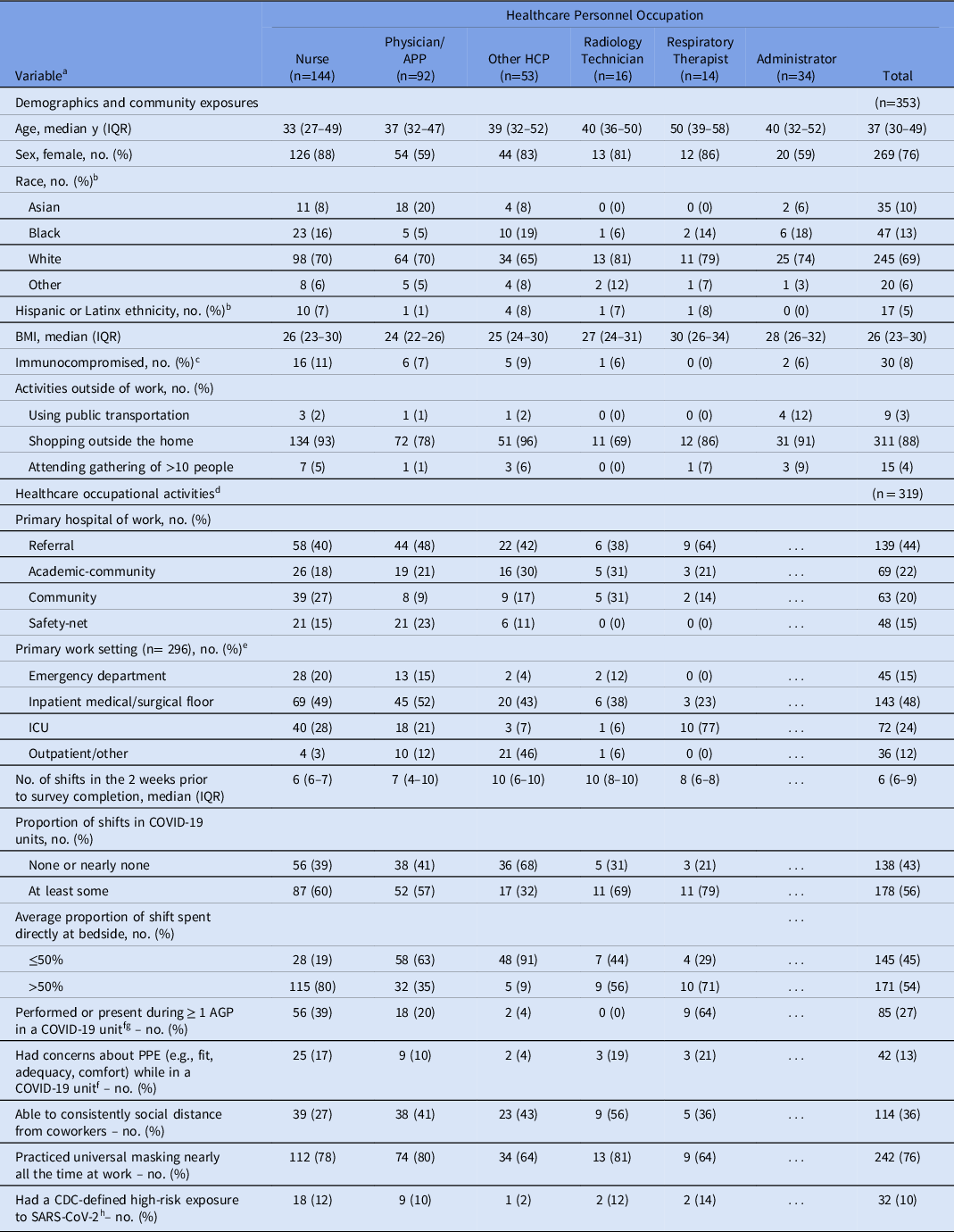
Note. APP, advanced practice provider; HCP, healthcare personnel; IQR, interquartile range; BMI, body mass index; ICU, intensive care unit; AGP, aerosol-generating procedure; PPE, personal protective equipment; CDC, Centers for Disease Control and Prevention.
a All questions about occupational activities refer to the 2 weeks prior to the survey completion date.
b Survey options for race included: American Indian or Alaska Native, Asian, black or African American, Native Hawaiian or other Pacific Islander, white, other race, or prefer not to answer. Due to small numbers, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander and other were collapsed into 1 category. We excluded participants who preferred not to answer. Ethnicity was examined separately from race.
c HCP were considered immunocompromised if they had an autoimmune or rheumatologic disorder, active malignancy, solid-organ or hematologic stem cell transplant, or other self-reported immunosuppressive condition or medication.
d These questions were not asked for the HCP classified as an administrator, so they were excluded from the new denominator (n=319).
e Excludes HCP where primary location was not able to be determined due to multiple locations being written in.
f Only asked for participants who worked at least some shifts in COVID-19 units; percentages calculated with denominators equal to only participants who were asked the question.
g The following procedures were specifically asked about as AGPs: airway suctioning, noninvasive positive pressure ventilation, manual (bag) ventilation, nebulizer treatments, intubation, cardiopulmonary resuscitation, chest physiotherapy, mini-bronchoalveolar lavage, breaking ventilation circuit, sputum induction, bronchoscopy, high-flow oxygen delivery.
h A high-risk occupational exposure to SARS-CoV-2 was defined according to the CDC guidelines as having prolonged close contact with a patient(s) on a non-COVID-19 unit that later was found to have SARS-CoV-2 while (1) the HCP was not wearing a respirator or face mask, (2) the HCP was not wearing eye protection while the patient was not wearing a facemask or intubated, or (3) the HCP was not wearing all recommended PPE (gown, gloves, eye protection and respirator) while performing an AGP. 10
Overall, 23 participants (6.5%) had SARS-CoV-2–specific IgG. A higher proportion of participants with direct patient care roles had SARS-CoV-2 antibodies, compared with administrators (6.9% vs 2.9%); however, this proportion was not statistically significant (OR, 2.5; 95% CI, 0.5–45.1). In a multivariable analysis, black race (OR, 8.4; 95% CI, 2.7–27.4) and working >50% of a typical shift directly at the bedside (OR, 3.4; 95% CI, 1.2–10.5) were independently associated with SARS-CoV-2 seropositivity (Table 2). Job category, work location, universal masking or social distancing at work, performing or being present during ≥1 AGP in COVID-19 units, and community incidence of COVID-19 in the participant’s residential ZIP code were not associated with SARS-CoV-2 seropositivity (Table 2).
Table 2. Factors Associated With SARS-CoV-2 Seropositivity in Healthcare Personnel
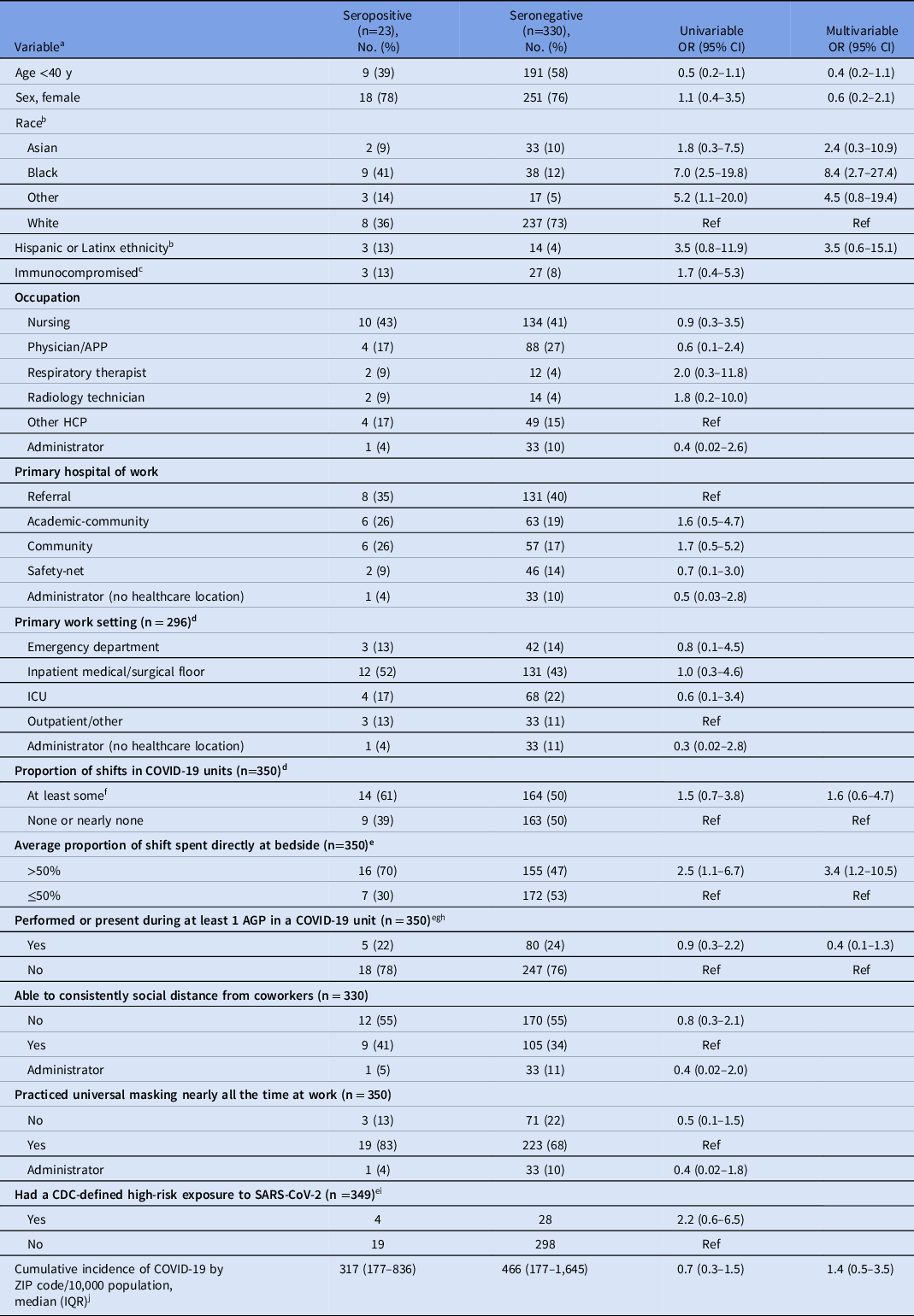
Note. OR, odds ratio; CI, confidence interval; APP, advanced practice provider; HCP, healthcare personnel; IQR, interquartile range; BMI, body mass index; ICU, intensive care unit; AGP, aerosol generating procedure; PPE, personal protective equipment; CDC, Centers for Disease Control and Prevention.
a All questions about occupational activities refer to the 2 weeks prior to the survey completion date.
b Survey options for race included: American Indian or Alaska Native, Asian, black or African American, Native Hawaiian or other Pacific Islander, white, other race, or Prefer not to answer. Due to small numbers, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander and other were combined. We excluded participants who preferred not to answer. Ethnicity was examined separately from race.
c HCP were considered immunocompromised if they had an autoimmune or rheumatologic disorder, active malignancy, solid-organ or hematologic stem cell transplant, or other self-reported immunosuppressive condition or medication.
d Excludes HCP where primary location was not able to be determined due to multiple locations being written in.
e Administrator group is included in the reference group.
f At least some was defined as participants reporting that they spent some, approximately half, more than half, or nearly all or all of their shifts in the previous 2 weeks in COVID-19 units.
g Information on AGP participation was only asked of participants who worked at least some shifts in COVID-19 units.
h The following procedures were specifically included as AGPs: airway suctioning, non-invasive positive pressure ventilation, manual (bag) ventilation, nebulizer treatments, intubation, cardiopulmonary resuscitation, chest physiotherapy, mini-bronchoalveolar lavage, breaking ventilation circuit, sputum induction, bronchoscopy, high-flow oxygen delivery.
i A high-risk occupational exposure to SARS-CoV-2 was defined based on the CDC guidance as having prolonged close contact with a patient(s) with SARS-CoV-2 infection while (1) the HCP was not wearing a respirator or facemask; (2) the HCP was not wearing eye protection while the patient was not wearing a facemask or intubated; or (3) the HCP was not wearing all recommended PPE (gown, gloves, eye protection and respirator) while performing an AGP. 10
j The cumulative incidence of COVID-19 per residential ZIP code was calculated using data from the Georgia Department of Public Health (GDPH) and includes all reported cases of COVID-19 (confirmed and probable) up to 2 weeks prior to each participant’s blood draw; log base 10 of cumulative incidence was used per 10,000 population in the analysis.
Discussion
In this cohort of predominantly inpatient HCP, 6.5% were positive for SARS-CoV-2 antibodies early in the COVID-19 pandemic in Atlanta, Georgia. This proportion was higher than the concurrently estimated community seroprevalence in Atlanta (2.5%; 95% CI, 1.4–4.5), which suggests that HCP might be at greater risk than the general public. Reference Biggs, Harris and Breakwell6 However, in our study, HCP with direct patient care roles were no more likely to be seropositive than administrators. Similarly, we did not find an association between work in COVID-19 units and SARS-CoV-2 seropositivity, emphasizing the importance of adhering to IP practices in all areas of the hospital. A higher proportion of time spent at bedside was the only occupational factor identified as possibly increasing the risk of SARS-CoV-2 infection. Although universal masking was standardized, universal eye protection during patient care had not been instituted before study enrollment and may explain some of these findings. Spending more time at the bedside may be a surrogate for the intensity of patient contact and for how frequently HCP interact with other HCP, increasing risk of SARS-CoV-2 transmission.
Though not a prespecified end point, HCP who identified as black had 8 times the odds of having SARS-CoV-2 antibodies than HCP who identified as white, even after accounting for occupational factors. The COVID-19 pandemic has disproportionately affected Black or African American, Native American, and Latinx persons and has exposed issues of socioeconomic inequality, structural racism, and access to healthcare in our society. HCP are not spared from this reality. Reference Tai, Shah, Doubeni, Sia and Wieland7 We were unable to evaluate the effect of other community factors such as living environment, socioeconomic status, or known community-based SARS-CoV-2 exposures.
In this study, we prospectively evaluated detailed occupational exposures at 4 diverse hospitals, but several limitations must be considered. First, we surveyed a small convenience sample of HCP, limiting generalizability. Second, 6.5% may represent a minimum seroprevalence estimate due to uncertainty of antibody persistance. Reference Long, Tang and Shi8,Reference Iyer, Jones and Nodoushani9 Third, survey answers are subject to recall bias, and reported activities in the 2 weeks before survey completion may not reflect fluctuating job responsibilities during the pandemic. Thus, all associations between occupational activities and SARS-CoV-2 infection should be interpreted cautiously. Lastly, ZIP code level data on COVID-19 incidence is likely insufficient to account for all community factors involved in SARS-CoV-2 infection risk.
In summary, 6.5% of HCP enrolled in our cohort from May through June 2020 had serologic evidence of prior SARS-CoV-2 infection. HCP spending a greater amount of time performing direct bedside care may be at increased risk for SARS-CoV-2 infection, and this will be critically assessed in longitudinal measurements over the next 12 months.
Acknowledgments
We acknowledge all healthcare personnel working during the COVID-19 pandemic, and we are grateful to those that volunteered for this study. We thank Monica Godfrey and Ellie Butler from the Hope Clinic of the Emory Vaccine Center for their assistance with study operations and Susan Ray for her invaluable help with study recruitment. We thank Jens Wrammert for support in establishing our serologic testing. We thank the Emory General Clinical Research Center (GCRC) and members of the Emory All of Us team who supported recruitment and study visits for specimen collection. We thank Laura Edison and Melissa Tobin-D’Angelo from the Georgia Department of Public Health for enabling our team to analyze ZIP code level data on COVID-19 incidence. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
Financial support
This study was supported by the Centers for Disease Control and Prevention through a cooperative agreement with the Georgia Emerging Infection Program (grant no. U50CK000485). J.H.A. is currently supported by the Antibacterial Resistance Leadership Group fellowship (National Institute of Allergy and Infectious Diseases grant no. UM1AI104681).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.54





