1. Introduction
The earliest Eocene is marked worldwide by a well-documented positive temperature peak coined the Palaeocene–Eocene Thermal Maximum (PETM) (McInerney & Wing, Reference McInerney and Wing2011), followed by additional, more progressive warming events, all together forming the Early Eocene hyperthermals (Zachos et al. Reference Zachos, McCarren, Murphy, Röhl and Westerhold2010). This ‘climatic optimum’ is known to coincide with the emergence of several mammal orders (Bowen et al. Reference Bowen, Clyde, Koch, Ting, Alroy, Tsubamoto, Wang and Wang2002; Gingerich, Reference Gingerich2006), and is thought to have been accompanied by a wide latitudinal expansion of intertropical and tropical biomes (McInerney & Wing, Reference McInerney and Wing2011). The extent of this expansion, however, is inconsistently supported, with conflict mainly arising between palaeobotanical and palaeoclimatic modelling evidence (Kiehl & Shields, Reference Kiehl and Shields2013; Herold et al. Reference Herold, Buzan, Seton, Goldner, Green, Müller, Markwick and Huber2014; Willard et al. Reference Willard, Donders, Reichgelt, Greenwood, Sangiorgi, Peterse, Nierop, Frieling, Schouten and Sluijs2019; Huurdeman et al. Reference Huurdeman, Frieling, Reichgelt, Bijl, Bohaty, Holdgate, Gallagher, Peterse, Greenwood and Pross2021). Direct palaeoentomological evidence for the PETM, on the other hand, has been scarce and relatively under-utilized compared to other palaeontological sources in constraining palaeobiomes of the earliest Eocene.
In this context, Oise amber from the Parisian Basin, France, proves to be an invaluable palaeontological resource, not only because of the exceptional quality and abundance of its fossils (over 21,000 arthropod inclusions; Nel & Brasero, Reference Nel, Brasero and Penney2010), but because it constitutes a direct window into the earliest Eocene (Nel et al. Reference Nel, de Plöeg, Dejax, Dutheil, de Franceschi, Gheerbrant, Godinot, Hervet, Menier, Augé, Bignot, Cavagnetto, Duffaud, Gaudant, Hua, Jpssang, de Lapparent de Broin, Pozzi, Paicheler, Beuchet and Rage1999; Bowen et al. Reference Bowen, Maibauer, Kraus, Röhl, Westerhold, Steimke, Gingerich, Wing and Clyde2015). Although detailed work on the myrmecofauna of this assemblage remains largely unpublished, preliminary insights have shown that Oise amber preserved the first occurrence of many modern ant genera (Aria, Reference Aria2010; Aria et al. Reference Aria, Perrichot and Nel2011), before their occurrences in Late Eocene and Miocene ambers, and coinciding with the documented latitudinal extension of tropical and intertropical biomes (Wing et al. Reference Wing, Harrington, Smith, Bloch, Boyer and Freeman2005; Willard et al. Reference Willard, Donders, Reichgelt, Greenwood, Sangiorgi, Peterse, Nierop, Frieling, Schouten and Sluijs2019). A tropical forest is indeed suggested for the Oise amber palaeohabitat, as the resin-producing tree and resin chemistry show affinities with extant members of the Fabaceae, specifically the intertropical genus Daniellia (de Franceschi & de Plöeg, Reference de Franceschi and de Plöeg2003; Jossang et al. Reference Jossang, Bel-Kassaoui, Jossang, Seuleiman and Nel2008; Nohra et al. Reference Nohra, Perrichot, Jeanneau, Le Pollès and Azar2015). Here we describe a new fossil Gesomyrmex species, and we show its relevance in retracing the evolution of biome distribution during the Cenozoic.
2. Material and methods
2.a. Fossil material
The new species described here is based on four specimens preserved each in distinct pieces of amber that originate from a lowermost Eocene (Ypresian) stratum dated c. 55/52 Ma, i.e. equivalent to the Sparnacian level MP7 of the mammal fauna of Dormaal (Nel et al. Reference Nel, de Plöeg, Dejax, Dutheil, de Franceschi, Gheerbrant, Godinot, Hervet, Menier, Augé, Bignot, Cavagnetto, Duffaud, Gaudant, Hua, Jpssang, de Lapparent de Broin, Pozzi, Paicheler, Beuchet and Rage1999; de Franceschi & de Plöeg, Reference de Franceschi and de Plöeg2003; Nel & Brasero, Reference Nel, Brasero and Penney2010), and excavated at the Le Quesnoy farm in Chevrière, near Creil, Oise department, northern France. Oise amber comprises more than 400 ants representing at least 40 morphotypes among six subfamilies (Aria, Reference Aria2010; Aria et al. Reference Aria, Perrichot and Nel2011). The subfamily Formicinae, to which the fossils examined herein belong, is represented by 16 morphotypes and 73 specimens (Aria, Reference Aria2010; CA, pers. obs.).
2.b. Microphotography
The amber pieces containing the studied specimens were polished to remove the weathered opaque surface, and some fragments were either embedded in Canada balsam between microscopic slides, or immersed into water or 60/70 % glucose solutions to minimize light scattering during the observations and image capture. Several stereomicroscopes were used for examination, magnifying up to ×200. Photographs were taken with a Nikon D800 camera coupled with a Nikon SMZ25 stereomicroscope, or a Canon 5D Mark II camera coupled to a Leica MZ APO stereomicroscope, and image stacks were merged using Helicon Focus 6.7 software (Helicon Soft Ltd.). The figures were composed with Adobe Illustrator CC2019 and Photoshop CC2019 software.
2.c. Repositories
The primary material of Oise amber is housed in the palaeontological collections of the Museum National d’Histoire Naturelle (MNHN), Paris, France. Reference was also made to AntWeb (https://www.antweb.org, version 8.81, last accessed 18 October 2022) and the following amber collections for comparison with extinct and extant species: JCW – Jörg Wunderlich collection, Hirschberg, Germany. NHMW – Naturhistorisches Museum Wien, Vienna, Austria.
2.d. Morphometric analyses
Data was gathered from Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009, Reference Dlussky, Rasnitsyn and Perfilieva2015), then handled and displayed graphically with R (R Core Team, 2022).
2.e. Acronyms
Acronyms for measurements (all in mm) and indices are as follows. The first four measurements were taken in the same plane, that is, with head in full face view. HL: head length, in a straight line from the midpoint of anterior clypeal margin to the midpoint of the occipital margin; HW: maximum head width measured behind the eyes; MFC: maximum distance between frontal carinae; SL: the maximum straight-line length of the scape, excluding radicle; ED: maximum diameter of eye as measured in lateral view of the head to show full surface of eye; AL: mesosoma length in dorsal view, from juncture with the petiole to anterior border of pronotum; PW: maximum width of pronotum in dorsal view; PtL: length of petiole in lateral view; PtH: height of petiole in lateral view; FL: length of profemur, measured along its long axis in posterior view; CI: cephalic index (HW/HL × 100); FCI: frontal carinae index (MFC/HW × 100); SI: scape index (SL/HW × 100); OI: ocular index (ED/HL × 100); PtI: petiolar index (PtH/PtL × 100); FI: profemur index (FL/AL × 100); AI: mesosoma index (PW/AL × 100).
3. Results
3.a. Fossil description (systematic palaeontology)
Order Hymenoptera Linnaeus 1758
Family Formicidae Latreille 1809
Subfamily Formicinae Latreille 1809
Genus Gesomyrmex Mayr (Reference Mayr1868)
Type species. Gesomyrmex hoernesi Mayr (Reference Mayr1868: 52), pl. 2, figs 38–41.
Gesomyrmex gallicus Aria, Jouault, Perrichot, and Nel, sp. nov.
LSID: urn:lsid:zoobank.org:act:4463CCAB-BDBF-4E1B-AE2D-5C11E0EEB99D.

Fig. 1. Gesomyrmex gallicus sp. nov. (a, b), Holotype MNHN.F.A32941, minor worker: (a) habitus in left lateral view; (b) habitus in ventral view. (c, d) Paratype MNHN.F.A32938, major worker: (c) habitus in dorsal view; (d) head in dorsal view. (e–g) Paratype MNHN.F.A32939, minor worker: (e) habitus in dorsal view; (f) habitus in ventral view; (g) head in frontal view. Scale bars: 1 mm (a–e, f), 0.25 mm (g).

Fig. 2. Line drawing of Gesomyrmex gallicus sp. nov., holotype MNHN.F.A32941 (minor worker (a, b)) and paratype MNHN.F.A32938 (major worker (c)). (a) Habitus in left lateral view; (b, c) heads of minor and major workers. Scale bars: 0.5 mm.
Material examined. Holotype MNHN.F.A32941 (minor worker), paratypes MNHN.F.A32938 (major worker), MNHN.F.A32939 (minor worker), MNHN.F.A32940 (minor worker).
Etymology. From the Latin gallicus, meaning ‘Gallic’ (from Gaul).
Locality and horizon. Le Quesnoy farm, commune of Chevrière, near Creil, department of Oise, France; Lower Eocene (Ypresian), level MP7 of the mammal fauna of Dormaal.
Diagnosis (workers). The new species is readily distinguishable from all extant and extinct species by its petiolar node with dorsal margin acute instead of rounded in profile view; and from all species except the extinct G. hoernesi by the masticatory margin of mandibles with nine teeth. It differs from the latter by its external mandibular margins (sensu Richter et al. (Reference Richter, Keller, Rosumek, Economo, Hita Garcia and Beutel2019)) straight to feebly convex in full-face view (medially concave in G. hoernesi), and the petiolar node with dorsal margin medially concave (flat or weakly convex in G. hoernesi). See also ‘Remarks’ below.
Description. Minor worker (Figs 1a, b, e–g, 2a, b, 3d–f, h, k; Supp. Fig. 1). Body 2.95 mm long, head slightly longer than broad, trapezoid-shaped, with convex sides, round posterior corners and tenuously concave posterior margin. Large and elongate eyes with straight lateral borders, somewhat more than 0.5 times the size of the head, converging anteriorly. Malar area and cheeks short. Small but distinct ocelli. Frontal and clypeal areas smooth but finely laminated antero-dorsally. Frontal triangle neat. Frontal carinae poorly visible, slightly bulging, diverging and ending on indistinct, tenuous medial torular arches covering half of condylar bulbs. Clypeus slightly bulged, projecting in an anteriorly long, rounded lobe, covering almost half of the mandibles’ length; long, decussate mandibles with eight nearly homodont teeth and a slightly longer apical tooth, whose extremity strongly curves downward; masticatory margin with setae; in full-face view, mandibular external margins straight to feebly convex. Short, incrassate, antenna covered with setae, with eight antennomeres, ending in a proto-club formed by apical two flagellomeres, apicalmost flagellomere longest (twice as long as broad); scape approximately as long as eye; palp formula 2/4, labial and maxillary palps short, covered with minute setae.

Fig. 3. Comparative illustration of diagnostic characters for Gesomyrmex gallicus sp. nov. (a–f) Dorsal view of mandibles in minor workers of Gesomyrmex (black arrows in (b, c) point to the concave external margin): (a) G. luzonensis (specimen ANTWEB1008527); (b) G. hoernesi (specimen FANTWEB00075, JCW coll.); (c) G. hoernesi (specimen FANTWEB00009, NHMW coll.); (d–f) G. gallicus sp. nov.: (d) paratype MNHN.F.A32938; (e) holotype MNHN.F.A32941; (f) paratype MNHN.F.A32939. (g–l), Dorsal and lateral views of petiole in minor workers of Gesomyrmex: (g, j) G. luzonensis (specimen ANTWEB1008527); (h, k) G. gallicus (holotype MNHN.F.A32941); (i, l) G. hoernesi (specimen FANTWEB00075, JCW coll.). Scale bars: 0.1 mm.
Mesosoma rather elongate but small in comparison to head size, punctate. Promesonotal articulation distinct, assumed inflexible. Constriction in posterior half-part of mesonotum; prominent, round metathoracic spiracles located at posterodorsal corners of mesonotum, just anterior to mesopropodeal margin. Propodeum short, with rather straight dorsum and slightly concave declivity subequal in length. Metapleural gland large, oriented latero-posteriorly. Short, rather slender legs, with sparse setae on apical tibial part and tarsi. Fore tibia short. Hind leg with a tiny tibial spur. Pretarsal claws without pre-apical tooth but with distinct, medial seta; arolium massive.
Squamiform, unpedunculate petiole; in profile view, petiolar node with anterior and posterior faces convex, meeting at acute angle dorsally; in posterodorsal view, dorsal margin slightly concave medially; inserted at ventral base of helcium. Gaster elongate, elliptical, mostly glabrous except scarce setae ventrally and on pygidium. Four distinct tergo-sternal complexes. Acidopore well developed.
Major worker (Figs 1c, d, 2c; Supp. Fig. 2). Blackish body, 4.7 mm long, flattened dorso-ventrally, gaster elongate. Head subrectangular and glabrous, except mandibles and a few scarce and minute white setae on top; occipital margin rather straight, with round corners; dorsal and lateral head cuticular sculpture consisting of thin parallel lines of striation, eventually diverging on clypeus. Apical tooth of mandible as long as second one, so that masticatory margin ends in a double-toothed complex; remaining visible part of masticatory margin damaged; long setae on ventral surface of mandibles. Presence of mandibular lobes above clypeo-mandibular joints. Clypeus very large, smooth; its anterior margin straight, slightly wrinkled medially; posterior clypeal margin narrow, surmounted by a small frontal triangle. Frontal carinae almost flat, of convex shape and strongly diverging on either lateral margin of clypeus, ending in slight laterally oriented prominences. Medial torular arches assimilate with tip of frontal carinae, situated near angles formed by lateral and anterior clypeal margins; condylar bulbs of antennae covered in great part by frontal carinae/lobes. Eight antennomeres; scape short, not reaching posterior eye margin, less than half as long as head capsule (excluding mandibles); funiculus inversely incrassate, antepenultimate flagellomere as long as broad, apical flagellomere distinctly largest. Eyes very elongate, twice as long as wide, placed rather anteriorly, covering nearly one-third of head length. Ocelli absent. Palp formula: 4,2; palp segments scarcely pubescent, approximately of equal length.
Mesosoma glabrous, shagreened, globally flat. Promesotonal and mesopropodeal articulations visible but supposedly inflexible. Propodeal spiracle large, with thick border, placed on lateral top of a conspicuous and bulged metapleuron ending with metapleural gland opening as large as the spiracle and bordered with short setae; latero-ventral extremities of propodeum form prominent and large, circular, awning-like structures covering hind coxa with rather long, marginal setae. Slight constriction in posterior half-part of mesonotum. Legs rather short, almost glabrous; tarsomeres 1–4 of all legs with —two to three apicoventral setae inserted on each side; protibial spur ogive-shaped; metatibial spur tiny; heavy protarsal pubescence, initiated just above insertion of spur; pretarsal claws simple, with a medial distinct setae; arolium massive.
Petiole sub-nodiform, in lateral view with anterior face strongly convex, dorsal margin acutely angled, posterior face weakly convex; in posterodorsal view with dorsal margin slightly concave medially; petiole without tergosternal fusion. Gastral tergites extending latero-ventrally. Short and scattered setae on ventral face of tergosternites I, II and III. Pygidium and hypopygium covered with long, thick hairs. Acidopore distinct.
Measurements. Minor worker (holotype in brackets). BL 2.5 (2.95) HL 0.75–0.81 (0.92), HW 0.62–0.66 (0.72), EL 0.43–0.43 (0.45), MFC (0.36), SL 0.43 (0.40), ED 0.17–0.2 (0.18), AL 0.9 (0.95), PW 0.4–0.4 (0.52), PtL 0.17–0.18 (0.17), PtH 0.28 (0.30), FL 0.5 (0.53), CI 76–82 (78), FCI (50), SI (55), OI 21–222 (19), PtI 164 (176), FI 55 (55), AI 0.44 (54). Major worker. BL 4.7, HL 1.10, HW 0.98, EL 0.51, MFC 0.38, SL 0.50, ED 0.23, AL 1.45, PW 0.60, PtL 0.30, PtH 0.40, FL 0.6, CI 0.89, FCI 50, SI 51, OI 20, PtI 113, FI 41, AI 41.
Remarks. Workers of G. gallicus sp. nov. share a diagnostic nine-toothed mandible with G. hoernesi, contrasting with the five, six or eight teeth in workers of extant species (when known), but differs from G. hoernesi in its straight to slightly convex external mandibular margins (vs medially concave), which is more similar to extant species (Fig. 3). Gesomyrmex gallicus sp. nov. also differs from G. hoernesi and all extant species in its petiolar node with dorsal margin angled between anterior and posterior faces (vs rounded), and from G. hoernesi, G. chaperi, G. howardi or G. luzonensis in its petiolar node with dorsal margin emarginate medially (vs flat or slightly convex) (Fig. 3). Based on the findings by Peeters et al. (Reference Peeters, Ito, Wiwatwitaya, Keller, Hashim and Molet2017), we assign the new larger morph to a soldier cast. A ‘supersoldier’ would be characteristically defined by a sub-rectangular head and relatively smaller eyes. Additionally, the new species differs from all other known Gesomyrmex species by its temporal range. Théobald (Reference Théobald1937) described a series of specimens from Eocene–Oligocene rock-imprints of Kleinkems (Germany) he assigned to the genus Gesomyrmex, but this type material was subsequently lost and the description precludes their confident placement within this genus. The alate specimen illustrated by Théobald (Reference Théobald1937: pl. XIV, fig. 22) has a wing venation strikingly different from that of the other representatives of the genus, inter alia because of the absence of the vein Rs distad pterostigma and of the small rhomboidal along Rs+M. Therefore, the species is considered as incertae sedis in Formicidae (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009). Species erected based on gynes from Eocene and Miocene compression deposits (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009, Reference Dlussky, Rasnitsyn and Perfilieva2015) also require a critical reappraisal, developed hereafter.
2.b. Taxonomy and morphometrics of fossil Gesomyrmex gynes
In order to assess problematic fossil species based on gynes from compression Lagerstätten (see Discussion below), we explored available morphometric data, especially those related to head shape (HR) and relative eye diameter (HER), because they were used as comparative criteria (see Dataset 1). Sorting the data based on head proportions (head length/head width) reveals different groupings among these species (Fig. 4). Gesomyrmex hoernesi, G. curiosus and G. incertus display similar HER in correlation to an overall increase in head length. In the absence of compelling diagnostic features for both G. curiosus and G. incertus, these taxa should be considered possible hoernesi. Gesomyrmex germanicus and G. macrops have similar head lengths and shapes, and likewise have not been properly diagnosed. We tentatively offer a diagnosis of G. germanicus based on morphometric values, and consider G. macrops to be G. cf. germanicus. Gesomyrmex breviceps, G. flavescens and G. pulcher have relatively disparate morphometrics and are not easily distinguished, also based on a lack of original diagnoses. We place these species as species inquirendae into Gesomyrmex sp. Gesomyrmex magnus can be safely diagnosed based on its much larger size compared to all other gyne-based taxa, as well as G. hoernesi and extant species, as originally stated (Dlussky et al. Reference Dlussky, Rasnitsyn and Perfilieva2015). Gesomyrmex bremii (originally described as Myrmica bremii, Heer, Reference Heer1849) (Dlussky & Putyatina, Reference Dlussky and Putyatina2014) does not preserve the eyes and therefore was not included in our analysis; however, the diagnosis provided by Dlussky and Putyatina is insufficient and the specimen too incomplete to justify erection of a new species.
Gesomyrmex cf. hoernesi Mayr (Reference Mayr1868)
Gesomyrmex curiosus Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009), syn. nov.
Remark. No formal diagnosis. Only one head in frontal view is preserved. The only comparative comment is: ‘[the head] is very different from all other fossil and living species’. From Messel.
Gesomyrmex incertus Dlussky et al. (Reference Dlussky, Rasnitsyn and Perfilieva2015), syn. nov.
Remark. No formal diagnosis. Comparative note is ‘Differs from all known species of Gesomyrmex by combination of elongate head with concave occipital margin and position of eyes’. The diagnostic value of the elongate head is not ascertained by our morphometric investigation (see Dataset 1 and Fig. 4), and other characters are questionable based on taphonomy. From Svetlovodnaya.

Fig. 4. Cephalic morphometrics of gynes for relevant fossil Gesomyrmex species. (a) Head length (HL). (b) Normalized head shape (head length/head width, HRN, squares) and normalized relative eye diameter (eye diameter/head length, HERN, diamonds). Abbreviations: flav., flavescens; germ., germanicus; hoern., hoernesi.
Gesomyrmex germanicus Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009)
Diagnosis. Head shape (head length/head width): 1.30 ± 0.1, Relative eye diameter (head length/eye diameter): 0.32 ± 0.01.
Remark. No original formal diagnosis. No original comparative comments. The species was erected based on its Eocene age, without comparison to extant taxa. From Eckfeld.
Gesomyrmex cf. germanicus Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009)
Gesomyrmex macrops Dlussky et al. (Reference Dlussky, Rasnitsyn and Perfilieva2015), species inquirenda
Remark. No formal diagnosis. Differentiated from other Svetlovodnaya species by the eye size, but from G. germanicus and G. pulcher by the shape of head margins; however, the latter are taphonomically deformed in these compression fossils and not reliable. From Svetlovodnaya.
Gesomyrmex sp. Mayr (Reference Mayr1868)
Gesomyrmex flavescens Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009), species inquirenda
Remark. No formal diagnosis and no comparative comments, only key of determination. According to Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009), the diagnostic character for this taxon is said to be a straight anterior clypeal margin, but this trait is ambiguous in the fossil specimen. From Eckfeld.
Gesomyrmex pulcher Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009), species inquirenda
Remark. No formal diagnosis, no comparative comments, only key of determination. Defining traits based on head shape and colour, which are unreliable in these fossils. From Messel.
Gesomyrmex breviceps Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009), species inquirenda
Remark. No formal diagnosis, no comparative comments, only key of determination. Head seemingly relatively short in specimen, but fossil is poorly preserved and taphonomic sample is limited. From Messel.
Gesomyrmex bremii Heer (Reference Heer1849), species inquirenda
Remark. Diagnosed by Dlussky and Putyatina (Reference Dlussky and Putyatina2014) based on ‘head longer than in G. flavescens (1.2 times as long as wide in G. flavescens and 1.4 times in G. bremii)’. Head length alone is not diagnostic given the overall data (see Dataset 1 and Fig. 4). From Radoboj.
Gesomyrmex magnus Dlussky et al. (Reference Dlussky, Rasnitsyn and Perfilieva2015)
Diagnosis. Body length c. 20 mm.
Remark. No original formal diagnosis, but comparative comment based on overall size.
4. Discussion
4.a. Identification as Gesomyrmex and current status of the genus
Gesomyrmex encompasses today only seven species, all endemic to SE Asian rainforests, north of the Wallace line (Antmaps.org, Janicki et al. Reference Janicki, Narula, Ziegler, Guénard and Economo2016). The genus was in fact first described by Mayr (Reference Mayr1868) from Baltic amber fossils, assigned to Gesomyrmex hoernesi, some 24 years before the first extant species, Gesomyrmex chaperi André, Reference André1892, was published. Wheeler later provided a more thorough description of this species in his monograph on the ants of the Baltic amber (Wheeler, Reference Wheeler1915), and synonymized Dimorphomyrmex André, Reference André1892 with Gesomyrmex (Wheeler, Reference Wheeler1929 b), noting remarkable polymorphism among workers. Dlussky and colleagues (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009, Reference Dlussky, Rasnitsyn and Perfilieva2015) more recently raised the number of fossil species to eleven, following the description of eight new gynes preserved as compression fossils from Eocene limestones of Germany, Croatia and Russia (Table 1).
Table 1. Diversity and distribution of extinct Gesomyrmex species

A set of morphological characteristics atypical among formicine workers allows for a straightforward identification of Gesomyrmex: eyes reniform and massive relative to head size; short, eight-segmented antennae inserted far anterior on the head, so that the scapes pass below the eyes in their regular resting position; long, triangular, decussate mandibles in at least minor workers; and a large clypeus rounded anteriorly and projecting over the base of the mandibles (Fig. 2). Distinctions among fossil morphotypes based on body size, anterior clypeal margin and occipital cephalic margin support in general a radiation of the genus during the early Cenozoic, as documented by previous authors. However, species erected based on dubious characters from compression fossils, such as colour or ‘head shape’ (prone to taphonomic deformation and changes due to orientation of entombment in these fossils) (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009, Reference Dlussky, Rasnitsyn and Perfilieva2015), do suggest some overestimation in palaeodiversity. Generic apomorphies are also missing or concealed in certain cases, such as in G. flavescens Dlussky et al. (Reference Dlussky, Wappler and Wedmann2009), questioning the affinity of these fossils with Gesomyrmex—see above.
Two fossil species of Gesomyrmex were described from the Oligocene of Germany based on incomplete compressions (Théobald, Reference Théobald1937) but were excluded from this genus by Dlussky et al. (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009): G. expectans Théobald (Reference Théobald1937) was transferred to Eoformica Cockerell, Reference Cockerell1921; and G. miegi Théobald, Reference Théobald1937 was treated as incertae sedis in Formicidae (Table 1).
2.b. Known ecology and lifestyle, and significance for a fossil-inclusive phylogeny
Although our understanding of the ecology and biology of Gesomyrmex remains scarce, observations have been made by some authors pointing to a diurnal arboreal lifestyle (reviews in Dlussky et al. Reference Dlussky, Wappler and Wedmann2009; Peeters et al. Reference Peeters, Ito, Wiwatwitaya, Keller, Hashim and Molet2017). Gesomyrmex typically nests in both live and dead stems or branches, co-opting tunnels burrowed by other insects or digging their own (Peeters et al. Reference Peeters, Ito, Wiwatwitaya, Keller, Hashim and Molet2017). Colonies were initially reported to have a single queen and relatively few individuals (150 in one of the nests studied), whereas a more recent study found them to be polydomous (Peeters et al. Reference Peeters, Ito, Wiwatwitaya, Keller, Hashim and Molet2017). Likewise, the worker population has initially been considered to encompass a broad polymorphism with morphological overlaps, but would instead be characterized by a subdivision into three distinct asexual castes including supersoldiers (Peeters et al. Reference Peeters, Ito, Wiwatwitaya, Keller, Hashim and Molet2017). Soldier morphs had been already recognized in G. hoernesi and in some modern species in which asexual individuals closely resemble their gynes (Bolton, Reference Bolton1994; Dubivikoff, Reference Dubivikoff2004; Dlussky et al. Reference Dlussky, Wappler and Wedmann2009). These recent findings (Peeters et al. Reference Peeters, Ito, Wiwatwitaya, Keller, Hashim and Molet2017) would be in accordance with recent phylogenomic analyses placing Gesomyrmex closer to other polymorphic lineages among Formicinae (Blaimer et al. Reference Blaimer, Brady, Schultz, Lloyd, Fisher and Ward2015), and therefore suggesting more complex eusocial habits, even if geographical or specific variations cannot be excluded. Interestingly, Gesomyrmex is found to be sister genus to Oecophylla (Blaimer et al. Reference Blaimer, Brady, Schultz, Lloyd, Fisher and Ward2015), also typically arboreal, but with a much broader contemporaneous distribution spanning SE Asia, India, Oceania and Africa (http://antmaps.org).
Wheeler (Reference Wheeler1929 a) had already deduced an arboreal lifestyle for the fossil species G. hoernesi based on the presence of well-developed pretarsal claws and clypeal traction setae—such setae being also present in extant species. Clypeal traction setae are not visible in G. gallicus, but we cannot discount that this is a taphonomic artefact. Whether this character could have phylogenetic importance is therefore uncertain. The shared presence of nine-toothed mandibles in both G. hoernesi and G. gallicus sp. nov., compared to five, six or eight teeth in extant taxa (note: teeth count unknown in some species; based on AntWeb images, at least seven in G. kalshoveni, five in G. spatulatus and eight in G. luzonensis), otherwise conflicts with the shape of the aboral mandibular margins, which are more similar between extant species and G. gallicus sp. nov (Fig. 3a–f). As mentioned, compression morphotypes unfortunately do not provide useful information with respect to this conundrum. This means that affinities among or between fossil and extant species of Gesomyrmex cannot be resolved further at present.
2.c. Palaeoenvironmental implications
Nonetheless, because this genus exclusively inhabits SE Asian warm and humid forests today, its presence in the fossil record can also have important palaeogeographical and palaeoecological implications. The discovery of Gesomyrmex in the lowermost Eocene amber of Oise correlates in particular with the spread of rain- and other warm climate forests to higher latitudes during the PETM and subsequent Eocene hyperthermals (Wing et al. Reference Wing, Harrington, Smith, Bloch, Boyer and Freeman2005; Zachos et al. Reference Zachos, McCarren, Murphy, Röhl and Westerhold2010; Willard et al. Reference Willard, Donders, Reichgelt, Greenwood, Sangiorgi, Peterse, Nierop, Frieling, Schouten and Sluijs2019) and which are known to characterize this locality (de Franceschi & de Plöeg, Reference de Franceschi and de Plöeg2003; Jossang et al. Reference Jossang, Bel-Kassaoui, Jossang, Seuleiman and Nel2008; Nohra et al. Reference Nohra, Perrichot, Jeanneau, Le Pollès and Azar2015).
Eocene warming events have been investigated in part via modelling approaches (Lunt et al. Reference Lunt, Dunkley Jones, Heinemann, Huber, LeGrande, Winguth, Loptson, Marotzke, Roberts, Tindall, Valdes and Winguth2012), with or without fossil calibration. A number of these results predict drier intertropical biomes (Kiehl & Shields, Reference Kiehl and Shields2013; Herold et al. Reference Herold, Buzan, Seton, Goldner, Green, Müller, Markwick and Huber2014) than palaeobotanical evidence would suggest (Willard et al. Reference Willard, Donders, Reichgelt, Greenwood, Sangiorgi, Peterse, Nierop, Frieling, Schouten and Sluijs2019; Huurdeman et al. Reference Huurdeman, Frieling, Reichgelt, Bijl, Bohaty, Holdgate, Gallagher, Peterse, Greenwood and Pross2021). Herold et al. (Reference Herold, Buzan, Seton, Goldner, Green, Müller, Markwick and Huber2014), for instance, presented a general biome model for the Eocene based on simulated vegetation distribution and topographic reconstructions, which indeed recovered the presence of rainforests as well as typically intertropical biomes across Eurasia, with the Arctic covered by warm to temperate forests. The model also predicted a large area of drought in the middle palaeo-Eurasian continent forming a great barrier between European and SE Asian rainforest expansions (Fig. 5a).
As G. gallicus sp. nov. and the distribution of other Gesomyrmex fossils show (Fig. 5b), this genus must have radiated worldwide during one of the early Eocene hyperthermals, probably the PETM, and therefore such a barrier might not have been present, at least initially. A scenario of a more contiguous zone of warm and humid forest coverage is preferred here to long-distance dispersal (LDD), although dispersal by sea was also possible. According to Helms (Reference Helms2018), the maximum recorded flight distance for ants is 32.2 km, in the invasive genus Solenopsis. In the context of the Australian dispersal of Leptomyrmex, for instance, Lucky (Reference Lucky2011) stresses the importance of ‘rafting’ for cross-continental dispersal, where flight propagation is not possible. This probably also applies to other, more global cases of radiation in the pre-anthropocene (Blaimer Reference Blaimer2012), before extreme LDD could be mediated by human travel (Suarez et al. Reference Suarez, Holway and Case2001). Across large landmasses, however, spatial propagation implies habitat contiguity within maximum flight range, especially in the case of genera such as Gesomyrmex with relatively narrow ecological tolerance. As discussed above, Gesomyrmex has so far been exclusively associated with trees of evergreen South Asian forests (Dlussky et al. Reference Dlussky, Wappler and Wedmann2009; Peeters et al. Reference Peeters, Ito, Wiwatwitaya, Keller, Hashim and Molet2017), consistent with the known geographical range of the taxon (http://antmaps.org).
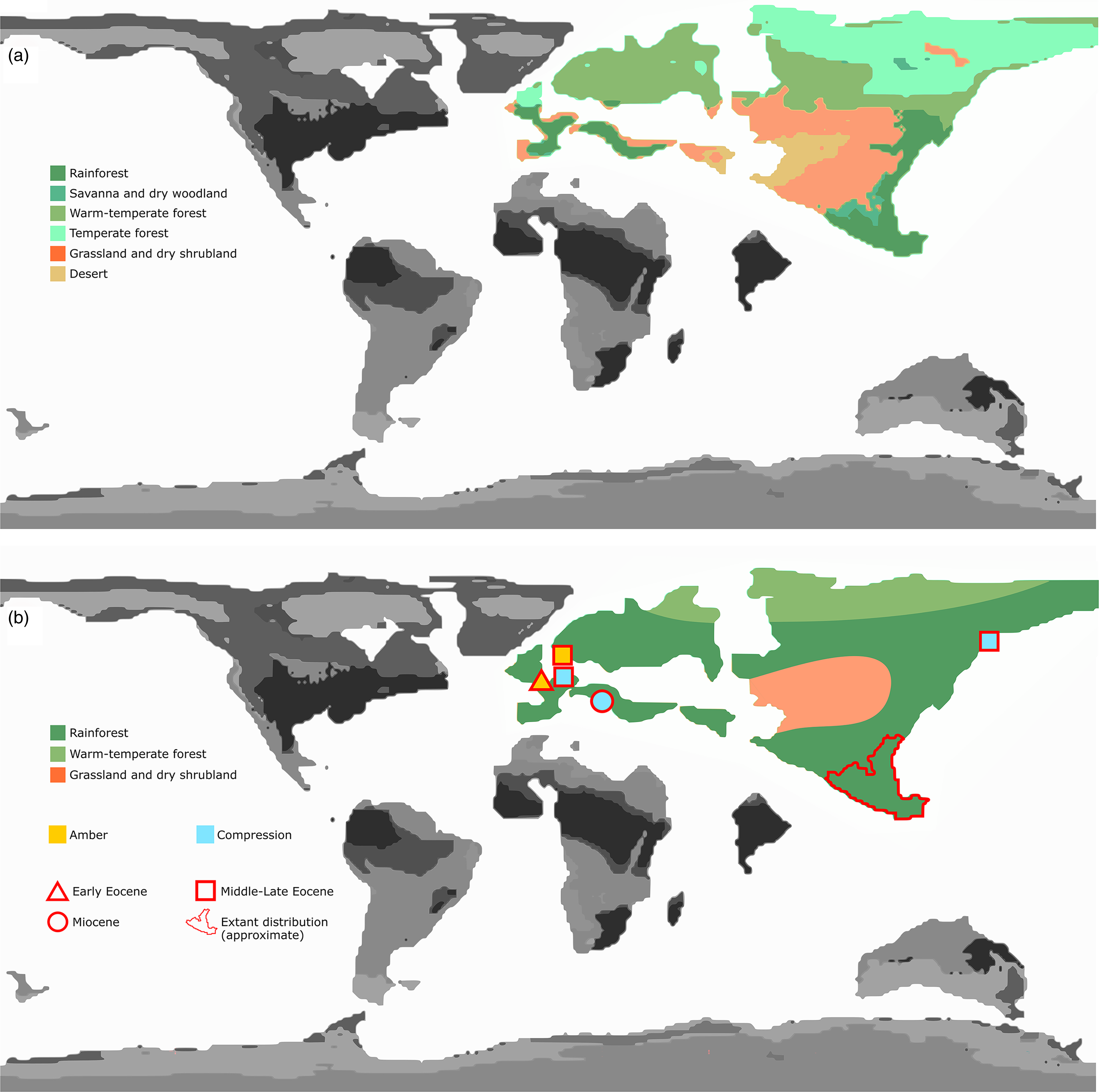
Fig. 5. Changes in the Eocene biome palaeomap in light of the distribution of Gesomyrmex species. (a) General Eocene biome zoning modelled by Harold et al. (Reference Herold, Buzan, Seton, Goldner, Green, Müller, Markwick and Huber2014); (b) expected gross biome coverage at the heart of the PETM based on the distribution of Gesomyrmex. Areas outside Eurasia are not considered here and are greyed out.
Herold and colleagues (Reference Herold, Buzan, Seton, Goldner, Green, Müller, Markwick and Huber2014) acknowledged a number of biases implied by their methodological approach to reconstructing early Eocene vegetation. Notably, they used a single climate simulation forced with Eocene topography and bathymetry to drive their vegetation model, with input parameters from temperature, precipitation and cloud cover. Their results were not directly calibrated with the palaeofloral record, but were compared to existing data (Morley, Reference Morley2007; Utescher & Mosbrugger, Reference Utescher and Mosbrugger2007) and considered in adequation. However, the reference studies used specifically pointed to uncertainties about early Eocene mid-latitudinal Asian vegetation types, because of both a lack and overlap of data. Morley (Reference Morley2007) advocated for marked vegetational zonation from the equator to mid-latitudes, but recognized that the character of the broadly equatorial vegetation was poorly constrained. Hard climatic evidence for the mid-latitudinal Asian region is shown only in the form of evaporitic deposits. Similarly, Utescher and Mosbrugger (Reference Utescher and Mosbrugger2007) compiled data over the entire Eocene and recognized themselves that the data overlap was likely to cause inaccuracies – which was in fact reflected by their own results. For the specific mid-latitudinal Asian region, therefore, neither of these studies particularly supported the simulation results of Harold and colleagues.
The Eocene as a whole – but especially the early Eocene – is likely to resist characterization by a single climatic map more than some other geological periods, because of its characteristic cyclicity of major hyperthermal events. Archibald and colleagues (Reference Archibald, Johnson, Mathewes and Greenwood2011) already pointed this out in the case of another Eocene ant record. In that study, the large, extinct Formiciidae were considered thermophilic based on palaeobotanic evidence and a correlation between temperature and size in extant taxa, and thus the known distribution of these fossil ants across Europe and North America was interpreted as evidence for cross-Arctic dispersal during the Ypresian. In light of known palaeotemperature data, such northern dispersal would have been possible only during global hyperthermals, estimated to have raised arctic temperatures by 5–10 °C (Archibald et al. Reference Archibald, Johnson, Mathewes and Greenwood2011).
Since palaeomyrmecological evidence in the Palaeocene is limited (Aria et al. Reference Aria, Perrichot and Nel2011; LaPolla et al. Reference LaPolla, Dlussky and Perrichot2013; Jouault & Nel, Reference Jouault and Nel2022) and because it lacked hyperthermal events, even if closed-canopy megathermal rainforests first became widespread during that period (Morley, Reference Morley2007), it seems more likely that Gesomyrmex radiated within a homogeneous and highly connected biome composed of warm and humid forests across most of Eurasia at the onset of the Eocene (Fig. 4b). We consider sea dispersal to an isolated patch of evergreen forest at the Svetlovodnaya latitude to be a much less parsimonious hypothesis. This scenario is consistent with the distribution pattern of other ant genera, especially when considering affinities between the fossil Palaearctic and modern Indomalaysian faunas (Guénard et al. Reference Guénard, Perrichot and Economo2015), and corroborates previous observations based on the myrmecological fossil record (Archibald et al. Reference Archibald, Johnson, Mathewes and Greenwood2011). The presence of rainforests at high latitudes during Eocene hyperthermals is also strongly corroborated by recent pollen assemblages documenting the presence of palms and subtropical taxa in the Arctic during these events, with mixed conifer–broadleaf forests present during cooler intervals (Willard et al. Reference Willard, Donders, Reichgelt, Greenwood, Sangiorgi, Peterse, Nierop, Frieling, Schouten and Sluijs2019), as well as direct proxy record of meso-megathermal rainforests at c. 30° palaeolatitude (Huurdeman et al. Reference Huurdeman, Frieling, Reichgelt, Bijl, Bohaty, Holdgate, Gallagher, Peterse, Greenwood and Pross2021). This evidence does not preclude patchiness and more complex zonation, but points at least to gross Eurasian continuity of megathermal forests (Fig. 5b), including evergreen tropical rainforests, mangroves and tropical halfevergreen rainforest / raingreen monsoon forest (Utescher & Mosbrugger, Reference Utescher and Mosbrugger2007).
We propose here that biological barriers across Eurasia in the form of relatively drier biomes more likely occurred later during the Eocene. Following the model proposed by Herold et al. (Reference Herold, Buzan, Seton, Goldner, Green, Müller, Markwick and Huber2014), a central desertic/shrubland (for grassland see Strömberg, Reference Strömberg2011; Herold et al. Reference Herold, Buzan, Seton, Goldner, Green, Müller, Markwick and Huber2014) area in the Palaearctic would have separated east and west faunas, and may have constituted a first step in the isolation of the easternmost populations of the genus Gesomyrmex. The presence of the Gesomyrmex species at relatively high latitude (Sikhote-Alin, Russian Far East) during the Late Eocene (Dlussky et al. Reference Dlussky, Rasnitsyn and Perfilieva2015) suggests that an extensive rainforest coverage persisted or reoccurred frequently during most of that geological period.
5. Conclusion
The occurrence of the megathermal arboreal ant genus Gesomyrmex in the earliest Eocene of France not only confirms previous evidence for the presence of rainforests in Europe during the PETM, but, together with the remainder of the palaeontological data, requires the existence of a continuous band of a warm and humid forest cover across Eurasia at that time. Modern distribution suggests that droughts occurring later during the Eocene contributed to the current South Asian isolation and likely loss of diversity for Gesomyrmex.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756822001248
Acknowledgements
We thank the Lafarge-Granulat company for their help with fossil sampling, and the Langlois-Meurinne family for permission to work on their property. We are also grateful to Jörg Wunderlich (Hirschberg, Germany) and curators of amber collections at the Museum für Naturkunde Berlin (Christian Neumann) and Naturhistorisches Museum Wien (Mathias Harzhauser) for access to specimens of G. hoernesi for comparison and imaging on AntWeb. We further thank Roberto Keller and the AntWeb community for availability of high-resolution images of various Gesomyrmex species. This work was part of CA’s Master thesis in 2010, and is also included as part of CJ’s current doctoral dissertation.
Conflict of interest
None.








