The transmission of respiratory viruses by droplet or contact routes in healthcare settings is a principal dogma of infection prevention. The transmission-based precautions as illustrated in the recommendations for isolation precautions in hospitals by the Centers for Disease Control and Prevention (CDC) were based on this dogma since 1996.1,Reference Siegel, Emily Rhinehart and Jackson2 However, transmission of respiratory viruses by airborne route has been implicated in community settings over the past decades, including transmission of influenza A in a commercial airlinerReference Moser, Bender, Margolis, Noble, Kendal and Ritter3 or within household,Reference Cowling, Ip and Fang4 and transmission of rhinovirus among game card players.Reference Dick, Jennings, Mink, Wartgow and Inhorn5 Spread of respiratory syncytial virus (RSV) by aerosol was also suggested in the healthcare setting.Reference Kulkarni, Smith, Lee Ddo, Hirst, Easton and O’Callaghan6 During the outbreak of severe acute respiratory syndrome (SARS) in 2003 by SARS coronavirus 1 (SARS-CoV-1), airborne transmission of SARS-CoV-1 was observed in both community and healthcare settings.Reference Yu, Li and Wong7,Reference Booth, Kournikakis and Bastien8 With the emergence of coronavirus disease 2019 (COVID-19) due to SARS coronavirus 2 (SARS-CoV-2), airborne transmission has been increasingly reported in the healthcare and community settings.Reference Birgand, Peiffer-Smadja, Fournier, Kerneis, Lescure and Lucet9–14
Since we have been performing air sampling to detect of SARS-CoV-2 RNA in the airborne infection isolation room (AIIR) of hospitals and community treatment facilities during the COVID-19 pandemic,Reference Cheng, Wong and Chen15–Reference Wong, Leung and Tong18 we would like to know whether air dispersal also occurs in patients infected with common respiratory viruses other than SARS-CoV-2. Here, we performed room air sampling of pediatric and adolescent patients with laboratory-confirmed respiratory viral infection. These findings may have implications in infection prevention and public health measures.
Methods
Setting
This study was conducted in a pediatric ward of Queen Mary Hospital, a 1,700-bed, university-affiliated, teaching hospital in Hong Kong. The pediatric ward contains 28 beds arranged as 6 double-bed AIIRs (room 1–6), 1 single-bed (room 7), and three 5-bed cubicles (room 8–10) without pressure difference between the cubicles and the common area (Fig. 1). The air changes per hour in the AIIRs and the cubicles are 12 and 6, respectively. The temperature and humidity of the AIIRs are set at 22oC and 65%, respectively. The AIIR is prioritized to care for patients aged ≤17 years and infected with pathogens of airborne transmission. Other patients who are aged ≤17 years and with fever and respiratory symptoms will also be admitted through the emergency department to this pediatric ward. This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Hospital Cluster.
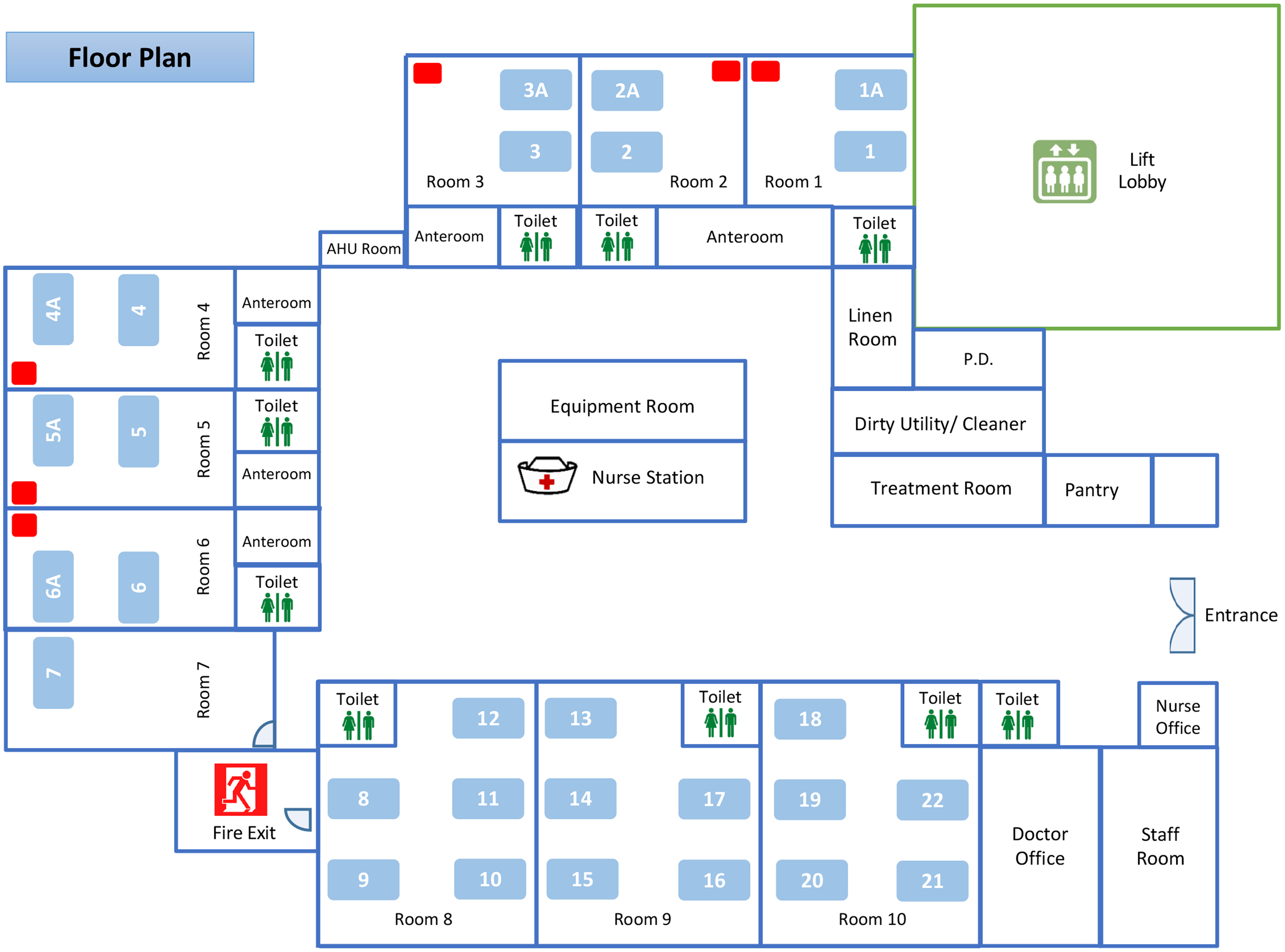
Fig. 1. The floor plan of a pediatric ward of Queen Mary Hospital. Note. The pediatric ward contains 28 beds in 6 double-bed airborne infection isolation rooms (AIIRs) (bed numbers 1, 1A, 2, 2A, 3, 3A, 4, 4A, 5, 5A, 6, and 6A), 1 single-bed room (bed number 7), and three 5-bed cubicles (bed numbers 8–22) without pressure difference between the cubicles and the common area. The air sampler is denoted as a red rectangle placed at the corner of the AIIRs at a distance >2 m from the patient’s head.
Microbiological diagnosis of patients with respiratory symptoms
Upon admission, nasopharyngeal aspirates (NPA) were collected for rapid molecular diagnostic test. The NPA in viral transport medium (VTM) were simultaneously tested for 23 pathogens using the BIOFIRE FILMARRAY Respiratory 2.1 plus Panel (bioMérieux, Marcy l’Étoile, France).19
Collection of air sample from patients with respiratory viral infection
Patients infected by a single virus detected by BIOFIRE FILMARRAY Respiratory 2.1 plus Panel were eligible for this study. Patients with newly diagnosed respiratory viral infection and singly isolated in AIIRs were selected. If >1 eligible patient was available on the day of air sample collection, only 1 patient was chosen at random. In addition, air sampling in double-bed AIIRs with cohort patients was performed. Repeated air samples for the same cohort of patients may be performed to monitor the change in viral load during hospitalization. Verbal consent was obtained from each patient or their parent.
We collected the air samples using an AerosolSense Sampler (Thermo Fisher Scientific, MA) as previously described.Reference Wong, Yuen and Chan17 Briefly, the air sample was collected through an omnidirectional inlet and was directed toward the collection substrate through an accelerating slit impactor at a flow rate of 200 L per minute for 6 hours. Air samples of 72,000 L were collected. The samples were sent to the microbiology laboratory within 30 minutes for further processing. The air sampler was placed at the corner of the AIIR at a distance >2 m from the patient’s head (Fig. 1).
Viral load assessment of air and clinical samples
Upon receiving the air samples, the collection substrate was immersed in 2 mL VTM, and 1 mL medium was used for total nucleic acid extraction using the eMAG extraction system (bioMérieux, Marcy-l’Etoile, France) following the manufacturer’s instructions. Quantifications of viral RNA or DNA in the air samples were performed using in-house real-time reverse transcription polymerase chain reaction (RT-PCR) as previously described.Reference To, Lu and Yip20–Reference Dupouey, Ninove and Ferrier22 The choice of in-house RT-PCR depended on the virological finding of clinical sample.
For the NPA specimens, total nucleic acid extraction was performed using 250 µL of the specimen. RT-PCRs for specific respiratory viruses were performed for viral load assay as described above.
Epidemiological characteristics of patients with air dispersal of respiratory viruses
The demographics, clinical symptoms, presence of underlying diseases, and the medical treatment among patients with or without detectable viral genome by air samples were analyzed. The use of surgical mask by patients during air sample collection was recorded. A case–control analysis was performed to analyze the factors associated with air dispersal of respiratory viruses. Case and control were defined as patients with or without air dispersal of respiratory viruses, respectively.
Statistical analysis
The factors associated with air dispersal of respiratory viruses were analyzed using the Student t test or Fisher’s exact test where appropriate. A 2-sided P value <.05 was considered statistically significant.
Results
Setting
Between December 3, 2021, and January 26, 2022, air sampling was conducted on 30 working days in the pediatric ward for 34 patients. Their NPA revealed parainfluenza virus 3 (PIF3) in 14 patients (41.1%), RSV in 9 patients (26.5%), human rhinovirus/enterovirus in 7 patients (20.6%), and adenovirus in 4 patients (11.8%). Of these 34 patients, 20 patients were singly isolated in AIIRs and another 14 patients shared double-bed AIIRs in which the patients were place near the air supply.
Epidemiological characteristics of patients with air dispersal of respiratory viruses
Of 20 singly isolated patients, 9 (45%) were male. The median age was 30 months (range, 3 months–15 years). Their NPA revealed PIF3 in 10 patients (50%), human rhinovirus/enterovirus in 5 patients (25%), RSV in 3 patients (15%), and adenovirus in 2 patients (10%). Rhinovirus-specific RT-PCR confirmed that the 5 patients with human rhinovirus/enterovirus detection had rhinovirus in their NPA specimens. Of 20 patients, 7 (35%) had air dispersal of the same respiratory viruses (Table 1). None of these 20 patients wore a surgical mask during air sampling. In the case–control analysis, case patients had a significantly higher mean viral load in the NPA than the controls (Table 2). Of 7 patients with air dispersal of respiratory viruses, the mean viral load in the air samples was 1.58 ×103 copies/mL (range, 63–7.60×103 copies/mL).
Table 1. Epidemiological Characteristics of Patients Who Were Singly Isolated in Airborne Infection Isolation Room With Respiratory Tract Infection Associated With Detectable Viral Genome by Air Sampler

Note. NPA, nasopharyngeal aspirates; PIF, parainfluenza virus; RN, running nose; RSV, respiratory syncytial virus; SOB, shortness of breath.
a 72,000 L of air was collected over a 6-h period for each air sample. All patients did not wear surgical mask during air sample collection. During the viral load assay for air samples, the collection substrate was immersed in 2 mL of viral transport medium. Therefore, the viral load in air is expressed as the copy of viral genome per mL of viral transport medium.
b Detectable viral RNA in air.
c Detectable viral DNA in air.
Table 2. Case–Control Analysis of Patients With or Without Air Dispersal of Respiratory Viruses During Respiratory Tract Infection

Note. NPA, nasopharyngeal aspirates; SD, standard deviation; SOB, shortness of breath.
a Units unless otherwise indicated.
b Viral load of NPA ≥ 5 log10 indicates high viral load in the clinical specimens.
c Each patient had one air sample collection during hospitalization.
Of another 14 patients shared double-bed AIIRs, 6 (42.9%) were male. The median age was 15 months (range, 65 days–10 years). These 14 patients were grouped into 7 pairs with the same virological diagnosis in each double-bed AIIR (Table 3). Of these 14 patients, the mean viral load of respiratory viruses in their NPA was 4.64×107 copies/mL (range, 5.33×103 to 1.68×108 copies/mL). Except for a 10-year-old girl with RSV infection, all patients in the double-bed AIIRs did not wear surgical mask during air sampling. All air samples were positive, with a mean viral load of 1.02×104 copies/mL (range, 10–4.99×104 copies/mL). The mean viral load in air samples was significantly higher in AIIRs housing 2 patients than in AIIRs for singly isolated patients (1.02×104 copies/mL vs 1.58×103 copies/mL; P = .020).
Table 3. Epidemiological Characteristics of Patients Under Cohort Nursing in Airborne Infection Isolation Room With Respiratory Tract Infection Associated With Detectable Viral Genome by Air Sampler

Note. AIIR, airborne infection isolation room; CDH, congenital heart disease; DD, developmental delay; NPA, nasopharyngeal aspirates; PIF, parainfluenza virus; RN, running nose; RSV, respiratory syncytial virus; SOB, shortness of breath.
a 72,000 L of air was collected over a 6-h period for each air sample. Except for a 10-year-old girl, all patients did not wear surgical masks during the air sample collection. During the viral load assay for air samples, the collection substrate was immersed in 2 mL viral transport medium. Therefore, the viral load in air is expressed as the copy of viral genome per mL of viral transport medium.
b Detectable viral RNA in air.
c Detectable viral DNA in air.
Discussion
Air dispersal of respiratory viruses including PIF3, RSV, rhinovirus, and adenovirus were documented by the detection of viral load in the 72,000 L of air samples collected inside the AIIRs occupied by patients with symptomatic infections. In addition to the previous reports of airborne transmission of respiratory viruses,Reference Dick, Jennings, Mink, Wartgow and Inhorn5,Reference Kulkarni, Smith, Lee Ddo, Hirst, Easton and O’Callaghan6 air dispersal of PIF3 was also recognized. Instead of collecting the exhaled air from the individual patientsReference Fabian, Brain, Houseman, Gern and Milton23–Reference Leung, Chu and Shiu26 or performing the air sampling in the settings of emergency room or outpatient clinics with various confounding factors in the environment,Reference Tseng, Chang and Li27,Reference Lindsley, Blachere and Davis28 this study is the first to demonstrate air dispersal in singly isolated patients with environmental control of air change, flow, temperature, and humidity in the AIIRs. Of 20 infected patients singly isolated in AIIRs, air dispersal was detected in 35%. The presence of air dispersal was only associated with the viral load in NPA but was not related to demographic characteristics, clinical symptoms, or use of bronchodilator and inhaled corticosteroids among the singly isolated patients. In addition, the mean viral load of respiratory viruses in room air sample was significantly higher in the AIIR caring for 2 patients with the same viral etiology than that in the AIIR caring for a single patient. This finding suggests that the burden of viral load among symptomatic infected cases was associated with the air dispersal of respiratory viruses.
The finding of air dispersal of respiratory viruses may have implications in infection prevention. Given the mean viral load in air samples of 1.58×103 copies/mL among the singly isolated patients, the total number of viral copies was 3.16×103 over a collection time of 6 hours because the collection substrate was immersed in 2 mL VTM. Assuming that the rate of air dispersal of respiratory viruses is static, 9 copies of viral genome were dispersed in the air per minute, which is comparable with the amount of air dispersal of SARS-CoV-2 RNA using the same air sampler in the same setting of AIIR.Reference Wong, Yuen and Chan17 The infectious dose of respiratory viruses demonstrated in human volunteer studies by aerosol exposure varied from 0.68 median tissue culture infectious dose (TCID50) for rhinovirus, to 0.5 TCID50 for adenovirus, to 30–40 TCID50 for RSV.Reference Karimzadeh, Bhopal and Nguyen Tien29 Using the correlation of 1 TCID50 to 103 copies/mL,Reference Lam, Leung and Zhang30 we estimated the infectious doses of rhinovirus (6.8×102 copies/mL), adenovirus (5.0×102 copies/mL), and RSV (3.0×104 copies/mL). Considering the air sampling collection for 6 hours in AIIR, we translated the infectious dose in the room air in the AIIR to the number of viral copies for rhinovirus (1.36×103 copies), adenovirus (1.0×103 copies), and RSV (6.0×104 copies). Based on these findings, the maximum viral copies detected in 72,000 L of air in 6 hours in the AIIRs singly isolated for RSV was 1.24×103 copies, which may be lower than the infectious dose. However, our study was conducted in the AIIRs with 12 air changes per hour, which may have facilitated the dilution of infectious virus-laden particle in the air. Presumably, the viral copies in the air may be double in the general ward setting, which has 6 air changes per hour. Thus, outbreaks of respiratory viruses would be very common in general ward during the winter season when respiratory viruses are highly prevalent in patients with mild or no symptoms. Further investigation is needed to understand the degree of air dispersal of viral genome in the general ward setting with air ventilation of 6 air changes per hour, as well as in the community setting with poor indoor air dilution, which is also a risk factor for SARS-CoV-2 transmission.Reference Wong, Chen, Lung, Ho, Yuen and Cheng31,Reference Wong, Au and Chen32 Although the clinical significance of airborne transmission of respiratory viruses other than SARS-CoV-2 remains to be determined, the enforcement of infection control practice in the hospitals, including hand hygiene and universal masking, has successfully prevented nosocomial transmission of respiratory viruses and SARS-CoV-2 before the emergence of the omicron BA.2 variant.Reference Wong, AuYeung and Lam33,Reference Wong, Lam and AuYeung34
This study had several limitations. We did not perform viral culture of the air samples. The demonstration of viral DNA or RNA may not correlate with the presence or level of viable virus. These sophisticated experiments have been performed in the investigation of airborne transmission of RSV.Reference Kulkarni, Smith, Lee Ddo, Hirst, Easton and O’Callaghan6 We could not include influenza A virus in this study because universal masking and enhancement of hand hygiene practice likely minimized the influenza activity in both community and hospital settings since the outbreak of COVID-19.Reference Wong, Lam and AuYeung34,Reference Cheng, Wong and Chuang35 The relative location of patients to the air sampler may have varied over the sampling time; some of our patients were pediatric cases who may have moved around in the bed. The time lag from the collection of NPA to air samples may affect the correlation of viral loads between the clinical and air samples. In addition, our study was not adequately powered to measure all factors associated with air dispersal of respiratory viruses. However, given the small sample size, our findings clearly demonstrate that the viral load of the patient is an important factor. Further study to investigate the phenomenon of air dispersal of respiratory viruses is warranted.
Acknowledgment
We thank the frontline staff of pediatric ward of Queen Mary Hospital for facilitating this study.
Financial support
This study was supported by the Health and Medical Research Fund (HMRF) Commissioned Research on Control of Infectious Disease (Phase IV), CID-HKU1-16, Food and Health Bureau, Hong Kong SAR Government.
Conflict of interest
All authors report no conflicts of interest relevant to this article.







