To the Editor—Intensive care units (ICUs) are an important target for antimicrobial stewardship (AMS) programs due to their high usage of antimicrobials. Reference Luyt, Bréchot, Trouillet and Chastre1,Reference Chiotos, Tamma and Gerber2 Audit and feedback, with face-to-face education within the ICU is associated with a short-term decrease in antimicrobial consumption, beneficial effects on resistance rates, and a reduction in antimicrobial costs. Reference Taggart, Leung, Muller, Matukas and Daneman3-Reference Katsios, Burry and Nelson6 We previously demonstrated the impact of an EMR-integrated AMS-ICU ward round over a 9-month period. Reference Devchand, Stewardson and Urbancic7 In this current study, we have demonstrated the long-term (>12 months) sustained improvement in antimicrobial prescribing following the implementation of an EMR-integrated AMS-ICU ward round. Additionally, we explored clinical and patient factors that affect the decision to provide AMS recommendations.
In August 2017, a new 5-day-per-week, EMR-integrated, AMS-ICU ward round was implemented at Austin Health (Melbourne, Australia), a tertiary referral hospital with a 29-bed mixed medical-surgical ICU. Reference Devchand, Stewardson and Urbancic7 From August 2017 to July 2019 (inclusive), we audited the results of this intervention. These recommendations were categorized according to the previously published “Five Moments” metric. Reference Devchand, Stewardson and Urbancic7 Compliance with AMS recommendations was reviewed 24 hours after each ward round by the AMS pharmacist. Multidrug-resistant colonization monitoring was performed routinely in the ICU (see definitions in the Supplementary Material online), and subsequent data were collected.
To monitor antimicrobial use 2 years before and after the intervention, defined daily dose (DDD) per 1,000 occupied bed day (OBD) data were obtained from the National Antimicrobial Utilisation Surveillance Program (NAUSP) (see definitions in the Supplementary Material online). 8 Appropriateness scores from the yearly point-prevalence National Antimicrobial Prescribing Survey (NAPS; see definitions in the Supplementary Material online) were also compared before implementation (2015–2016) and after implementation (2017–2018) of the EMR-integrated AMS-ICU ward rounds. Appropriateness scoring was undertaken by the same infectious diseases clinician each year, independent from this intervention. Reference James, Upjohn and Cotta9
A sensitivity analysis was performed utilizing additional data collected over a 9-week period (February–April 2019). The analysis consisted of all ICU antimicrobials reviewed by the AMS-ICU service within 48 hours of prescription to determine factors (patient vs clinician) associated with recommendations made as a result of the AMS-ICU ward rounds. Adjusted odds ratios (aORs) were calculated for these factors, correcting for Charleston comorbidity index and ICU physician years of experience (<5 years and ≥5 years). Antimicrobial classes were compared against piperacillin/tazobactam and amoxicillin/clavulanate (IV). Infectious syndromes were compared with infections of unclear source. This study was approved by the Austin Health Ethics Committee (no. CD 18-004).
In the 2-year postintervention period, 1,992 AMS recommendations were given as a result of the AMS-ICU ward rounds for the 916 patients reviewed (Supplementary Table 1 online). The rates of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant gram-negative (MDR-GN) colonization before and after the intervention are shown in Supplementary Table 2 (online). We detected a significant increase in MRSA colonisation after the intervention versus before the intervention (2.29% vs 0.06%; P < .0001), with no difference noted for VRE or MDR-GN isolation (P > .05).
Duration of the ward round was collected from November 2017 onward; the median duration was 0.5 hours (IQR, 0.38–0.65). All of the ward rounds had at least an infectious diseases consultant or a registrar present. During the first year after the intervention, 876 (87.6%) of 1,000 recommendations were implemented by the ICU, compared with the second year, during which 886 (89.3%) of 992 recommendations were implemented (P = .23). Recommendations and acceptance rates are outlined in Supplementary Table 3 (online).
Utilizing DDD per 1,000 OBD data, we demonstrated an immediate decrease in the use of ceftriaxone, meropenem, piperacillin/tazobactam, and vancomycin. Additionally, we demonstrated an ongoing significant reduction in the use of vancomycin and ciprofloxacin after the intervention; however, we detected no significant long-term change in the utilization of ceftriaxone or piperacillin/tazobactam postintervention (Fig. 1). Prescribing appropriateness (utilizing yearly NAPS appropriateness scores) increased from 41 (51%) of 80 during the preintervention period (2015–2016) to 62 (73%) of 85 after the intervention was implemented (2017–2018) (P = .0061).
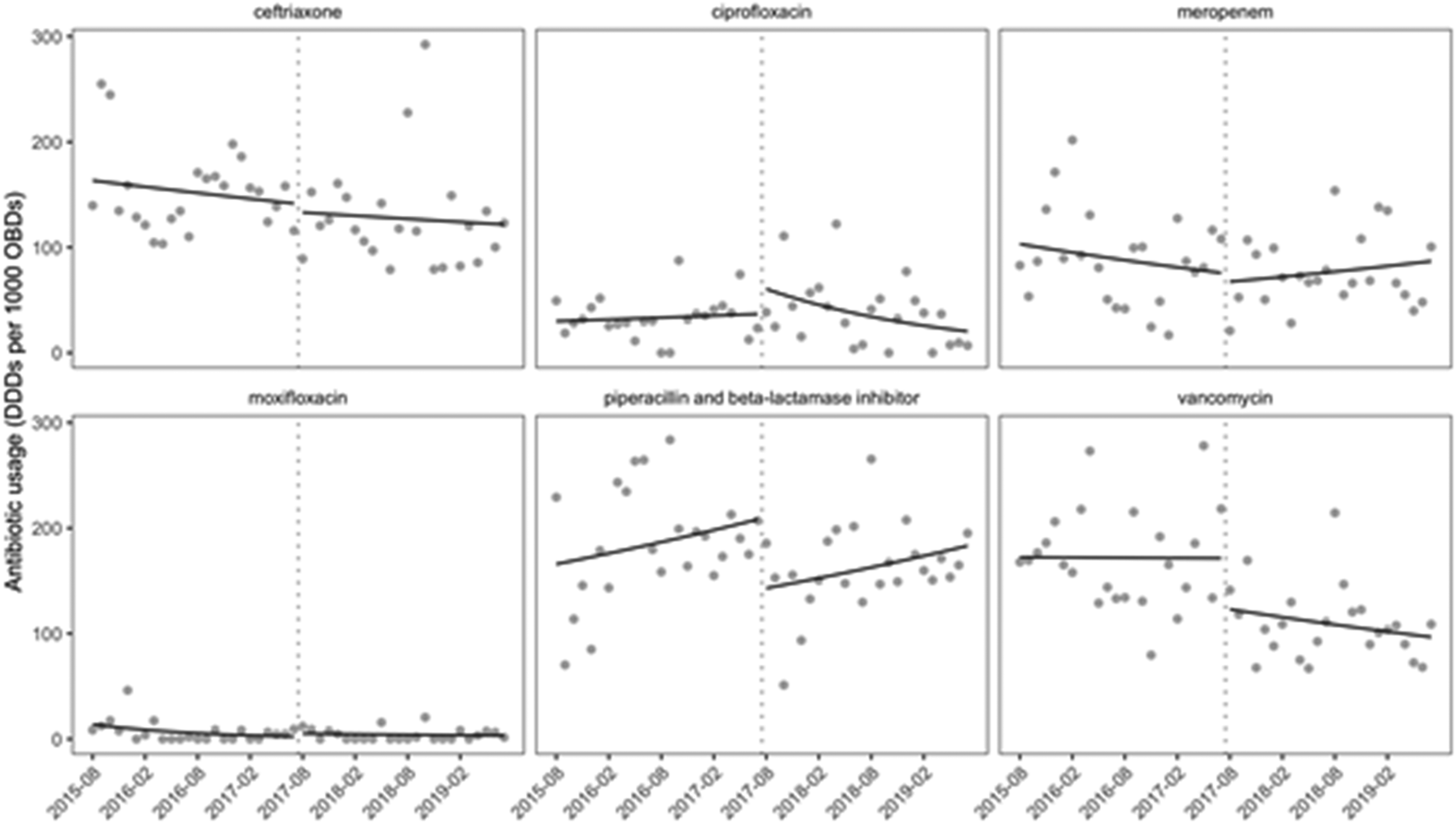
Fig. 1. Change in antimicrobial use after implementation of AMS-ICU intervention. Dotted vertical lines represent commencement of intervention. Solid lines represent preintervention and postintervention trends in antimicrobial use estimated using Poisson segmented regression. The dots on the graph are raw data points. Note. ICU, intensive care unit; AMS, antimicrobial stewardship; DDDs, defined daily doses; OBDs, occupied bed days.
The additional sensitivity analysis was performed for 184 patients (Supplementary Table 4 online) to examine factors that affected the decision to make AMS recommendations. This analysis revealed that significantly more recommendations were made for IV therapy than for oral therapy: adjusted odds ratio (aOR), 2.64 (Supplementary Table 5 online). No antimicrobial class was associated with an increased ratio of recommendations (Supplementary Table 5 online). Pneumonia was the only infectious syndrome associated with a higher ratio of recommendations (aOR, 6.85, IQR, 1.83–25.63).
Similarly to other studies, our audit has demonstrated an immediate decrease in the utilization of our target antimicrobials (ceftriaxone, meropenem, piperacillin/tazobactam and vancomycin) after the intervention. Reference Taggart, Leung, Muller, Matukas and Daneman3,Reference Morris, Bai and Burry4 In addition, we demonstrated an ongoing significant reduction in vancomycin and ciprofloxacin use after this intervention. Interestingly, during the audit period, we noted a significant increase in MRSA colonization. Nevertheless, we achieved an ongoing reduction in vancomycin use. We hypothesize that this is due to our low rates of MRSA before and after the intervention, which allowed us to stratify risk when prescribing vancomycin. In addition, our institution has a strong focus on antibiotic allergy assessment, which could account for some of the ongoing reduction in ciprofloxacin and vancomycin use. Reference Trubiano, Chen, Cheng, Grayson, Slavin and Thursky10
Our study was limited by the provision of a Monday–Friday AMS-ICU service. However, this study included an exploratory sensitivity analysis to examine factors associated with recommendations being made by the AMS-ICU ward rounds. We observed a greater proportion of recommendations for IV compared to oral antimicrobial therapy. Also, 87.2% of prescriptions during this period were for IV therapies, a key target for “de-escalation” and “switch.” Prescriptions for patients with pneumonia were more likely to receive a recommendation supporting earlier findings that pneumonia is a target for AMS-ICU programs. Reference Devchand, Stewardson and Urbancic7
This audit demonstrates that the benefits of an EMR-integrated AMS-ICU ward-round intervention can be sustained in the long term. Future research should focus on risk-stratifying patients who would most benefit from an AMS review within the ICU.
Acknowledgments
The authors are grateful to Elizabeth A Grabsch for assistance with obtaining data.
Financial support
J.A.T. is supported by a National Health and Medical Research Council Early Careers Fellowship (grant no. APP1139902). AJS is supported by an Australian National Health and Medical Research Council Early Career Fellowship (GNT1141398). Other authors report no financial support relevant to this article.
Conflicts of interest
AJS reports having received an investigator-initiated research grant funded by Merck Sharp & Dohme Corp. All other authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.71