To the Editor—The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for COVID-19, which emerged in Wuhan, China, in December 2019. The current pandemic appears to be characterized by human-to-human transmission; it occurs through cough, sneeze, droplet inhalation, and direct contact of hands with mouth, nose, and eyes. The virus resides in the mucous membranes and it is transmitted through the saliva and the respiratory droplets. Although prevention of person-to-person transmission is the key to limiting the pandemic, so far, little attention has been given to the events taking place immediately after the onset of the first symptoms.
To prevent the spread of the virus, in February 2020, the Italian government issued a recommendation, among the methods of sanitizing the environments, for the use of 0.5% hydrogen peroxide.1 Hydrogen peroxide is already widely used as an environmental, surgical disinfectant and as an oral disinfectant in the treatment of gingivitis.Reference Cortelyou2,Reference Urban, Rath and Radtke3 SARSCoV-2 is spread by human-to-human transmission; the infection is estimated to have an average incubation period of 6.4 days and a base reproduction number of 2.24–3.58.Reference Lai, Shih, Ko, Tang and Hsueh4 Furthermore, scientific studies have proven that the virus persists for 2 days on the mucous membranes of macaquesReference Liu, Wei and Nishiura5 before the subsequent spread of the virus to the lower respiratory tract. This delay represents a window of therapeutic opportunity.
The efficient inactivation of coronaviruses (eg, SARS and MERS) on inanimate surfaces using hydrogen peroxide (H2O2 0.5% for 1 minute) was assessed by Kampf et al.Reference Kampf, Todt, Pfaender and Steinmann6 Based on their findings, and after reviewing the current literature concerning hydrogen peroxide, we propose that hydrogen peroxide, as an antiseptic agent, could play a pivotal role in reducing the hospitalization rate and COVID-19–related complications. The antiseptic efficacy of hydrogen peroxide 3% against SARSCoV-2 on oral and nasal mucosa can be reasonably hypothesized. The antiseptic action is due not only to the known oxidizing and mechanical removal properties of hydrogen peroxide but also to the induction of the innate antiviral inflammatory response by overexpression of Toll-like receptor 3 (TLR3).,Reference Koarai, Sugiura and Yanagisawa7 Thus, the overall progression of the infection from the upper to the lower respiratory tract can be reduced.
Therefore, we advise an off-label use of H2O2 3% and 1.5 % (10 volumes) by oral and nasal washing respectively, performed immediately after the onset of the first symptoms and the presumptive diagnosis of COVID-19 and during the illness in home quarantine or by hospitalized patients not requiring intensive care.
We propose a regimen of gargling 3 times per day for disinfection of the oral cavity and nasal washes with a nebulizer twice daily (due to a greater sensitivity of the nasal mucosa). Hydrogen peroxide (H2O2) is safe for use on the mucous membranes as gargling or as a nasal spray; in fact, it is already commonly used in otolaryngology. Figure 1 shows the epithelial of oral mucosa treated with H2O2 3% for a period of 6 months. No damage was observed on oral mucous membranes or their microvilli after ongoing gargling treatment with H2O2 3%. Another route for SARSCoV-2 is through nasolacrimal ducts; thus, we advise the use of iodopovidone 0.5%–0.6% as eye drops (1 drop 3 times daily on conjunctiva of both eyes) due to its antiseptic action against SARS-CoV-2 within 1 minute.
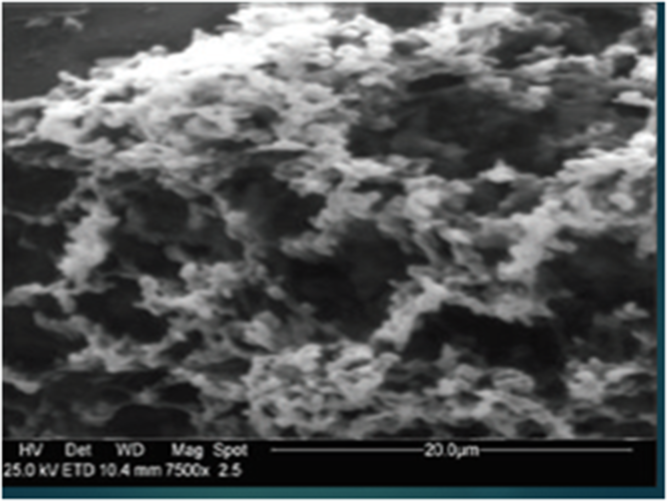
Fig. 1. Mouth mucous membranes after administration of H2O2 3% (10 vol) over a period of six months (Scraping cytology and scanning electron microscopy; SEM 7500 Cambridge MARK 250 SEM).
In our opinion, the effectiveness of this regimen will be verified through a significant reduction of the rate of hospitalization and respiratory complications in patients positive for SARS-CoV-2 with and without mild-to-moderate symptoms. We strongly encourage the rapid development of randomized controlled trials including both SARS-CoV-2–positive and –negative participants to study the benefits of H2O2 3% in the reduction of pulmonary complications and hospitalization rates.
Acknowledgments
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




