Nearly 21 million surgical procedures are performed annually in the United States.Reference Steiner, Karaca, Moore, Imshaug and Pickens 1 Nearly 500,000 of these procedures (~2%–3%) are complicated by surgical site infections (SSIs) every year.Reference Anderson 2 SSIs lead to longer hospital stays, Reference Anderson 2 higher rates of unplanned reoperation,Reference Chow, Merkow, Cohen, Bilimoria and Ko 3 higher rates of hospital readmission,Reference Vogel, Dombrovskiy and Lowry 4 – Reference Merkow, Ju and Chung 7 and a 2- to 4-fold higher risk of death.Reference Kirkland, Briggs, Trivette, Wilkinson and Sexton 8 , Reference Kaye, Anderson and Sloane 9 Annually, SSIs are responsible for nearly 10,000 avoidable deathsReference Klevens, Edwards and Richards 10 and $9 billion in excess healthcare costsReference Scott 11 in the United States.
Endogenous microflora cause a substantial number of these SSIs. Preoperative skin antisepsis, which decreases the concentration of bacteria colonizing the surgical field, is an SSI preventative intervention recommended by public health authorities and professional societies.Reference Berríos-Torres, Umscheid and Bratzler 12 – Reference Ban, Minei and Laronga 15 These recommendations call for the use of topical preoperative skin preparations that contain alcohol for immediate cutaneous microbial reduction, combined with another agent with residual antiseptic activity to inhibit microbial regrowth during the surgical procedure.Reference Maiwald and Chan 16 Current evidence does not support the use of one alcohol-containing topical skin antiseptic over anotherReference Maiwald and Widmer 17 ; accordingly, the spectrum of distinct alcohol-containing agents used for skin antisepsis in the United States is small.
ChloraPrep (CP, Becton Dickenson, Franklin Lakes, NJ), 2% chlorhexidine gluconate (CHG) with 70% isopropyl alcohol, is currently one of the most widely used preoperative topical skin preparations in the United States. 18 CHG is active against a wide array of common skin organisms. However, its widespread use may lead to the emergence of clinically significant resistance that can limit its future utility.Reference Madden and Sifri 19 In addition, rare but serious allergic reactions have been reported with antiseptic products containing CHG.Reference Williamson, Carter and Howden 20 Consequently, continued development of new and effective antiseptic formulations is imperative.
We evaluated the antimicrobial efficacy and basic safety profile of a newly developed topical preoperative skin antiseptic ZuraGard (ZG; Zurex Pharma, Middleton, WI), which contains isopropyl alcohol as an active ingredient as well as the functional excipients citric acid, alkyl para-hydroxybenzoates, methylene blue (as a colorant), and purified water.
Methods
Study population
For trial 1 and trial 2, respectively, and prior to the recruitment of study participants, the Gallatin and MicroBiotest institutional review boards reviewed and approved these study protocols. Healthy, adult male and female participants with skin surfaces free of tattoos, dermatoses, abrasions, cuts, lesions or other skin disorders within 15.24 cm (6 inches) of the test site were recruited at 2 US study sites. Potential participants whose bacterial counts were <1.3×103 CFU/cm2 on the abdomen and/or 1.0×105 CFU/cm2 on the inguinal region during pretreatment screening were excluded. Additional exclusions criteria included a history of skin allergies, skin cancer within 15.24 cm (6 inches) of the study site, or any sensitivity to natural rubber latex, adhesive skin products (eg, adhesive dressings, medical tapes), isopropyl alcohol, or any ZG excipients. Participants currently using products containing CHG were also excluded. All female participants of childbearing potential were required to have negative urine pregnancy test results prior to treatment/testing.
Study design
We conducted 2 phase 2 studies in which ZG was compared to CP and/or an alcohol-free ZG vehicle (ZV). Our overall aim was to assess the immediate and persistent activity of ZG against natural bacterial flora on the skin of adult human participants and to compare these outcomes with those obtained with CP as a reference and ZV as a negative control.
On the day of treatment, participants providing informed consent were assigned a unique code which specified the randomly assigned application locus of test solutions used in these studies. Participants underwent sampling to measure baseline cutaneous microbial counts at abdominal and inguinal sites prior to application of study test solutions. In each study, the primary investigator or a designated clinical team member topically applied test solutions per protocol to each test region by scrubbing over a 3.8×12.7-cm (1.5×5-inch) area at inguinal sites (rich in sebaceous glands) and over a 12.7×12.7-cm (5×5-inch) area at abdominal sites (poor in sebaceous glands). Each test site was air dried, and the posttreatment weight of the applicator was recorded. Following application of test solutions, cutaneous samples for culturing were obtained from study skin sites using the time intervals noted below.
In the first phase 2 study (trial 1), the primary outcome measure was a comparison of mean log10 reductions in colony-forming units per cm2 [log10 CFU/cm2]) from baseline for 2 different ZG application times (recommended and abbreviated) versus equivalent ZV and standardized CP application times. Inguinal and abdominal sites on each side of each participant’s body were randomly assigned (1) recommended ZG application times (2-minute inguinal scrub and 30-second abdominal scrub); (2) abbreviated ZG application times (1-minute inguinal scrub and 15-second abdominal scrub); (3) standardized CP application times (2-minute inguinal scrub and 30-second abdominal scrub); or (4) recommended ZV application times (2-minute inguinal scrub and 30-second abdominal scrub). Postapplication cutaneous samples were collected at 10 minutes, 6 hours, and at either 12 or 24 hours after application.
The second phase 2 study (trial 2) was designed similarly with 3 important modifications. First, a negative control was not used, and ZG and CP test solutions were compared directly against each other. Second, the abbreviated 15-second ZG application time was used only for abdominal sites; the recommended 2-minute ZG application time was used for inguinal sites. Third, the antimicrobial effectiveness of the test solutions was assessed as 10-minute responder rates (RR) where RR was the proportion of participants with a ≥2 log10 CFU/cm2 reduction from baseline at the abdominal site and a ≥3 log10 CFU/cm2 reduction from baseline at the inguinal site 10 minutes after test solution application. Bacterial counts at both loci were required to remain below baseline counts 6 hours later. The primary objective was to meet or exceed a 70% RR.
Immediately after the 10-minute skin sample was taken in both studies, skin testing sites on the abdomen or groin targeted for 6-hour, 12-hour, and 24-hour testing were covered with sterile, semiocclusive gauze taped to the skin to cover and protect the test site. All participants were instructed not to remove the dressing, to limit their physical activity, to avoid sweating, and to avoid any potential test site contamination. This procedure is in line with studies that do not house participants for the entire testing period and consistent with preoperative study designs. No washing of the product application sites and no friction with clothing occurred.
Bacterial sampling procedures
All microbial specimens were collected from each skin site in each study by the testing site’s clinical team using a sterile cylinder containing 3.0 mL sterile stripping and suspending fluid with product neutralizers. Once in contact with the skin, the area surrounding the cylinder was massaged to enhance collection of cutaneous flora. All samples were transferred to a sterile counting tube. A second aliquot sample was collected in the same manner immediately afterward. Both were combined and serially diluted in Butterfield’s phosphate buffer containing product neutralizers. Plated cultures were prepared from each of these dilutions on tryptic soy agar with product neutralizers (TSA+) and incubated at 30°C for ~72 hours, or until the appearance of sufficient bacterial growth. Colonies were counted on culture plates by technicians blinded to the sample origin. Cutaneous bacterial counts at baseline and at each postapplication time were recorded for each test area of skin surface in colony-forming units (CFU) per square centimeter (cm2).
Statistical methods
A modified intent to treat (mITT) approach, restricted to participants with baseline cutaneous microbial counts >1.3×103 CFU/cm2 at abdominal sites and >1.0×105 CFU/cm2 at inguinal sites on the day of treatment was used for statistical analysis. Differences in counts between baseline and each programmed postapplication period were calculated as log10 CFU/cm2 data. Descriptive statistics for bacterial count reductions were computed for each sampling site and for each postapplication assessment period using mean, median, standard deviation (SD), and minimum/maximum recovery count. In trial 2, the log recovery counts were converted to binary measures for each participant in the primary analyses to achieve a binomial distribution for RR data. The RR itself was derived from the number of successes at a given time point divided by the number of measurable values for that time point (eg, 18 successes at 24 time points = 75% RR). All results were reported as a net change and 95% confidence intervals (CI) calculated using least-square means. Minitab and the SAS version 8.2 statistical software (SAS Institute, Cary, NC) were used for all statistic calculations.
Safety
Safety was monitored for both studies by cutaneous reactivity at each test site. All local and systemic adverse events (AEs) observed or reported to the investigators were evaluated and followed to resolution along with intensity, duration, and causal relationship to the tested agent. Adhesive reactions as well as acute responses to the sampling techniques were likely and expected and included mild skin irritation and erythema, along with possible allergic reactions.
Results
Overall, 100 participants were enrolled and randomized. Notably, 4 participants enrolled in trial 1 who had previously met the necessary baseline cutaneous microbial count thresholds during the screening visit had microbial counts below these required thresholds on the day of treatment and were excluded from the final analyses. Consequently, 96 participants were included in the final mITT analyses.
Trial 1 participants were 47 males and 17 females aged 18–74 years. Most (ie, 52) were of European-American (white) ethnicity, 1 was African-American (black), 6 were Hispanic, 2 were Asian, and 3 self-identified as “other” ethnicities. Trial 2 participants comprised 20 males and 16 females aged 20–67 years. Furthermore, 20 participants self-identified as European-American, 2 as African-American, 2 as Hispanic, 10 as Asian, and 2 as “other.”
Trial 1
In trial 1, 60 participants were randomized and treated. We report those treated sites which passed baseline entry criteria and were included for analysis. Because the database used was acceptable sites rather than participants, there were frequently a different number of sites for each time point in each trial conducted. As shown in Table 1, 10-minute postapplication cutaneous microbial counts at inguinal sites dropped an average of 2.97 log10 CFU/cm2 following ZG application for the recommended time, and an average of 3.061 log10 CFU/cm2 following the abbreviated (1-minute) ZG application. Average reductions in inguinal counts remained at ≥3.0 log10 CFU/cm2 at 6 hours, 12 hours, and 24 hours after ZG application. Although both applications of ZG produced greater mean reductions in microbial flora than did a 2-minute application of CP, these differences did not achieve statistical significance (Fig. 1).
As shown in Table 2, at the 10-minute postapplication period, cutaneous microbial counts at abdominal sites were reduced by an average of 3.181 log10 CFU/cm2. This period followed the recommended (30-second) ZG application time. A reduction of 2.707 log10 CFU/cm2 followed the abbreviated (15-second) ZG application time. Average reductions in abdominal counts exceeded 2.0 log10 CFU/cm2 at 6 hours, 12 hours, and 24 hours.
Application of the CP test solution resulted in a mean reduction in abdominal cutaneous microbial counts of 2.07 log10 CFU/cm2 after 10 minutes. Although reductions in cutaneous microbial counts were below 2.0 log10 CFU/cm2 12 hours following application, reductions exceeding threshold were observed at 6 hours and 24 hours, respectively. Thus, although average abdominal reductions with ZG exceeded those with CP at all time points, these benchmark differences were not statistically significant (Fig. 2). Application of ZV did not achieve target reductions in cutaneous microbial counts at either the inguinal or abdominal sites.
Table 1. Log10 Reductions from Baseline, Inguinal Site
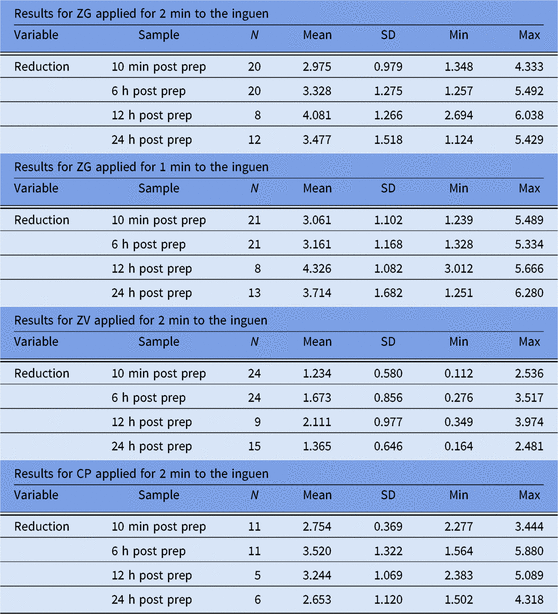
Note. SD, standard deviation; Min, minimum; Max, maximum; ZG, ZuraGard; ZV, ZuraGard vehicle; CP, Chloraprep.
aLog10 reductions from CFU baseline counts assessed 10 minutes, 6 hours, 12 hours, and 24 hours after exposure of the inguinal test area to either ZG, ZG-abbreviated, ZV, and CP in trial 1.
Table 2. Log10 Reductions from Baseline, Abdominal Site a
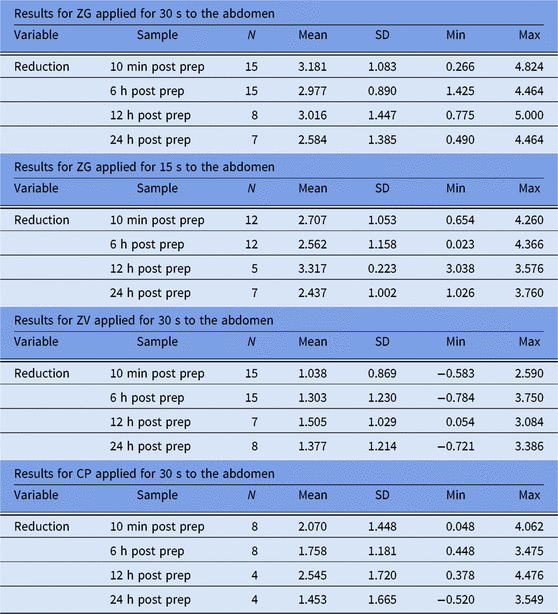
Note. SD, standard devtiation; Min, minimum; Max, maximum ZG, ZuraGard; ZV, ZuraGard Vehicle; CP, Chloraprep.
a Log10 reductions from CFU baseline counts assessed 10 min, 6 h, 12 h, and 24 h after exposure of the abdominal test area to either ZG, ZG-abbreviated, ZV, or CP in trial 1.
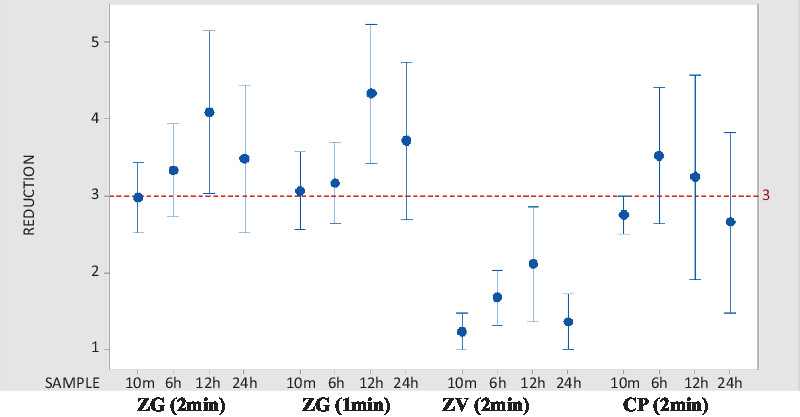
Fig. 1. Comparative interval plot of CFU reductions from baseline at 95% CI for the mean, inguinal site. Log10 CFU/cm2 reductions in CFU from inguinal site baseline are shown. Calculations were made at inguinal site 10 minutes, 6 hours, 12 hours, and 24 hours following application of the active agent. Note. CFU, colony-forming units; CI, confidence interval; ZG, ZuraGard; ZV, ZuraGard vehicle; CP, Chloraprep.
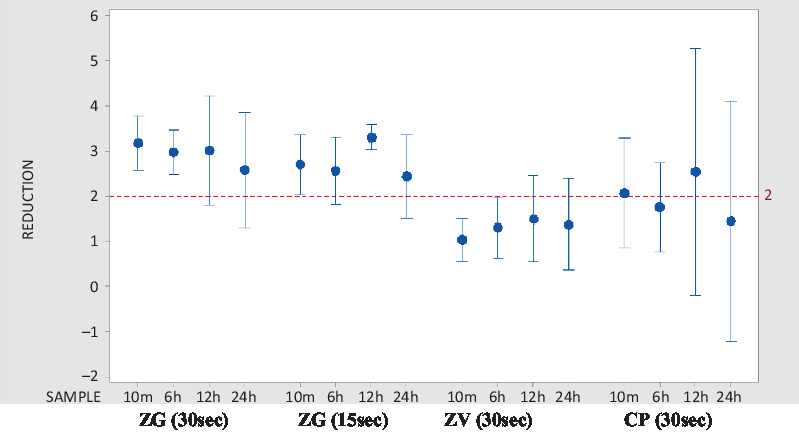
Fig. 2. Comparative interval plot of CFU reductions from baseline at 95% CI for the mean, abdominal site. Log10 CFU/cm2 reductions in CFU from abdominal site baseline are shown. Calculations made at abdominal site 10 minutes, 6 hours, 12 hours, and 24 hours following application of the active agent. Note. CFU, colony-forming units; CI, confidence interval; ZG, ZuraGard; ZV, ZuraGard vehicle; CP, Chloraprep.
No skin irritation or other AEs were observed before baseline, after test agent application, or before sample collection time points for all treated participants.
Trial 2
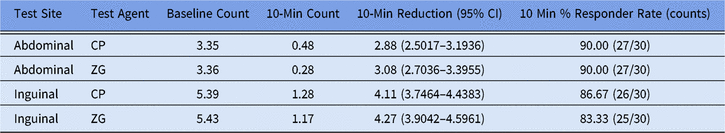
In trial 2, 36 participants were randomized and treated. At the 10-minute sampling period, abbreviated ZG abdominal application times yielded an average reduction in cutaneous microbial counts of 3.1 log10 CFU/cm2 from baseline, although recommended ZG application to inguinal sites yielded an average reduction of 4.3 log10 CFU/cm2 from baseline (Table 3). Standardized abdominal and inguinal CP applications yielded comparative count reductions of 2.9 and 4.1 log10 CFU/cm2, respectively. Both ZG and CP applications exceeded the primary end-point objective of a 70% responder rate (see Table 3). No AEs were reported, and there was no evidence of an increase in cutaneous irritation after application of either test solution.
Table 3. Mean Log10 CFU Counts and Responder Rates at 10 Minutes a
Note. CFU, colony-forming units; CI, confidence interval; ZG, ZuraGard; CP, Chloraprep.
a Mean Log10 CFU counts and responder rates at 10 min as calculated for ZG and CG in both abdominal and inguinal sites at baseline and 10 min after application. Reduction, change, and CI were calculated using least-squares means and are thus slightly different than numerical mean values.
Discussion
Reducing cutaneous bacterial counts at procedural sites is an effective strategy for reducing SSI risk. Existing guidelinesReference Berríos-Torres, Umscheid and Bratzler 12 – Reference Ban, Minei and Laronga 15 recommend the application of alcohol-containing products to prepare the skin prior to any surgical procedure because they act more rapidly than do other aqueous solutions.Reference Maiwald and Chan 16 , 24 Here, we describe the results of 2 phase 2 trials that demonstrate the effectiveness of a novel preoperative antiseptic, ZuraGard, in reducing bacterial counts on the skin of healthy volunteers. ZuraGard reduced microbial counts at both inguinal (≥3 log10 CFU/cm2 reduction) and abdominal (≥2 log10 CFU/cm2 reduction) test sites, caused no observable skin irritation or other AEs, and performed equivalently to CP, currently the most widely used presurgical antiseptic skin preparation in the United States. 18
Perhaps most notably, ZG achieved targeted 10-minute postapplication microbial reductions regardless of whether a 1- or 2-minute application time was employed at inguinal sites or a 15- or 30-second application time was employed at abdominal sites. The reductions in cutaneous bacterial counts following application of ZG were sustained for up to 24 hours, and no skin irritation was observed. These findings may have important practical significance because shorter application times have been correlated with higher levels of staff adherence to surgical site preparation protocols in at least 1 study.Reference El-Othmani, Mahmood, Pearson, Xi, Delfino and Saleh 38
In view of the potential for regrowth of skin bacteria during the surgical procedure, and given the short duration of alcohol-based antiseptic activity, guidelines recommend that agents used for surgical site preparation contain an additional component to promote prolonged residual activity, typically povidone-iodine or CHG. Although CHG may possesses residual activity that is more pronounced than povidone-iodine,Reference Maiwald and Chan 16 , Reference Hibbard, Mulberry and Brady 39 there is insufficient evidence to preferentially support one type of alcohol-containing preparation over another or to suggest that addition of another antimicrobial could contribute to initial efficacy.Reference Maiwald and Widmer 17 , Reference Dumville, McFarlane, Edwards, Lipp, Holmes and Liu 26 Nevertheless, alcohol and CHG combinations have become the preferred surgical site preparation in the United States. 18 There are compelling reasons to pursue the development and evaluation of alternatives to conventional preoperative antiseptics. For example, IgE-mediated anaphylactic/anaphylactoid reactions triggered by CHG exposure, Reference Garvey, Krøigaard and Poulsen 27 although rare, are increasingly described in association with surgical procedures.Reference Krishna, York and Chin 28 Hypersensitivity reactions mediated by other mechanisms are also well known, Reference Abdallah 29 and given the myriad of opportunities for CHG exposure, there is a legitimate concern that the number of surgery patients with an allergy to this agent will increase.Reference Moka, Argyra, Siafaka and Vadalouca 30 Moreover, the burgeoning number of CHG-containing healthcare products, including medical devices, hand soaps, body washes, surgical irrigation products, and oral rinses, raise concerns about the potential for selection and spread of resistance to this particular biocide. Emerging CHG resistance has already been suggested in several outbreaks of healthcare-associated infections in the United States,Reference Horner, Mawer and Wilcox 31 , Reference Kampf 32 and there are indications that high-frequency exposure to sublethal concentrations of CHG may enhance acquired resistance in organisms such as Acinetobacter spp, K. pneumoniae, and Pseudomonas spp, all of which are known for their virulence and adaptability to antibiotics.Reference Kampf 32 Notably, in terms of functional excipients used to support persistence and shelf-life, ZG contains a citric acid and sodium citrate solution as well as trace methyl- and propyl-parabens, all of which exhibit mild antimicrobial properties. Taken together, these findings strongly support the need for continued diversification of our topical antiseptic armamentarium.
In summary, ZG is a novel preoperative skin antiseptic that, in preliminary studies, reduces bacterial contamination of the skin and preforms similarly to CP.Reference Sharp, Green and Rose 21 These results justify larger-scale studies to examine the effectiveness and safety of this product in randomized clinical trials and in more diverse patient populations.
Acknowledgments
Author ORCIDs
Thomas G. Hedberg MSc, PhD 0000-0003-2058-240X
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.







