In 2016, an estimated 264,400 cases of healthcare-associated Clostridioides difficile infection (CDI) were reported in the United States, representing an incidence of 83 cases per 100,000 persons. Reference Guh, Mu and Winston1 These estimates were based on disease surveillance data from the Emerging Infections Program (EIP), which comprises 10 health departments located in California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Risk factors for CDI include, but are not limited to, age (with the elderly being more frequently affected), prior use of antibiotics, prior use of gastric acid suppressors, and exposure to healthcare settings. Reference Eze, Balsells, Kyaw and Nair2 EIP data also indicate disparities in healthcare-associated CDI by race, with higher rates seen among white people than among people from other racial or ethnic groups. 3
Although CDI is associated with demographic factors like race or ethnicity on the national level, Reference Eze, Balsells, Kyaw and Nair2,3 the relationship between these factors and infection may be a consequence of disparities in treatment and access to care across demographic groups. Notably, household income and private health insurance are also positively associated with CDI on the national level. Reference Mao, Kelly and Machan4 One study, based on a national all-payer claims database, reported that white patients had higher rates of CDI than black, Hispanic, Asian, and Native American patients. Reference Mao, Kelly and Machan4 However, when examining a subpopulation of patients with similar healthcare access and antibiotic exposure, no differences by race or ethnicity were found. This finding suggests that national differences in infection by race and ethnicity could be explained by differences in access to healthcare and treatment, which may in turn be determined by socioeconomic context.
Individual socioeconomic indicators are often not available in health records. However, by linking geographic data with census data, it is possible to describe and account for area-level socioeconomic context when investigating CDI using hospital records. These indicators may be summarized as a social deprivation index (SDI), a summary score based on several socio-demographic domains associated with health outcomes. Reference Messer, Laraia and Kaufman6
Urban safety-net hospitals disproportionately serve patients who are low income, black and/or Hispanic, and rely on public health insurance. Reference Gaskin and Hadley5 Among patients served by urban safety-net hospitals, where low-income black and/or Hispanic patients may represent the majority, disparities in treatment by race may look different from what is observed nationally, leading to patterns of CDI that diverge from the national norm.
Hahnemann University Hospital (HUH) was a tertiary-care facility located in the heart of Philadelphia, and it served as a safety-net hospital to its predominantly black patient base prior to its closure in 2019. The goals of this study were (1) to investigate the association between CDI and race or ethnicity, insurance type, referral location, and SDI at HUH and (2) to compare demographic patterns of infection at HUH with what has been observed nationally.
Methods
Overall study design
A case–control design was used to investigate the occurrence of healthcare-associated CDI among patients aged 18 years and older at HUH. Hospital-wide data corresponding to CDI, billing address, race/ethnicity, primary insurer, type of referral to the hospital, prior use of antibiotics, and prior use of proton-pump inhibitors were abstracted from electronic medical records for August 1, 2014, through May 1, 2018. Institutional review board approval (no. 1403002707) for this project was granted by the Drexel University Human Research Protections Office.
Cases were patients who showed symptoms of CDI at least 72 hours after admission to the hospital and whose infection was confirmed by testing fecal samples using a rapid enzyme immunoassay (C Diff Quik Check). Controls were selected from admissions records for patients who had a length of stay of at least 72 hours and who had no positive C. difficile test on record between admission to discharge. Controls were frequency matched to cases at a ratio of 2 controls to every 1 case by age, length of inpatient visit, and admission date (5 days before to 14 days after admission).
In total, 228 cases and 454 controls were selected. We restricted our analysis to patients who lived within Philadelphia County, resulting in the exclusion of 58 cases and 134 controls who lived outside the county. The final sample included 170 cases and 324 controls (n = 494). This restriction was made to focus on the core patient base for HUH, representing Philadelphia residents who were more likely to be primarily or solely dependent on HUH for care. To contextualize the differences between residents and nonresidents, we conducted a sensitivity analysis comparing key attributes of the 2 groups.
Individual-level factors
Covariables of interest included individual race or ethnicity, insurance type, type of referral to the hospital, prior use of antibiotics, prior use of proton-pump inhibitors, and census-tract–level deprivation index. Insurance type was classified based on the primary insurer as private, Medicare, Medicaid, or other/unknown. Referral type described where the patient came from prior to their arrival at HUH, and referral could be classified as home, acute care or rehabilitation, long-term care or nursing facility, or other/unknown. Prior use of antibiotics and prior use of proton-pump inhibitors were parameterized as dichotomous variables based on use of these drugs over the previous 6 months.
Neighborhood-level factors
Patients’ billing addresses were geocoded and mapped to census tracts; 6 patients reported a postal box instead of a home address and all but 1 of these postal box addresses were outside of the city of Philadelphia. The location of 31 other patients could only be resolved to the street where they lived, and each were assigned a home location at the midpoint of that street.
Derived SDI scores
The SDI was calculated on the census tract level using the approach described by Messer et al. Reference Messer, Laraia and Kaufman6 Characteristics that inform this score are education, poverty, occupation, household crowding, use of public assistance or food stamps, unemployment, and proportion of households that are female-headed with dependents. The SDI is a z score standardized to have a mean of 0 and a standard deviation of 1. Therefore, if a Philadelphia resident was assigned a score higher than 0, it indicates they lived in a census tract with demographic characteristics that indicate deprivation above the Philadelphia average. A score lower than 0 indicates below-average deprivation for the city. Population data were obtained from the 2018 American Community Survey 5-year estimates for Philadelphia County. 7
Analytic approach
We examined the univariable relationship between CDI and the covariables of interest using the Pearson χ Reference Eze, Balsells, Kyaw and Nair2 test for categorical covariables and the t test for continuous covariables. Multivariable estimates of association were calculated using generalized linear mixed-effects models with census tract-level random intercepts. We fit a total of 5 multivariable models. Because we were primarily interested in demographic indicators of infection, we began with an empty model containing only tract-level random intercept, adding deprivation index in model 2, race or ethnicity in model 3, insurance and referral type in model 4, and prior use of antibiotics and proton-pump inhibitors in model 5. For each model, we used a median odds ratio to assess area level variance as described by Merlo et al. Reference Merlo, Chaix and Ohlsson8
The demographics of CDI according national surveillance data from the EIP were also tabulated and compared to the distribution observed in our sample. EIP data, including case counts and population estimates, were taken from the 2016 Annual Report for the Emerging Infections Program for Clostridium difficile infection. 3 To approximate the demographic distribution of the population served by HUH, we pulled data from the 2018 American Community Survey 5-year estimates 7 for all Philadelphia census tracts containing at least 1 study participant. We estimated the expected case distribution at HUH by multiplying the demographic-specific rates observed in the EIP data by these American Community Survey population statistics.
Geocoding was conducted using the DeGauss geocoder version 2.3 software. Reference Brokamp, Wolfe, Lingren, Harley and Ryan9 All analyses were conducted in R version 3.6.2 software (R Foundation for Statistical Computing, Vienna, Austria). Generalized linear mixed models were fit using the lme4 package version 1.1.21 software. Reference Bates, Mächler, Bolker and Walker10 All computational code is available at https://github.com/daniel-vader/cdiff-safety-net-hosp.
A priori design considerations
Using a 2:1 case–control matching ratio and assuming an α of 0.05, power of 0.80, and 20% risk factor prevalence, we determined a priori that 221 cases and 442 controls were needed to detect a 50% increase in risk factor prevalence among controls.
Results
Distribution of patient characteristics by case–control status is described in Table 1. Overall, 60.1% of patients were non-Hispanic black, 25.1% were non-Hispanic white, and 4.9% were Hispanic. Comparing controls to cases, the proportion of patients who were white (25.3% vs 24.7%), black (58.6% vs 62.9%), Hispanic (5.2% vs 4.1%), or of another racial or ethnic group (10.8% vs 8.2%) were approximately equal (P = .71). Mean age among cases (58.9 years) and controls (59.5 years) was also similar (P < .19), which was expected because cases and controls were frequency matched by age. Differences were observed by insurance type (P < .01); a greater proportion of controls (52.5%) than cases (35.3%) used private insurance. No association between referral location and infection was indicated (P = .17), with controls and cases having approximately equal proportions of patients referred from home (69.1% vs 68.2%) or acute care or a rehabilitation facility (11.7% vs 12.4%). A smaller percentage of controls had used antibiotics (14.5% vs 35.9%; P < .001) or proton-pump inhibitors (17.9% vs 32.4%; P < .001) in the past 6 months. We detected little difference in mean SDI z score between controls and cases (0.37 vs 0.34; P = .77), suggesting that cases and controls lived in areas with similar levels of deprivation.
Table 1. Distribution of Covariables by Case–Control Status
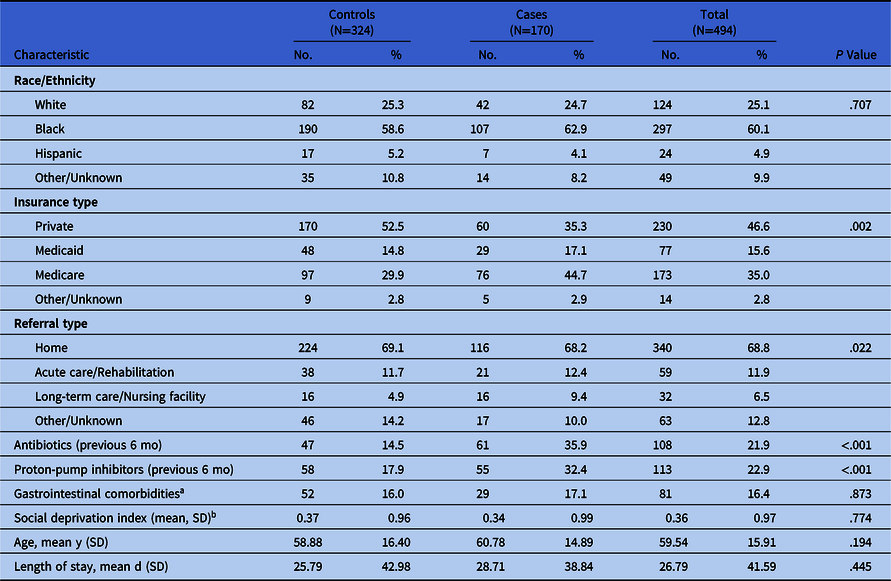
Note. SD, standard deviation.
a Presence of irritable bowel syndrome, Crohn’s disease, ulcerative colitis, or gastroesophageal reflux disease.
b Deprivation index is a z-score calculated at the census tract level and based on all tracts in Philadelphia County.
Figure 1 illustrates cases, controls, and deprivation index by census tract. Cases and controls appear to live in similar areas of Philadelphia, with the greatest density of patients in both groups living just north of HUH. Figure 2 displays the distribution of SDI z score in the general population of Philadelphia and in the study sample. The mean SDI in the sample was 0.36 standard deviations (SD) higher than in the general population.

Fig. 1. Social deprivation index (SDI) and patient count by census tract, Philadelphia County.

Fig. 2. Density plot of deprivation index in the sample and general population of Philadelphia.
Descriptive statistics comparing patients identified as Philadelphia residents to nonresidents are presented in the supplement (Supplementary Table S1 online). A higher proportion of nonresidents were white (57.4% vs 25.1%; P < .001) and, on average, lived in areas with less social deprivation (SDI, −0.12 vs 1.21; P < .001). Differences by insurance type and referral type were also observed.
Considering multivariable models (Table 2), the odds of CDI among black patients were 1.04 times (95% CI, 0.62–1.73) as high as among white patients in a model that included census tract as a random intercept, SDI, insurance type, referral type, antibiotics, and proton-pump inhibitors (model 5). Likewise, no association was indicated between SDI and infection (OR, 1.00; 95% CI, 0.79–1.25). The median odds ratio for census-tract–level variation was 1.22 (95% CI, 0.69–1.68), indicating little evidence of tract-level variation in the outcome. These null associations were consistent across all models.
Table 2. Estimates of Association With Clostridioides difficile Infection by Model
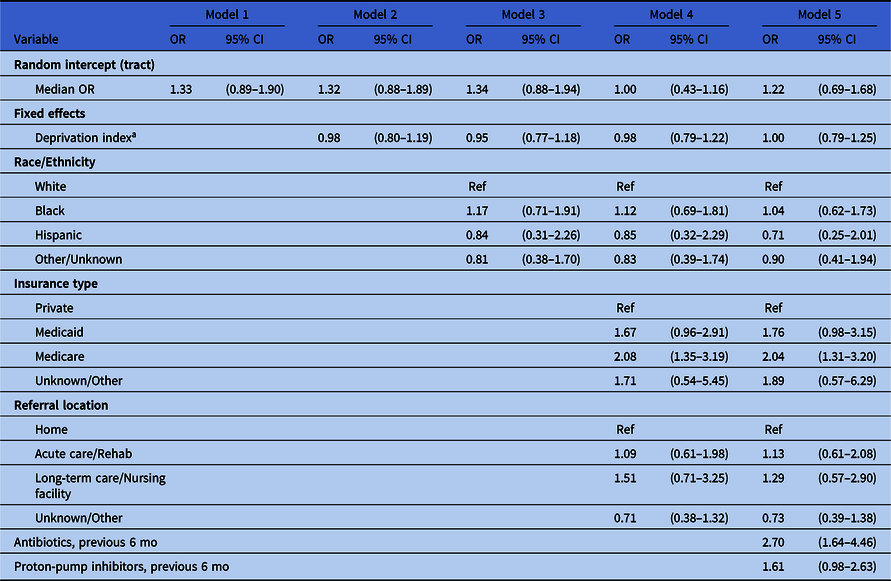
Note. CI, confidence interval.
a Deprivation index was calculated at the census tract level and assigned to patients based on their location of residence.
Patients insured through Medicaid had 1.76 times (95% CI, 0.98–3.15) the odds of having CDI as patients on private insurance. The odds ratio for patients insured through Medicare compared to private insurance was 2.04 (95% CI, 1.31–3.20). Patients who had records of receiving antibiotics in the past 6 months had 2.70 times (95% CI, 1.64–4.46) the odds of CDI as patients who did not, and the odds ratio for those using proton-pump inhibitors was 1.61 (95% CI, 0.98–2.63).
The demographic distribution of cases in national surveillance data compared to the distribution of cases at HUH (along with the populations from which these cases were drawn) are described in Table 3. Differences in the observed and expected distribution were seen by age group and race. At HUH, 48% of cases occurred among patients aged 45–64 years, and 39% occurred among patients aged 65 and older. However, the expected distribution of cases was 27% and 56% respectively. Also, 75% of hospital cases occurred among nonwhite patients, but the expected proportion was only 56%.
Table 3. National, Local, and Expected Local Clostridioides difficile Infection (CDI) Case Distribution by Sex, Age Group, and Race

a Numbers from the 2016 Annual Report from the National Emerging Infections Program.
b Case numbers from patients included in this study. Population numbers calculated using 2018 American Community Survey 5-year estimates for Philadelphia census tracts that contained at least 1 study participant (case or control).
c Expected cases calculated by multiplying the rate observed in national surveillance data by the corresponding local population.
Discussion
HUH was one of the oldest safety-net hospitals in the United States, and individuals from low-income black communities made up a large portion of its patient base prior to its closure in 2019. Also, 60.1% of patients in our sample were black, and only 46.6% were using private insurance. Differences in patient demographics between safety-net and non–safety-net hospitals may also indicate differences in the conditions underlying patient decisions to seek care. Poverty is associated with elevated use of emergency room services at hospitals in the United States, Reference Hunt, Weber, Showstack, Colby and Callaham11 and 91% of 15,572 inpatient admissions at HUH in 2017 started as emergency room visits. Less than a mile away at Jefferson University Hospital, only 49% of 39,973 inpatient admissions started in the emergency room. Reference Brubaker12
Coverage with public verses private insurance is strongly correlated with income in the United States; 71.2% of individuals with an annual household income <$25,000 were covered by public insurance compared to 34.4% of individuals from all income groups. Reference Berchick, Barnett and Upton13 Medicare was associated with CDI at HUH despite controlling for known risk factors like age, length of inpatient stay, prior use of antibiotics, and prior use of proton-pump inhibitors. Reference Badger, Ledeboer, Graham and Edmiston14 Because direct individual-level economic indicators were not available in these data, public insurance may have served as a proxy for those indicators in our analysis.
Because patients living in the HUH catchment were the focus of this study, we chose to exclude patients living outside of Philadelphia from our analysis. An examination of the 2 groups revealed that they came from different socioeconomic contexts. In contrast to city residents, nonresidents were predominantly white, lived in socially advantaged areas, and were less likely to be on Medicaid (Supplementary Table S1 online).
Differences in the occurrence of CDI as reported by the EIP and in our data indicate that descriptive analysis of CDI using national surveillance data may not be transferable to the communities served by safety-net hospitals like HUH. Nearly 50% of cases in our sample occurred among those aged 45–64 years, and 39% occurred among patients aged 65 and older. Given the population served by HUH, the distribution was expected to be 27% and 56%, respectively. No model in our analysis indicated a difference in the odds of CDI between black and white patients, but the national incidence of CDI in 2011 was estimated to be 104.7 per 100,000 white persons and 61.8 per 100,000 nonwhite persons. Reference Lessa, Mu and Bamberg15 These disparities in the rate of infection between white and nonwhite people persist throughout the most recent data from the EIP in 2016. 3
One explanation for the racial disparities observed in national data may be that white people tend to have better access to healthcare, leading to differences in treatment and diagnosis. Reference Mao, Kelly and Machan4,Reference Argamany, Delgado and Reveles16 The use of antibiotics, a cause of hospital-associated CDI, is one pathway through which differences in treatment may lead to differences in the incidence of CDI. Nationally, antibiotics are more frequently used among white people; one study estimated that white people fill twice the number of outpatient antibiotic prescriptions as nonwhite people. Reference Olesen and Grad17,Reference Gahbauer, Gonzales and Guglielmo18 Controlling for antibiotic use in our study had no impact on the estimated association between race or ethnicity and infection, suggesting that differences in prescription and use on the national level may not be equivalent to practice at HUH.
The demographic distribution of CDI at safety-net hospitals may be of particular importance during the ongoing COVID-19 pandemic. In the United States, the same black communities that are disproportionately served by safety-net hospitals like HUH are also disproportionately affected by COVID-19. Reference Abrams and Szefler19 As of June 15, 2020, the Philadelphia Department of Public Health estimates that hospitalization rates were more than twice as high among black compared to white city residents. 20 As a hospital-associated infection, CDI has the potential to exacerbate differences in the impact of the pandemic on these groups. A report on hospital-acquired infection at Christian Hospital in St Louis, Missouri, and Mt Sinai Morningside Hospital in New York, New York, showed a decrease in the overall rate of CDI, potentially due to increased attention to infection control. Reference McMullen, Smith and Rebmann21 However, the report lacked information on the demographic distribution of infection and does not discuss whether CDI surveillance has been affected by the pandemic.
This study has several strengths, including the frequency matching on age and length of inpatient visit, consideration of variation in the occurrence of CDI by census tract of residence, and comparison with national surveillance data using population adjusted estimates of case distribution. One consequence of age-matching cases and controls was that we were unable to investigate the relationship between age and infection at HUH. Although age is an established risk factor for CDI, further examination may have been beneficial given the concentration of cases among patients 45–64 years old. This study also lacked data on individual-level socioeconomic indicators. Although these factors are indirectly measured through census tract-level SDI, it is difficult to tell how representative patients at HUH are of their census tracts. A limitation of hospital-based data is difficulty in defining the population of inference. The reasons why a patient might be treated at one hospital rather than another, particularly when there are multiple options nearby, are not restricted to factors like geography and condition. This limitation is further complicated by the closure of HUH in 2019, meaning that study of this population became impossible after that point. Finally, although we believe that restricting our study population to residents of Philadelphia County allowed us to better define our study population, the exclusion of these patients necessarily led to a loss in power relative to the a priori calculations.
In conclusion, Medicare insurance and prior use of antibiotics were associated with CDI at a safety-net hospital in Philadelphia, but statistical evidence did not indicate an association between infection and race or ethnicity or census tract social deprivation. These findings diverge from the association between race and infection observed in national surveillance data where the rate of infection is higher among white people than among nonwhite people. Furthermore, a greater proportion of CDI cases at HUH were aged <65 years than would be expected based on national data. Descriptive statistics from national surveillance data on CDI may not be transportable to safety-net hospitals, which disproportionately serve low-income nonwhite patients. Further research is needed to examine whether these findings persist at other safety-net hospitals and to investigate the cause of these potential differences.
Acknowledgments
We thank the medical students, residents, and fellows for their assistance with data abstraction and entry, in particular: David Tomajan, Ray Pajarillo, Andrew Quinn, Brian Lin, Jeffrey Kim, Ankit Amin, Bahar Adeli, Grace Decost, Sumedha Singh, Ahn Duc Mai, Cicily Vachaparambil, Billy Zhang, and Sarah Loughran.
Financial support
This project was supported by a sponsored research agreement grant from Pfizer to Drexel University (PI, M. Kutzler; coinvestigators, N. Goldstein, and S. Welles). This project was supported by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS grant no. 1 UB6HP31689-01-00). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS, or the US government.
Conflicts of interests
M. Kutzler receives grant funding, participates in industry collaborations, has received speaking honoraria, and honorarium for reviewing federal research grants at the National Institutes of Health and the US Department of Defense. Dr Kutzler is currently funded through a sponsored research agreement with Pfizer. M. Kutzler has received royalties from patent licensure with Inovio Pharmaceuticals for molecular adjuvants and DNA vaccine antigens that are not reported in this manuscript; thus, these financial relationships do not relate to the content of this manuscript and in no way pose a potential conflict of interest. All other authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.1324








