Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Gessler, Damian D. G.
1995.
The constraints of finite size in asexual populations and the rate of the ratchet.
Genetical Research,
Vol. 66,
Issue. 3,
p.
241.
Nordborg, Magnus
Charlesworth, Brian
and
Charlesworth, Deborah
1996.
The effect of recombination on background selection.
Genetical Research,
Vol. 67,
Issue. 2,
p.
159.
1997.
Metapopulation Biology.
p.
455.
Santiago, Enrique
and
Caballero, Armando
1998.
Effective Size and Polymorphism of Linked Neutral Loci in Populations Under Directional Selection.
Genetics,
Vol. 149,
Issue. 4,
p.
2105.
Gerrish, Philip J.
and
Lenski, Richard E.
1998.
Mutation and Evolution.
Vol. 7,
Issue. ,
p.
127.
Barton, N. H.
and
Charlesworth, B.
1998.
Why Sex and Recombination?.
Science,
Vol. 281,
Issue. 5385,
p.
1986.
Barton, Nick
and
Partridge, Linda
2000.
Limits to natural selection.
BioEssays,
Vol. 22,
Issue. 12,
p.
1075.
Gerrish, Philip
2001.
The rhythm of microbial adaptation.
Nature,
Vol. 413,
Issue. 6853,
p.
299.
Johnson, Toby
and
Barton, Nick H
2002.
The Effect of Deleterious Alleles on Adaptation in Asexual Populations.
Genetics,
Vol. 162,
Issue. 1,
p.
395.
Betancourt, Andrea J.
and
Presgraves, Daven C.
2002.
Linkage limits the power of natural selection in
Drosophila
.
Proceedings of the National Academy of Sciences,
Vol. 99,
Issue. 21,
p.
13616.
Bachtrog, Doris
and
Gordo, Isabel
2004.
ADAPTIVE EVOLUTION OF ASEXUAL POPULATIONS UNDER MULLER'S RATCHET.
Evolution,
Vol. 58,
Issue. 7,
p.
1403.
Bachtrog, Doris
and
Gordo, Isabel
2004.
ADAPTIVE EVOLUTION OF ASEXUAL POPULATIONS UNDER MULLER'S RATCHET.
Evolution,
Vol. 58,
Issue. 7,
p.
1403.
Gordo, Isabel
and
Campos, Paulo R. A.
2006.
Adaptive evolution in a spatially structured asexual population.
Genetica,
Vol. 127,
Issue. 1-3,
p.
217.
Perfeito, L.
Gordo, I.
and
Campos, P. R.A.
2006.
The effect of spatial structure in adaptive evolution.
The European Physical Journal B - Condensed Matter and Complex Systems,
Vol. 51,
Issue. 2,
Engelstädter, Jan
2008.
Constraints on the evolution of asexual reproduction.
BioEssays,
Vol. 30,
Issue. 11-12,
p.
1138.
Patwa, Z
and
Wahl, L.M
2008.
The fixation probability of beneficial mutations.
Journal of The Royal Society Interface,
Vol. 5,
Issue. 28,
p.
1279.
Barton, N. H.
2010.
Genetic linkage and natural selection.
Philosophical Transactions of the Royal Society B: Biological Sciences,
Vol. 365,
Issue. 1552,
p.
2559.
Barton, N. H.
2010.
Mutation and the evolution of recombination.
Philosophical Transactions of the Royal Society B: Biological Sciences,
Vol. 365,
Issue. 1544,
p.
1281.
Neher, R A
and
Shraiman, B I
2011.
Genetic Draft and Quasi-Neutrality in Large Facultatively Sexual Populations.
Genetics,
Vol. 188,
Issue. 4,
p.
975.
Hartfield, Matthew
and
Otto, Sarah P.
2011.
RECOMBINATION AND HITCHHIKING OF DELETERIOUS ALLELES.
Evolution,
Vol. 65,
Issue. 9,
p.
2421.
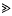 , can be approximated as a sudden reduction in the frequency of the favourable allele, by a fraction w = 1 −(s/S)r/s (where r is the recombination rate). An expression for the effect of a given sequence of such catastrophes is derived. This also applies to the ecological prxoblem of finding the probability that a small population will survive, despite occasional disasters. It is shown that if substitutions occur at a rate Δ, and are scattered randomly over a genetic map of length R, then an allele is unlikely to be fixed if its advantage is less than a critical value, Scrit = (π2/6)(2ΔS/(Rlog(S/s))). This threshold depends primarily on the variance in fitness per unit map length dueto substitutions, var(W)/R = 2ΔS/R. With no recombination, the fixation probability can be calculated for a finite population. If Δ > s, it is of the same order as for a neutral allele ( ≈ Δ/(2N(Δ−s))), whilst if
, can be approximated as a sudden reduction in the frequency of the favourable allele, by a fraction w = 1 −(s/S)r/s (where r is the recombination rate). An expression for the effect of a given sequence of such catastrophes is derived. This also applies to the ecological prxoblem of finding the probability that a small population will survive, despite occasional disasters. It is shown that if substitutions occur at a rate Δ, and are scattered randomly over a genetic map of length R, then an allele is unlikely to be fixed if its advantage is less than a critical value, Scrit = (π2/6)(2ΔS/(Rlog(S/s))). This threshold depends primarily on the variance in fitness per unit map length dueto substitutions, var(W)/R = 2ΔS/R. With no recombination, the fixation probability can be calculated for a finite population. If Δ > s, it is of the same order as for a neutral allele ( ≈ Δ/(2N(Δ−s))), whilst if 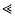 , fixation probability is much higher than for a neutral allele, but much lower than in the absence of hitch-hiking
, fixation probability is much higher than for a neutral allele, but much lower than in the absence of hitch-hiking 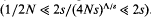 . These results suggest that hitch-hiking may substantially impede the accumulation of weakly favoured adaptations.
. These results suggest that hitch-hiking may substantially impede the accumulation of weakly favoured adaptations.