INTRODUCTION
The ongoing international outbreak of invasive Mycobacterium chimaera associated with HCD use during bypass surgery exemplifies the challenges faced by those dealing with complex medical devices and systems. While the number of confirmed cases is currently small relative to the large number of patients receiving open heart surgery, the extent of the outbreak is not yet known and this incidence may increase. Herein, we summarize our current understanding of the outbreak, including epidemiology, transmission pathway, clinical presentation, and potential prevention approaches.
THE OUTBREAK
Detection of a Healthcare-Associated Infectious Risk and a Novel Transmission Pathway
When 2 patients with disseminated M. chimaera infection following prosthetic valve surgery were detected at the University Hospital of Zurich in 2011, the implications of these findings were not immediately clear.Reference Achermann, Rossle and Hoffmann 1 Mycobacterium chimaera is a slow-growing, nontuberculous mycobacterium (NTM) included in the M. avium complex (MAC). These opportunistic human pathogens are known to cause lung infections in those with underlying lung disease and disseminated infection in severely immunocompromised patients only.Reference Tortoli, Rindi and Garcia 2 Randomly amplified polymorphic DNA–polymerase chain reaction (RAPD-PCR) was performed to determine the clonal relationship of the 2 M. chimaera isolates. In contrast to the diversity seen among pulmonary M. chimaera isolates, these 2 isolates had identical RAPD-PCR patterns.Reference Achermann, Rossle and Hoffmann 1 The hospital water system was investigated as a possible source, and M. chimaera was identified in the water circuitry of HCDs.Reference Sax, Bloemberg and Hasse 3
HCDs are stand-alone devices responsible for heat exchange in cardiopulmonary bypass machines (Figure 1). Direct infections via blood from cardiopulmonary bypass machines were described in the 1970s;Reference Geldof and Brom 4 however, this seemed an unlikely mechanism because water-to-blood leaks are rarely observed (0.003% of all procedures) in modern devices.Reference Mejak, Stammers, Rauch, Vang and Viessman 5 No M. chimaera was detected in an artificial patient circuit after 72 h of continuous cardiopulmonary bypass machine circulation while using an M. chimaera–contaminated HCD.Reference Götting, Klassen and Jonas 6 Additionally, a convenience sample of perioperative blood cultures of 32 patients collected during the outbreak period in Zurich remained negative for NTM.Reference Sax, Bloemberg and Hasse 3 Recognizing that NTMs can form aerosols in various environmental settings,Reference Falkinham 7 Sax et alReference Sax, Bloemberg and Hasse 3 found M. chimaera in the exhaust air of contaminated HCDs using an active air sampling method. RAPD-PCR patterns of airborne M. chimaera matched isolates from contaminated HCD water, and further experiments consistently showed that airborne M. chimaera could only be detected if both the HCD water was contaminated and the HCD was turned on.Reference Sax, Bloemberg and Hasse 3 This phenomenon was later confirmed in another center.Reference Götting, Klassen and Jonas 6 Indirect evidence for the link between contaminated air in the operating room (OR) and the development of clinical infection was elucidated by an investigation of the role of airflow management in the OR.Reference Sommerstein, Ruegg, Kohler, Bloemberg, Kuster and Sax 8 Laser particle counter and smoke experiments helped demonstrate the importance of the OR setup and HCD orientation in the room. The ultraclean air ventilation system failed to protect critical areas from contaminated air, especially if the HCD exhaust was directed towards the operating field.Reference Sommerstein, Ruegg, Kohler, Bloemberg, Kuster and Sax 8 Transmission to patients is likely a rare and stochastic event, potentially due to a single colony-forming unit settling on prosthetic material, followed by in-situ replication and subsequent dissemination.Reference Sommerstein, Ruegg, Kohler, Bloemberg, Kuster and Sax 8 These findings provided sufficient evidence to delineate the transmission pathway from a M. chimaera–contaminated HCD via air to the operative field. However, when and how M. chimaera first contaminates the water tank of the HCD device remains unknown. The exact location where aerosol forms inside the HCD is also unknown, but preliminary studies have attributed aerosolization to the second fan in the upper part of the HCD.Reference Götting, Klassen and Jonas 6
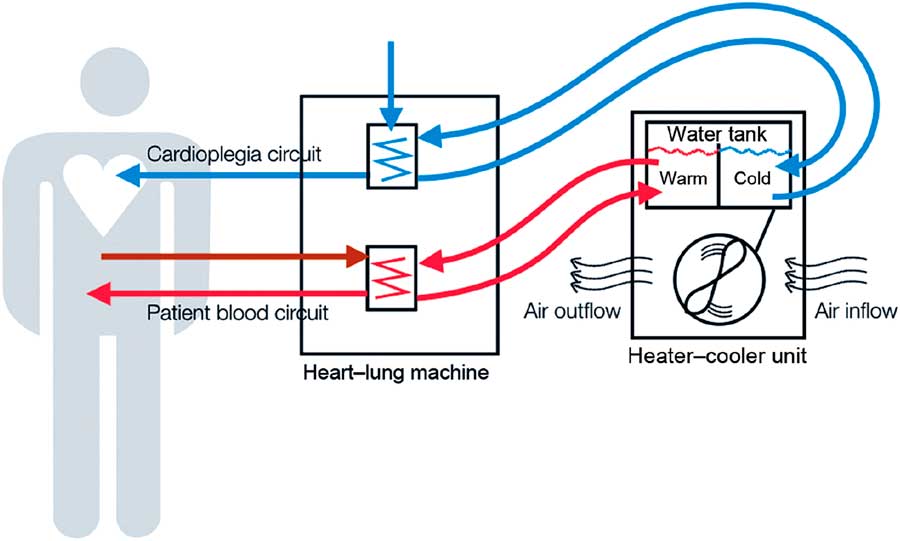
FIGURE 1 Heater-cooler devices, a functional part of extracorporeal circulation. As stand-alone devices connected to the cardiopulmonary bypass machine, heater-cooler devices (HCD) provide warming and cooling of blood and cardioplegia solution during open-chest heart surgery on extracorporeal circulation. They feature a water reservoir from where pumps supply the tubing of 3 circuits, a patient circuit to cool and warm the patient’s blood, a cardioplegia circuit to cool the cardioplegia solution, and a blanket circuit for additional external cooling and warming of the patient. In the former 2 circuits, the temperature is transferred to the patient’s blood and sterile cardioplegia solution, respectively, across a membrane that physically separates HCD water from the sterile circuits on the patient side. Water is an ideal heat transfer fluid, especially for cooling, but contamination remains an issue. The cooling of HCD water requires a radiator with a fan to dissipate superfluous heat. This cooling fan sustains a substantial airflow. HCD water systems are typically not airtight and have a complex inner tubing system. This schematic was reused with the permission of Emerging Infectious Diseases. Reference Sommerstein, Ruegg, Kohler, Bloemberg, Kuster and Sax 8
The Source of the Outbreak
Two institutions that reported contaminated HCDs and infected patients could not find M. chimaera in their water supply despite extensive sampling.Reference Sax, Bloemberg and Hasse 3 , Reference Götting, Klassen and Jonas 6 They both employed the LivaNova 3T HCD (London, United Kingdom; formerly Sorin Group, Milan, Italy). Investigators in the Netherlands also discovered M. chimaera–contaminated HCDs in all 9 Dutch institutions that relied on LivaNova devices.Reference Jakko 9 NTM contamination of other HCD brands was encountered in 7 institutions, but these NTMs were not M. chimaera. Reference Jakko 9 Results of whole-genome sequencing (WGS) showed that isolates from 4 Dutch patients and samples from the local LivaNova HCD were closely related (ie, clonal).Reference Jakko 9 Recently published WGS data from 3 US centers also showed that M. chimaera isolates from patients and from HCD water and air were closely related; these investigators concluded that their results “strongly suggest a point source contamination of LivaNova 3T HCDs with M. chimaera.”Reference Perkins, Lawsin and Hasan 10 Additionally, an outbreak investigation that included testing at the LivaNova manufacturing site in Germany confirmed the presence of M. chimaera in samples taken in the pump assembly area and in brand new HCD devices.Reference Haller, Holler and Jacobshagen 11 A recent FDA medical device report showed widespread contamination of different HCD brands with NTMs, but only LivaNova HCDs were associated with M. chimaera–infected patients. 12 These findings suggest that a hospital-independent, common source is responsible for most of the M. chimaera outbreak. LivaNova HCDs have a market share of 70% and thus are the predominant medical devices used for temperature regulation during cardiopulmonary bypass. 13 Nonetheless, NTMs are ubiquitous in water environments, and widespread colonization of water distribution systems in healthcare settings has been reported.Reference Galassi, Donato, Tortoli, Burrini, Santianni and Dei 14 Direct NTM contamination of HCDs through hospital water is also possible in addition to the likely point-source nature of the current global outbreak of M. chimaera.Reference Wallace, Iakhiaeva and Williams 15 Furthermore, the infectious risk from other HCD brands must not be ignored.
Clinical Presentation and Epidemiology
The number of invasive M. chimaera infections after open-chest heart surgery being reported by public health authorities in Switzerland, the European Union, and the United States is increasing. 16 – 18 Currently, the worldwide case count is at least 70 (as of October 2016), based on published reports and personal communications.Reference Perkins, Lawsin and Hasan 10 , Reference Haller, Holler and Jacobshagen 11 , Reference Tan, Sampath and Abu Saleh 19 , Reference Kohler, Kuster and Bloemberg 20 Mycobacterium chimaera infections after open-chest heart surgery usually present as prosthetic valve endocarditis, disseminated infections, or infections of vascular grafts.Reference Haller, Holler and Jacobshagen 11 , Reference Kohler, Kuster and Bloemberg 20 In a recent case series of 10 patients, common symptoms were fever, shortness of breath, and weight loss. Physical examination was nonspecific.Reference Tan, Sampath and Abu Saleh 19 , Reference Kohler, Kuster and Bloemberg 20 Ocular involvement has recently been described in 2 cases as a potential clinical marker.Reference Tan, Sampath and Abu Saleh 19 Frequent laboratory abnormalities were anemia, lymphopenia, and thrombocytopenia as well as elevated levels of C-reactive protein, lactate dehydrogenase, aminotransferases, and creatinine. The diagnoses were delayed between 3 months and 5 years (median 21 months) following cardiac surgery. 16 , 17 , 18 , Reference Kohler, Kuster and Bloemberg 20 Despite combination antibiotic therapy with at least 3 active agents, 50% of the patients with M. chimaera infection died due to complications of the infection. Mycobacterium chimaera still grew in tissue samples from some patients despite their prolonged prior antibiotic therapy.Reference Kohler, Kuster and Bloemberg 20 Due to the delayed, subacute presentation and granulomatous inflammation shown by histopathology results, Mycobacterium chimaera should be considered in any patient presenting with a diagnosis of sarcoidosis or culture-negative endocarditis after exposure to an HCD. Importantly, prosthetic material is not always a prerequisite for infection; a recently reported case occurred after coronary-artery bypass surgery with sternal wires as the only foreign material.Reference Haller, Holler and Jacobshagen 11 Further manifestations of M. chimaera infections can be surgical wound or organ-space surgical site infections, such as mediastinitis.Reference Kohler, Kuster and Bloemberg 20 In addition to recommendations for M. chimaera case detection proposed by Kohler et al,Reference Kohler, Kuster and Bloemberg 20 we consider patients with cardiac-assist devices or a history of heart transplantation to be at increased risk for infection (based on cases communicated by D.D. and M.E.). The Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) has also recently released guidelines for the identification of possible cases of HCD-associated NTM infections. 18
Preventive Measures
As insight into the M. chimaera outbreak grows, several preventive measures have been taken at the institutions involved. Here, we summarize current approaches regarding surveillance cultures, applying decontamination procedures, and optimizing airflow management.
Detection of M. chimaera in water and air
Environmental surveillance cultures have been used to determine the need for immediate preventive actions and to assess their effect, 21 but their utility is unclear outside of an outbreak investigation. Collected water and air volumes and air sampling techniques vary among published reports,Reference Sax, Bloemberg and Hasse 3 , Reference Götting, Klassen and Jonas 6 , Reference Garvey, Ashford and Bradley 22 and the lower limits of detection remain unknown. Culture results take up to 8 weeks to return, and repeated cultures of the same HCD often yield conflicting results. Furthermore, not every microbiology laboratory is capable of identifying M. chimaera, which may result in reporting a MAC without further speciation. Hospital water should be assumed to be NTM-contaminated (with only filtered or sterile water used to fill HCDs). Thus, sampling hospital water is unlikely to be helpful and may have poor negative predictive value.Reference Sax, Bloemberg and Hasse 3 , Reference Götting, Klassen and Jonas 6
Modified heater-cooler device maintenance and decontamination
Several factors make it very difficult to eradicate M. chimaera from HCD water systems: M. chimaera, like other MACs, forms biofilms that render it less susceptible to antibiotics or disinfection procedures.Reference Garvey, Ashford and Bradley 22 , Reference Vaerewijck, Huys, Palomino, Swings and Portaels 23 Also, the lipid-rich hydrophobic outer membrane of MAC confers resistance to common disinfectants such as chlorine, and causes MACs to concentrate on water surfaces, resulting in extremely high concentrations once aerosols are generated.Reference Taylor, Falkinham, Norton and LeChevallier 24 – Reference Parker, Ford, Gruft and Falkinham 26 In addition to M. chimaera, a mixture of microbial flora consisting of Pseudomonas aeruginosa, other gram-negative bacteria, and molds, may coexist.Reference Götting, Klassen and Jonas 6 , Reference Garvey, Ashford and Bradley 22 Routine decontamination procedures seem to fail.Reference Garvey, Ashford and Bradley 22 Despite an intensified maintenance protocol consisting of daily water changes with the addition of hydrogen peroxide and biweekly disinfection cycles, emergence of M. chimaera was identified in brand new LivaNova 3T HCDs delivered from the factory between January and September 2014 after 7–12 months of initially negative water cultures.Reference Schreiber, Kuster and Hasse 27 The successful decontamination of a LivaNova 3T HCD colonized with M. chimaera utilizing a combination of mechanical biofilm removal and replacement of several HCD parts followed by 2 decontamination cycles with peracetic acid was recently reported.Reference Garvey, Ashford and Bradley 22 This procedure was followed by a maintenance protocol of daily water changes using filtered water with addition of 100 mL 3% hydrogen peroxide and weekly disinfection cycles with filtered tap water with 450 mL of 3%–5% peracetic acid, which resulted in negative follow-up water cultures over 3 months.Reference Garvey, Ashford and Bradley 22 However, detectable regrowth of mycobacteria may occur with substantial delay, as shown in a longitudinal assessment of factory-new LivaNova HCDs.Reference Schreiber, Kuster and Hasse 27 HCD accessory devices, such as tubes and connectors, may lead to cross contamination or recontamination of other newly purchased devices with M. chimaera. Reference Sax, Bloemberg and Hasse 3
Airflow management
Considering the posited airborne transmission route and the fact that HCDs may also contain microorganisms other than M. chimaera, exposure of patients to the potentially contaminated airflow produced by HCDs must be avoided.Reference Sommerstein, Ruegg, Kohler, Bloemberg, Kuster and Sax 8 An ultraclean air ventilation itself may not prevent airborne transmission.Reference Sommerstein, Ruegg, Kohler, Bloemberg, Kuster and Sax 8 Several strategies have been described to achieve a strict separation of the HCD from operating room air. The construction of a custom-built housing for 3T HCD with active suction of the exhaust air out of the OR is one solution.Reference Sax, Bloemberg and Hasse 3 , Reference Schreiber, Kuster and Hasse 27 Placing the HCD outside of the operating room in a space with separate air ventilation represents another safe solution.Reference Götting, Klassen and Jonas 6 , Reference Sommerstein, Jenni, Carrel and Marschall 28 Implementation of this measure an extension of tube length and a longer time period to achieve the desired temperature in the HCD circuit.Reference Götting, Klassen and Jonas 6 Notably, an operating room door that cannot be completely closed may result in a failure of this set-up.Reference Götting, Klassen and Jonas 6
DISCUSSION
Practical Interim Suggestions and Challenges
Based on the published data and on the CDC guidelines for the identification of possible cases of HCD-associated NTM infections, 18 we offer several practical suggestions for clinicians, hospitals, public health agencies, and device manufacturers (Table 1). Until further knowledge of alternative preventive measures and safe HCD technology becomes available, strict separation of HCD exhaust air from operating rooms and other critical healthcare areas is the only means of guaranteeing patient safety. Hospitals that cannot immediately remove the HCD from the OR should position the device so that the airflow is directed away from the patient as an interim solution; however, these hospitals should be aware of the potentially increased infectious risk. For the long term, separation of the exhaust air of any potentially aerosol-generating device from critical areas in the OR should be achieved.
TABLE 1 Practical Interim Suggestions
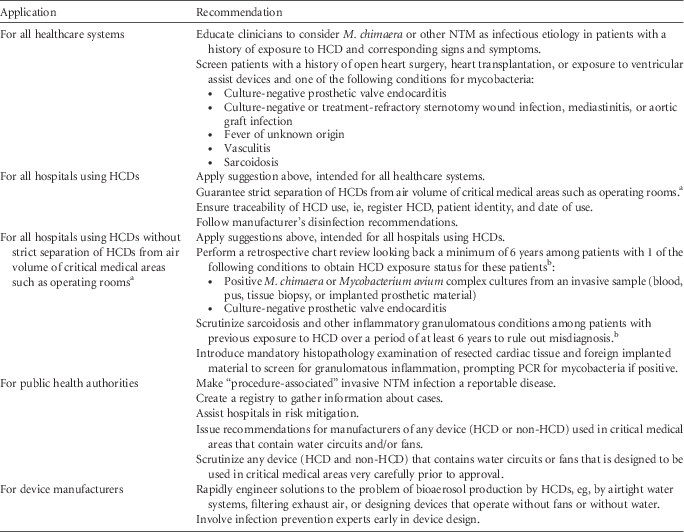
NOTE. NTM, nontuberculous mycobacteria; HCD, heater-cooler device; PCR, polymerase chain reaction.
a This interim suggestion applies especially to LivaNova/Sorin HCDs; HCDs of other brands have not definitely been linked to infections in cardiac surgery patients, nor has a potential link been formally excluded. Contamination of LivaNova 3T devices with M. chimaera at the production site before September 2014 was reported in a recent publication.Reference Haller, Holler and Jacobshagen 11 For the evaluation of solutions that guarantee strict separation of HCDs from the air volume of critical medical areas, hospitals must carefully evaluate the risks associated with this technology against the benefit to continue medical activity involving HCDs. Emerging scientific evidence and guidance by health authorities should be followed. Testing HCDs for M. chimaera and NTM may help to inform risk management decisions.
b Patients with previous HCD exposure and possible M. chimaera infection should undergo clinical evaluation.
Routine HCD water reservoir cultures for NTM are unlikely to be helpful because (1) few labs are well equipped to perform environmental mycobacterial cultures, (2) there is an 8-week lag time until culture results are available, and (3) the performance characteristics of the testing are not well defined (eg, the negative predictive value of cultures is probably poor). Because the possibility of transmission by direct water-to-blood leak cannot be ruled out entirely, a minimal acceptable water quality standard should be maintained. Monitoring after disinfection procedures by heterotrophic plate count could be considered; a count between 100 and 500 cfu/mL is considered acceptable. 12 Whether patients who were exposed to a running HCD but have no implanted foreign material in situ (eg, lung transplant patients) are at risk for invasive M. chimaera infection remains unknown. Whether disseminated M. chimaera infection is curable also remains unknown. Finally, more studies with HCD brands other than LivaNova are needed for additional risk assessment.
Summary
To our knowledge, many healthcare providers are unaware of the entity “invasive M. chimaera infection,” which may result in misdiagnosis or delayed diagnosis. Because most hospitals did not (or still do not) provide strict separation between OR air and HCD exhaust air, many more patients were (and will continue to be) at risk. Given the difficulty surrounding this diagnosis with a latency of up to 5 years and the absence of a standardized case identification strategy, it is likely that there are additional unrecognized cases.
Although a single-point contamination at the production site of LivaNova is the likely cause of the outbreak, additional contamination on a hospital level cannot be ruled out. Widespread water system colonization with NTM has been demonstrated. Filter effectiveness is uncertain, and HCDs are prone to persistent contamination with NTM.
A reliable disinfection protocol for the HCDs currently in use has not yet been defined; quick HCD design fixes seem out of reach; and waterless systems or other technical advances are not currently available. Any devices (not only HCDs) designed to be used in critical areas, such as operating rooms, that contain a water circuit or utilize a fan should undergo infection control review prior to approval and marketing; however, the details and the extent of this review still need to be defined.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.




