Safe and reliable vascular access is a necessary part of care for sick infants in the neonatal intensive care unit (NICU). Central venous catheters (CVCs) are commonly used to provide long-term venous access. They provide critical nutrition for growth as well as a portal for other vital intravenous fluids and medications. They provide improved nutrition and avoid repeated painful procedures associated with the use of peripheral venous cannulas.Reference Janes, Kalyn, Pinelli and Paes 1 , Reference Ainsworth, Clerihew and McGuire 2 CVCs used in the NICU include umbilical venous catheters (UVCs) and peripherally inserted CVCs (PCVCs). PCVCs are inserted via a peripheral vein and threaded so that the tip of the catheter lies in a large central vein. UVCs also have the tip sited in a large central vein but are inserted via the umbilical vein.
There are a number of complications associated with CVC use, varying from minor and easily treatable to life-threatening. The most frequent complication is central line–associated bloodstream infection (CLABSI). The Centers for Disease Control and Prevention defines a CLABSI as “a primary blood stream infection in a patient that had a central line within the 48-hour period before the development of the blood stream infection, and is not related to an infection at another site.”Reference O’Grady, Alexander and Burns 3 The incidence of CLABSI varies widely within the population studied and definition used, but is 1.6–15 per 1,000 central line–days in NICUs in high income countries.Reference Aly, Herson and Duncan 4 – Reference Milstone, Reich and Advani 6 Incidence increases with decreasing birthweight and gestational age.Reference Dudeck, Weiner and Allen-Bridson 7 Infection usually occurs via skin commensals that migrate via the catheter entry site or cannula hub, the predominant causative organism being coagulase-negative Staphylococcus.Reference Salzman, Isenberg, Shapiro, Lipsitz and Rubin 8 , Reference Salzman and Rubin 9 The umbilical stump is particularly heavily colonized, but a recent retrospective cohort analysis demonstrated that UVC CLABSI rate is similar to that in PCVC.Reference Shalabi, Adel and Yoon 10 CLABSI is responsible for 69% of all late-onset infections in preterm babies.Reference Kaplan, Lannon, Walsh and Donovan 11 Late-onset neonatal sepsis is a significant risk factor for increased mortality and prolonged hospital stays,Reference Stoll, Hansen and Fanaroff 12 although mortality is variable and related to the implicated pathogen.Reference Verstraete, Boelens and De Coen 13 In those who survive, there is poorer long-term growth and developmental outcomes,Reference Schlapbach, Aebischer and Adams 14 , Reference Stoll, Hansen and Adams-Chapman 15 with associated increased morbidity and increasing healthcare costs.Reference Payne, Carpenter, Badger, Horbar and Rogowski 16
Prevention of CLABSI is a key objective for improvement of patient safety and reduction of mortality, hospital stay, and costs. Preventing and controlling healthcare-associated infection is one of the 10 Australian National Safety and Quality Health Service standards, highlighting the national commitment to these preventable infections. 17 Fortunately, CLABSI has been shown to be highly modifiable with “bundles” of health care interventions.Reference Pronovost, Needham and Berenholtz 18 , Reference Blot, Bergs, Vogelaers, Blot and Vandijck 19 A bundle is defined as “a limited number of specific practices, each essential for effective and safe patient care and that, when implemented together, result in additional improvements in patient outcomes.”Reference Schulman, Stricof and Stevens 20 These multidisciplinary, evidence-based best practice recommendations are effective in reducing CLABSI in the NICU.Reference Schulman, Stricof and Stevens 20 , Reference Bizzarro, Sabo and Noonan 21
An audit of CLABSI rates in 2012 at our institutionReference Greenhalgh and Gordon 22 showed 8.5 CLABSI/1,000 central line-days, and 13.4/1,000 central line–days for infants less than 29 weeks’ gestation. Comparative data combining rates from all the other level 5 NICUs in New South Wales over the same period using the same definition of CLABSI showed a CLABSI rate of 8.3/1,000 central line–days at less than 29 weeks’ gestation.Reference Shein 23 The audit identified a number of areas for improvement: the implementation of standardized practices, central line policy revision, and a structured education program. Following this, a bundle of CLABSI prevention interventions was introduced. The aim of this study was to compare CLABSI rates before and after the introduction of the CLABSI prevention bundle to determine its effectiveness and to identify areas for further improvement.
METHODS
Study Setting and Design
This was a retrospective cohort analysis of prospectively collected data. Eligible infants were admitted to the Royal Prince Alfred Hospital NICU and had a CVC inserted. The study periods were January 1, 2012-December 31, 2012 (baseline) and August 1, 2013-July 31, 2014 (intervention). The bundle of interventions commenced during March-August 2013.The intervention period was chosen as the most recent fully audited data available. This hospital is a major obstetric tertiary referral center. The hospital currently averages approximately 5,500 deliveries per year and covers an inner-city, multicultural population. It provides a level 5 NICU service 24 with an average of approximately 900 admissions to the nursery each year. This study was approved prospectively by the Sydney Local Health District ethics committee.
Process and Interventions
Following the audit, a multidisciplinary team of staff with an interest in quality improvement and infection control was formed. Areas where CLABSI prevention had failed were reviewed, and solutions were proposed. These were based upon current best evidence, and largely upon the recommendations from the Provincial Infectious Diseases Advisory Committee, Ontario. 25 A fishbone diagram was used to highlight the issues (Figure 1).
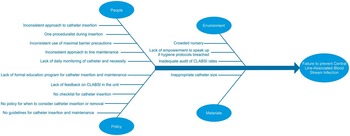
FIGURE 1 Fishbone diagram highlighting process failures in prevention of central line-associated bloodstream infection (CLABSI).
The baseline and intervention central line practices are documented in Table 1. It must also be noted that probiotics were introduced into routine newborn care from October 2012 for all infants less than 30 weeks’ gestation, or greater than 29 weeks’ gestation with additional risk factors placing them at higher risk for necrotizing enterocolitis. There were no other relevant changes in policy or definitions during the study periods.
TABLE 1 Baseline and Intervention Central Line Practices

NOTE. CLABSI, central line–associated bloodstream infection; CVC, central venous catheter; Fr, French; SCORPIO, Structured, Clinical, Objective Referenced, Problem-based, Integrated, and Organized; TPN, total parenteral nutrition; WHO, World Health Organization.
a Daily discussion included both initial decision to insert a CVC as well as ongoing requirement for it to remain in situ. There was a concerted effort to avoid CVC in larger and mature infants likely to tolerate enteral nutrition well.
Implementation Strategy
The bundle of interventions was implemented via a structured education program. There is a strong background within this unit with the SCORPIOReference Hill 26 (Structured, Clinical, Objective Referenced, Problem-based, Integrated, and Organized) method of teaching. This approach uses interactive problem-focused learning to teach specific skills and has been adapted for adult postgraduate learning.Reference Vaughan, Jeffery and Raynes-Greenow 27 It was the successful foundation of the SEA-URCHIN Project 28 (South East Asia–Using Research for Change in Healthcare-Associated Infections) that was also initiated by this unit, with which our bundle had similar objectives and some shared materials. Education sessions were conducted every 3 months, covering all new staff and providing updates to all staff at least yearly. There were 3 workshops, each lasting 1.5–2.5 hours and containing an introduction, a number of skills-focused stations, an objective structured clinical examination, and formative assessment. Each workshop had a coordinator and a number of facilitators, who were medical staff and nurse educators with an interest in infection control. The central line workshop contained an introduction with an overview of CLABSI and 4 stations: (1) preparation to handle or insert lines, (2) CVC insertion, (3) CVC maintenance, and (4) local and state audit feedback of CLABSI. The infection control workshop consisted of 4 stations: (1) hand hygiene, (2) infection control at birth, (3) nursery admission, and (4) nursery environment. A hand hygiene workshop consisted of an introduction with local audit feedback and 3 stations: (1) the World Health Organization’s 5 Moments for Hand Hygiene, 29 (2) effective hand hygiene, and (3) assertiveness training.
Data Collection
Information was identified and obtained from the neonatal clinical database. Data were extracted for every infant who had a CVC placed during his or her NICU admission. If further clarification was required, there was validation of data with the full medical record. Data regarding clinical characteristics, CVC use, and details of any bloodstream infection were collected. Infections are coded monthly by a committee of attending neonatologists and the hospital microbiologist using the following definitions.
Definitions
Central line was defined as a venous line inserted via either the umbilical or a peripheral vein, such that the line tip is placed into a large central venous vessel.Reference Dudeck, Horan and Peterson 30 Central lines included UVC and PCVC.
Proven bloodstream infection was defined as growth of a certain pathogen in blood and treated by the clinicians as infection. If the pathogen could be a potential contaminant, there must be a pure growth of that organism, with either confirmatory laboratory evidence or growth of the same organism on repeat culture.
CLABSI was defined as a proven bloodstream infection associated with a central venous line when a central line has been in use 48 hours before signs and symptoms of an infection with no apparent source other than the central line. A culture of the same organism in any sample within 13 days is counted as a single infection. This definition was unchanged throughout the studied periods.
Early-onset infection was defined as a proven bloodstream infection with initial symptoms occurring sooner than 48 hours after birth.
Late-onset infection was defined as a proven bloodstream infection with initial symptoms occurring at least 48 hours after birth.
Babies were analyzed as part of gestational cohort groups. These groups included less than 29, 29–31, 32–36, and at least 37 weeks’ gestation.
Statistical Analysis
Data were managed with an electronic spreadsheet (Excel 2013; Microsoft) and analyzed using Prism, version 5.0 for Windows (GraphPad). The central line utilization ratio was calculated as the number of central line–days/number of patient-days. CLABSI rates were calculated as number of CLABSI/central line–days×1,000.Reference Dudeck, Weiner and Allen-Bridson 7 Continuous data were expressed as mean (SD) or median (interquartile range) according to the distribution. Data were compared with the Mann-Whitney test or the unpaired t test as appropriate. Categorical data were expressed as count and proportion and compared with the χ2 or Fisher exact test. One-way analysis of variance (Kruskal-Wallis test) was used to compare more than 2 groups. All reported P values are 2 sided, and significance was assumed at P<.05.
RESULTS
Clinical Characteristics
Patient demographic characteristics are shown in Table 2. There were no significant differences between the groups in any of the parameters recorded.
TABLE 2 Characteristics of Infants With Central Lines Inserted

NOTE. Data are no. (%) of infants unless otherwise indicated. IQR, interquartile range.
Central Line Use
With regard to central line insertion, 353 CVCs (177 UVCs and 176 PCVCs) were inserted in 214 newborns during the baseline period, compared with 260 CVCs (142 UVCs and 118 PCVCs) inserted in 162 newborns during the intervention period. There were significantly fewer CVC inserted in the intervention period; the central line utilization rate was 0.11 in the intervention period vs 0.2 at baseline (Fisher exact test, P=.0001).
Overall, median dwell time of CVCs was significantly shorter in the intervention period compared with the baseline period; 4.4 (95% CI, 2.2–6.7) vs 5.0 (2.9–8.2) days (Mann-Whitney test, P=.01). A reduction in PCVC dwell time underlies this difference: 6 (5.0–11.8) vs 7.3 (4.0–10.4) days (Mann-Whitney test, P=.0004) (Figure 2). UVC dwell times were not significantly different in intervention and baseline groups (3.1 [1.8–4.8] vs 3.4 [1.7–5.4], P=.87). Dwell time was not different in babies less than 31 weeks’ gestation (5.9 [2.9–10.4] vs 5.5 [2.9–7.8] days, P=.19) but was significantly shorter for those babies at least 31 weeks’ gestation in the intervention period (3.7 [1.9–5.5] vs 4.2 [2.8–6.8] days, P=.05). Dwell time was longer in preterm infants in comparison with term and was inversely proportional to gestational age (1-way analysis of variance, P<.0001) (Figure 3).

FIGURE 2 Dwell time of central venous catheters in the baseline and intervention groups. PCVC, peripherally inserted central venous catheter; UVC, umbilical venous catheter.

FIGURE 3 Reduction in central venous catheter dwell time in the intervention period across gestational age cohorts.
Most babies less than 29 weeks’ gestation had more than 1 CVC placed during their admission, with the median number of lines placed in this cohort being 2. One infant in this cohort had 5 CVCs placed. After 29 weeks’ gestation, most infants had only 1 CVC placed. Compared with baseline, there was no difference in the number of CVCs per patient either overall or when analyzed per gestational age cohort.
Infection
There were 15 positive blood cultures with clinical signs of infection in the intervention period: 4 were early-onset and 11 were late-onset sepsis. Of the 11 with culture-positive late-onset sepsis, 3 were CLABSI, all with a pure growth of coagulase-negative Staphylococcus. One other patient had a CVC in situ at the time of deterioration but had a diagnosis of necrotizing enterocolitis with perforated bowel and blood culture grew Enterococcus faecalis. The remaining patients did not have a CVC in situ within 48 hours of bloodstream infection. At baseline, there were 22 bloodstream infections. One of these infections was an early-onset infection, confirmed from blood cultures of samples collected at birth with the same organism isolated in the mother. Another was in a baby without a central line following laser therapy for retinopathy of prematurity. The remaining 20 infections were CLABSI. Of 20 infections, 19 were caused by coagulase-negative Staphylococcus and 1 by Escherichia coli.
Overall there was a significant decrease in CLABSI rates from 8.5 per 1,000 central line–days to 2.3 per 1,000 central line–days (Fisher exact test, P=.004) (Table 3). Three (1.2%) of 260 CVC were implicated in infection compared with 20 (5.7%) of 353 CVC in the baseline data. The run chart of total CLABSI over time is shown in Figure 4 and was extended post hoc to include 2014–2015 data to highlight continued reduced CLABSI rates.

FIGURE 4 Run chart of central line–associated bloodstream infections (CLABSI) per 1,000 central line–days 2012–2015
TABLE 3 Central Line–Associated Infection Rates
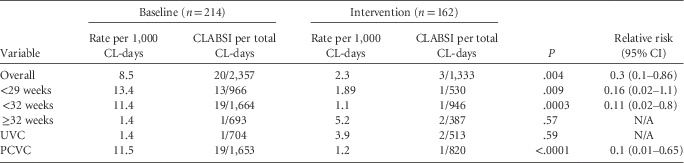
NOTE. CL, central line; CLABSI, central line–associated bloodstream infection; N/A, not applicable; PCVC, peripherally inserted central venous catheter; UVC, umbilical venous catheter.
Of interest, 2 of the 3 CVCs in the intervention group that caused clinical sepsis were UVCs, both in infants between 32–34 weeks’ gestation. There was only 1 CLABSI seen related to a PCVC, which was in an infant of 25 weeks’ gestation who had 5 PCVC in total. There were too few CLABSI in the intervention cohort to perform any further analysis.
DISCUSSION
The major findings of this study are that adoption of a coordinated training, education, and review program in a busy Australian neonatal unit has resulted in change in clinical practice, and that this behavioral change translated into significant reductions in clinical complications associated with CVC use. The reductions in the number of CVCs inserted, CVC dwell time, and CLABSI rates provide further data in support of CLABSI prevention intervention bundles. The reduction of healthcare-associated infection is a nationally important issue, and we have demonstrated the substantial impact that can be made within a single unit with a bundle of interventions.
The intervention package is generalizable to many NICUs in resource-rich countries. Although analyzed retrospectively, data are entered prospectively, infections are coded by the clinical team and the hospital microbiologist, and data are validated by a dedicated audit officer.
Limitations of this study include the relatively small data set, particularly regarding the number of CLABSI. The reduction in infection is, however, in keeping with similar infection prevention bundles in larger studies.Reference Schulman, Stricof and Stevens 20 , Reference Bizzarro, Sabo and Noonan 21 It is not a randomized trial and therefore is prone to bias. However, the demographic characteristics of infants in the baseline and intervention groups showed no significant differences. There was no measurement of compliance to the bundle, and in consequence we cannot confirm that the interventions described were adhered to and are directly responsible for the reduction in CLABSI. Documentation in medical records has been insufficient to provide other relevant process outcomes. There has been a reduction in sepsis statewide, which has been attributed to a focus on timely data feedback and quality improvement.Reference Shein 23 Therefore, it is possible that the reduction in CLABSI seen in this study is a reflection of the general trend seen across the state. However, our hospital has shown the largest and most consistent decrease in infection rates statewideReference Shein 23 and was the first to implement a bundle of interventions using a structured education program. Ongoing audit will be necessary to document sustained effects. We acknowledge that the introduction of probiotics is a potential confounder. It is possible that both by a direct beneficial effect of probiotics on the immune systemReference Rao, Athalye-Jape, Deshpande, Simmer and Patole 31 and by reducing times to full feeds,Reference Athalye-Jape, Deshpande, Rao and Patole 32 probiotics may have reduced CLABSI in vulnerable infants by reducing the need for or dwell time of central lines.
CVCs are necessary in many infants, in particular in preterm infants who may take prolonged periods to tolerate full enteral feeds. However, median dwell time of CVCs was 4.4 days overall and just 2.1 days in term infants. There are a number of clinical factors other than time to reach enteral feeds that may influence the decision to insert a CVC, such as difficult peripheral venous access, or the infusion of hyperosmolar or other irritant fluids or medications. The short dwell time seen in this study may reflect a high rate of CVC-related complications and subsequent reinsertion, although the reasons for insertion and removal of CVCs are not routinely collected in our unit. The short dwell time suggests that at least some CVCs were not necessary. CVC dwell time was significantly shorter in infants at least 31 weeks’ gestation in the intervention group than at baseline. This may be a consequence of the policy both to avoid CVCs, utilizing peripheral venous access instead, and to remove CVCs at the earliest opportunity, especially in those who are likely to tolerate and reach full feeds more quickly.
There were only 3 CLABSI in the intervention group and consequently too few to perform adjusted analysis or comment further upon the associations or impacts of the CLABSI seen in the intervention period. Only 1 PCVC was related to CLABSI in 1 year of data collection, in a 25-week infant. However, 2 UVCs were implicated in CLABSI, both in more mature babies. This contrasts with the baseline data, in which most cases of CLABSI were related to PCVC use. This may reflect the emphasis that has been placed on PCVC insertion and maintenance, with a focus on the smallest and most vulnerable infants. Ongoing quality improvement in this unit now focuses on both UVC and PCVC, and the education program emphasizes the importance of the maintenance and insertion bundles in all infants with central lines, regardless of gestation or line type. Further regular audit is important to continue to identify areas of change and should measure compliance to interventions.
Although global incidence of NICU CLABSI is decreasing,Reference Dudeck, Weiner and Allen-Bridson 7 , Reference Jaggi, Rodrigues and Rosenthal 33 central line infection still represents a major risk at an individual level and contributes significantly to length of hospital stay and associated costs. As incidence decreases, it is becoming more important to tackle CLABSI and institute best practices via neonatal networks, such as the Australia and New Zealand Neonatal Network or the Vermont Oxford Network. Walshe demanded that “our expectations of the evidence base for [quality improvement] methodologies should be on a par with our expectation in relation to other forms of healthcare interventions.”Reference Walshe 34 (p.153) These networks offer the potential to replicate successful education programs and perform robust interventional studies in this area of quality improvement.
ACKNOWLEDGMENTS
Financial support. None reported.
Potential conflicts of interest. Both authors report no conflicts of interest relevant to this article.









