To the Editor—In the past several decades, carbapenemases have emerged as the main carbapenem-resistance mechanism in Pseudomonas aeruginosa, Acinetobacter spp, and more recently, among Enterobacterales.Reference Bush and Jacoby1 In Brazilian hospitals, bla SPM-1 and bla IMP-1 have been described as the most prevalent metallo-β-lactamase (MBL) genes among P. aeruginosa isolates.Reference Perez, Antunes, Freitas and Barth2 However, efflux pumps may contribute to carbapenem resistance development in P. aeruginosa because accumulation of the drug is reduced due to its rapid extrusion from the bacterial cell.Reference Cabot, Ocampo-Sosa, Tubau, Macia, Rodriguez, Moya, Zamorano, Suarez, Pena, Martinez-Martinez and Oliver3 Considering the lack of activity of carbapenem agents in front of these mechanisms, drugs such as ceftolozane/tazobactam (CT), have recently been targeted for new therapeutic approaches. Thus, it is crucially important to determine the exact mechanism that is occurring to establish a better stewardship strategy because CT does not have action on MBL-producing P. aeruginosa.Reference Livermore, Mushtaq, Meunier, Hopkins, Hill, Adkin, Chaudhry, Pike, Staves and Woodford4
In P. aeruginosa, efflux pumps have been categorized into 5 superfamilies, 4 of which belong to the resistance-nodulation division (RND) family: MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY.Reference Cattoir5 Efflux pump overexpression, particularly MexAB-OprM and MexXY, may cause high levels of carbapenem resistance among P. aeruginosa isolates.Reference Poole6 Although carbapenemases and efflux pumps have an important role in determining resistance, it is particularly difficult to infer the exact influence of each other because multiple resistance mechanisms may occur simultaneously.
The aim of this study was to evaluate the meropenem minimum inhibitory concentration (MIC) among meropenem-nonsusceptible P. aeruginosa, producing or non-producing MBLs, in the presence of a specific pump inhibitor agent, the L-phenyl-L-arginine β-naphthylamide (PaβN).Reference Lamers, Cavallari and Burrows7 Moreover, ceftolozane/tazobactam (C/T) MICs were determined for all isolates included in this study.
A set of 91 meropenem-nonsusceptible P. aeruginosa were consecutively included in this study. Only 1 isolate per patient was considered, which had been recovered from distinct clinical specimens (endotracheal aspirate, n = 62; sputum, n =10; urine, n = 10; and others, n = 9) from hospitalized patients in southern Brazil from August 1, 2016, through February 2, 2017.
Meropenem MICs were determined by standard broth microdilution, according to the Clinical Laboratory Standards Institute (CLSI) protocol using P. aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 as strains for quality control.8 C/T MICs were determined by Etest (Liofilchem, Italy). For MBL gene detection, a real-time polymerase chain reaction (PCR) was performed as previously described.Reference Perez, Antunes, Freitas and Barth2 Broth microdilution using meropenem as substrate, ranging from 0.5 to 256 µg/mL, in the presence of 50 µg/mL PaβN, was performed to determine the MexAB-OprM overexpression activity, as described elsewhere.Reference Lamers, Cavallari and Burrows7 Efflux overexpression was determined when a decrease in meropenem MIC without inhibitor occurred at ≥2log2.Reference Mesaros, Glupczynski, Avrain, Caceres, Tulkens and Van Bambeke9 Isolates were evaluated in triplicate, and the results were visually screened after 24 hours of incubation at 35°C.
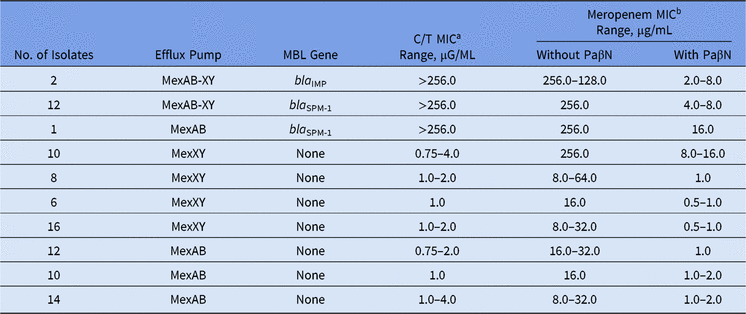
Among our set of isolates, 15 (16.5%) were MBL producers (13 bla SPM-1 and 2 bla IMP), and among them, high levels of meropenem resistance (MICs ≥ 128.0 µg/mL) were found. The remaining 76 (83.5%) isolates were non-MBL producers with meropenem MICs ranging from 8.0 to 256.0 µg/mL (Table).
In the presence of PaβN, all P. aeruginosa isolates showed a significant reduction in meropenem MICs, no matter the presence of any MBL gene (Table 1). Moreover, overexpression of the MexAB-OprM efflux pump was verified for all meropenem-resistant non–MBL-producing isolates. This result is in agreement with other studies, which have reported that >70% of the isolates can overexpress efflux pump mechanisms like MexAB-OprM.Reference Cabot, Ocampo-Sosa, Tubau, Macia, Rodriguez, Moya, Zamorano, Suarez, Pena, Martinez-Martinez and Oliver3
Table 1. Effect of the Efflux Pump Inhibitor on Ceftolozane/Tazobactam and Meropenem MIC Values Among Meropenem-Nonsusceptible MBL-producing and Non–MBL-producing Pseudomonas aeruginosa
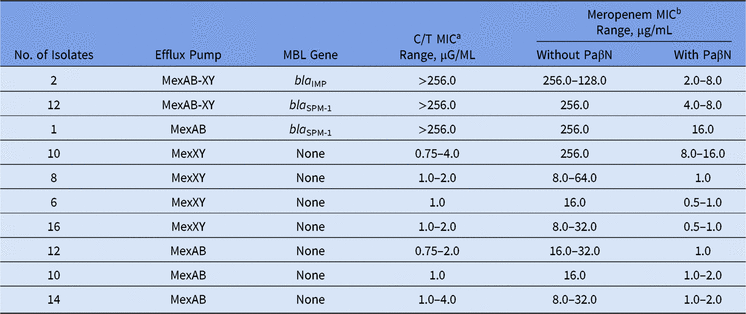
Note. MBL, metallo-β-lactamase; MIC, minimum inhibitory concentration; PaβN, L-phenyl-L-arginine β-naphthylamide.
a C/T (ceftolozane/tazobactam) break points are ≤4 μg/mL (susceptible) and >4 μg/mL (resistant).
b Meropenem break points are ≤4 μg/mL (susceptible) and ≥16 μg/mL (resistant).
This study highlights important issues: (1) the need to detect the exact carbapenem resistance mechanism involved (a simple phenotypic test such as blue-carba would be enough) because MBL producers are not elective for CT therapy; (2) better targeting of the use of new antimicrobials, such as CT, for those resistant isolates due to the overexpression of ampC enzyme associated with efflux pumps (ie, CT emerged as an important therapeutic agent with no resistance detected in this study and with low MIC50 and MIC90 of 1.0 and 2.0 μg/mL, respectively [data not shown]); and (3) epidemiology survey of Pseudomonas resistance (currently, carbapenem-resistance due to efflux plus porin loss represent nearly 70% of all carbapenem-resistant Pseudomonas isolates) for rapid adjustment in stewardship programs to avoid antimicrobial resistance.
In conclusion, the presence of an efflux pump inhibitor (PaβN) markedly decreases the level of meropenem resistance among P. aeruginosa isolates, Reference Lomovskaya and Bostian10 regardless the presence of MBL, highlighting the role of efflux pump as an important mechanism to keep a high level of meropenem resistance. Additionally, microbiology labs should detect and report the presence of the resistance mechanism to establish the best antimicrobial use, particularly to prevent antimicrobial resistance development.
Author ORCIDs
Leandro Reus Rodrigues Perez, 0000-0002-6662-6503
Acknowledgments
The author thanks Sophia Perez for technical support.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
The author report no conflicts of interest relevant to this article.