1. Introduction
Schemes for early detection of breast cancer in average-risk women include mammography screening every 2 years and a physician-guided breast exam every year from age 50 years. For high-risk women, in particular those with known mutations in the major breast cancer susceptibility genes, BRCA1, BRCA2 and a family history of cancer, who have a 20% or greater lifetime risk for developing breast cancer, most guidelines recommend annual breast MRI and a 6-month breast exam starting at age 30 years (Smith et al., Reference Smith, Cokkinides, Brooks, Saslow, Shah and Brawley2011; http://publications.nice.org.uk/familial-breast-cancer-cg164/). In addition, the Breast Cancer Risk Assessment Tool (BCRAT) is used to determine whether a woman meets the minimum risk threshold of a 5-year risk of at least 1·67% when tamoxifen treatment for primary chemoprevention is considered (Fisher et al., Reference Fisher, Costantino, Wickerham, Cecchini, Cronin, Robidoux, Bevers, Kavanah, Atkins, Margolese, Runowicz, James, Ford and Wolmark2005; Smith et al., Reference Smith, Cokkinides, Brooks, Saslow, Shah and Brawley2011). Thus, an objective assessment of a woman's breast cancer risk has obvious clinical implications that affect management decisions.
Several statistical models have been developed for assessing risk for developing breast cancer (Gail et al., Reference Gail, Brinton, Byar, Corle, Green, Schairer and Mulvihill1989, Reference Gail, Costantino, Pee, Bondy, Newman, Selvan, Anderson, Malone, Marchbanks, McCaskill-Stevens, Norman, Simon, Spirtas, Ursin and Bernstein2007; Claus et al., Reference Claus, Risch and Thompson1993; Antoniou et al., Reference Antoniou, Pharoah, Smith and Easton2004; Tyrer et al., Reference Tyrer, Duffy and Cuzick2004). These models are based on algorithms that take into account factors such as family history of breast/ovarian cancer, BRCA1/BRCA2 carrier status, and non-genetic risk factors, such as current age, age at menarche, age at first live birth, number of previous breast biopsies, history of atypical hyperplasia, race/ethnicity, height and weight, use of hormone replacement therapy and number of affected female first- and second-degree relatives (Gail et al., Reference Gail, Brinton, Byar, Corle, Green, Schairer and Mulvihill1989, Reference Gail, Costantino, Pee, Bondy, Newman, Selvan, Anderson, Malone, Marchbanks, McCaskill-Stevens, Norman, Simon, Spirtas, Ursin and Bernstein2007; Bondy et al., Reference Bondy, Lustbader, Halabi, Ross and Vogel1994; Costantino et al., Reference Costantino, Gail, Pee, Anderson, Redmond, Benichou and Wieand1999; Tyrer et al., Reference Tyrer, Duffy and Cuzick2004; Matsuno et al., Reference Matsuno, Costantino, Ziegler, Anderson, Li, Pee and Gail2011). For example, the BCRAT (also known as Gail model); the International Breast cancer Intervention study (IBIS- http://www.ems-trials.org/riskevaluator/) also known as the Tyrer–Cusick risk evaluator; the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA – http://ccge.medschl.cam.ac.uk/boadicea/). Notably, not all three models take into account all of these breast cancer risk factors or similarly weight them when assigning breast cancer risk. These risk assignment models have been validated in several cohorts, both average-risk and high-risk individuals in several ethnically diverse populations (Spiegelman et al., Reference Spiegelman, Colditz, Hunter and Hertzmark1994; Jacobi et al., Reference Jacobi, de Bock, Siegerink and van Asperen2009; Matsuno et al., Reference Matsuno, Costantino, Ziegler, Anderson, Li, Pee and Gail2011; Anothaisintawee et al., Reference Anothaisintawee, Teerawattananon, Wiratkapun, Kasamesup and Thakkinstian2012). None has been assessed in a Jewish Israeli high-risk population and in most cases the cohorts included both high-risk and average-risk women, and BRCA mutation carriers were not excluded. Since the BCRAT model has not been recommended for use in high-risk women or for women under the age of 35 years, we elected to evaluate the accuracy of the BOADICEA and IBIS models in high-risk women of that ethnic group.
2. Materials and methods
2.1 Study population
All women who underwent oncogenetic counselling from 1 June 1996 to 31 May 2000 at the Oncogenetics unit at the Sheba Medical Center, Tel-Hashomer were eligible if they fulfilled the inclusion criteria: were cancer free at the time of initial counselling; were considered high risk based on the accepted practiced clinical criteria, as previously described by us (Kushnir et al., Reference Kushnir, Laitman, Paluch-Shimon, Berger and Friedman2012); and were genotyped for the predominant mutations in BRCA1 and BRCA2 in Jewish women and tested negative. The study was approved by the local IRB of the Sheba Medical Center, and each participant gave her informed consent.
2.2 Assignment of risk for developing breast cancer
The 10-year and lifetime risks for developing breast cancer were calculated for all eligible participants using the freely available algorithms of BOADICEA (http://ccge.medschl.cam.ac.uk/boadicea/) and IBIS (http://www.ems-trials.org/riskevaluator/ – version 6).
2.3 Follow-up
Counselling date indicated the beginning of follow-up for each participant. End of follow-up was marked by breast cancer diagnosis date or by the end of the follow-up (1 May 2011), whichever came first.
2.4 Calculating the observed/expected ratio of breast cancer
Observed breast cancer rates were obtained from the Israeli National Cancer Registry (INCR) by cross referencing the ID numbers of all participants with the list of breast cancer diagnoses reported to the INCR. The INCR, a passive, national, population-based cancer registry, was established in 1960. Since 1982 reporting on all cancer cases to the INCR is compulsory by law. The INCR completeness with respect to solid tumours is over 93% (Fishler et al., Reference Fishler, Barchana, Keinan-Boker, Ifrah and Blau2008). The expected rates of developing breast cancer for the study participants were calculated separately from each algorithm. To that end, the predicted 10 year risk for each participant was divided by 10 to obtain the annual risk, and then multiplied by the number of years since initial counselling to the time of evaluation (11–16 years). The expected number of breast cancer cases in the cohort is the sum of all these predicted risks. The observed/expected ratio was then calculated. The 95% confidence interval was calculated by using the Poisson distribution.
3. Results
3.1 Study population
Overall, 358 Jewish Israeli women participated in the study: 205 (57·2%) were of Ashkenazi origin; age range at counselling was 20–75 years (mean age 46·76 ± 9·8 years). The average follow-up was 13·57 ± 1·45 years (range 11–16 years), with a total of 4·861 person-years of follow-up. During that time period 15 women (4·18%) were diagnosed with breast cancer, mean age at diagnosis was 57·06 ± 8·6 years (range 37–74 years).
3.2 IBIS and BOADICEA breast cancer risks
Table 1 shows the ranges and the means of the predicted risks for breast cancer, at 10-year, during the calculated study duration, and lifetime risk using both models for women who developed breast cancer and those who remained cancer-free at the end of the follow-up.
Table 1. Predicted risk for breast cancer at 10 years, for the duration of the study and lifetime, based on the IBIS and BOADICEA models, by health status
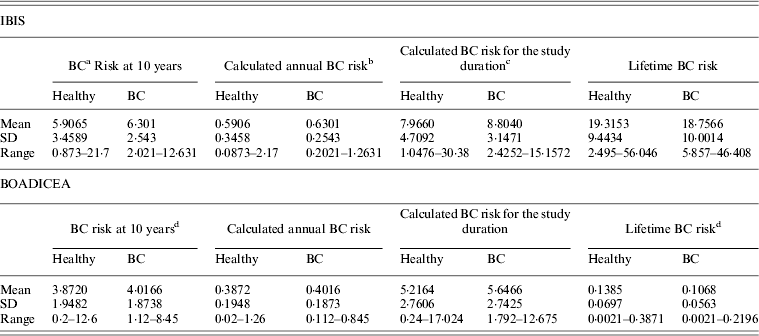
a BC – Denotes breast cancer.
b The 10-year risk divided by 10.
c The 10-year risk divided by 10 and multiplied by the duration of the study follow-up per individual.
d The 10-year risk and lifetime risk multiplied by 100.
3.3 Observed/expected breast cancer ratio
Using the IBIS model, the observed/expected ratio was 15/28·6 = 0·52 (95% CI 0·32–0·87); using the BOADICEA model the observed/expected ratio was 15/18·7 = 0·52 (95% CI 0·48–1·33).
4. Discussion
In the present study, where validation of two prediction models for breast cancer risk was carried out in high-risk Israeli women, both models overestimated the actual risks.
However, the BOADICEA model outperformed the IBIS model as a predictor of breast cancer risk in this population of Jewish Israeli high-risk women. Several factors may have contributed to these differences in accuracy of breast cancer risk prediction: the inability to assess cancers other than breast and ovary in family members in the IBIS model, the inability (in version 6) to evaluate a male breast cancer cases, and the overestimation of the non-genetic risk factors in that algorithm compared with the BOADICEA model. These differences may also relate to the specific risks of Jewish high-risk women. Despite the fact that both models allow for including Ashkenazi ethnicity as a covariate, neither has been validated in Jewish non-Ashkenazi women, until the present study. In other populations, both models were rated as equally accurate and outperforming other available breast cancer risk models (Jacobi et al., Reference Jacobi, de Bock, Siegerink and van Asperen2009).
Determining lifetime risk for developing breast cancer has a clinical implication in Israel as the national health basket covers breast MRI from age 30 years to all women who have a 20% risk or more for developing breast cancer. Thus, using the more accurate risks assigned by BOADICEA may help in targeting the individuals who seems to be at the highest level of breast cancer risk, with little overspending of the resources. One of the main purposes of using these models is to assess eligibility for offering genetic testing for the high-risk women. The current guidelines stipulate that anyone with a 10–15% chance of having a BRCA1/BRCA2 mutation should be offered genetic testing and genotyped (Smith et al., Reference Smith, Cokkinides, Brooks, Saslow, Shah and Brawley2011). The limited spectrum of BRCA1/BRCA2 mutations in Jewish individuals, primarily (but not exclusively) of Ashkenazi origin, coupled with the paucity of ‘private’, family-specific germline mutations in both genes, and the fact that the risks for harbouring BRCA1/BRCA2 mutations in consecutive breast and ovarian cancer cases in Jewish individuals is well established (Laitman et al., Reference Laitman, Borsthein, Stoppa-Lyonnet, Dagan, Castera, Goislard, Gershoni-Baruch, Goldberg, Kaufman, Ben-Baruch, Zidan, Maray, Soussan-Gutman and Friedman2011), makes the application of both these models as determining genetic testing eligibility in Israel of limited clinical utility.
This study has several inherent limitations: the number of women is limited, despite the long-term follow-up and the number of cumulative person-years; it is a retrospective cohort study with all the disadvantages of this study design. All participants were recruited from a single medical centre in Israel which may not adequately represent the entire high-risk population in Israel. The fact that BRCA mutations tested were predominantly detected in Ashkenazi (and to a lesser extent Iraqi) high-risk families is also a limitation. Yet the rate of ‘private’ family-specific mutations in the Ashkenazi and non-Ashkenazi high-risk women is 4·7 and 8·8%, respectively (Laitman et al., Reference Laitman, Borsthein, Stoppa-Lyonnet, Dagan, Castera, Goislard, Gershoni-Baruch, Goldberg, Kaufman, Ben-Baruch, Zidan, Maray, Soussan-Gutman and Friedman2011). Thus, the majority of women tested are indeed non-BRCA mutation carriers.
In conclusion, the BOADICEA model outperformed the IBIS model in Jewish Israeli high-risk women in determining breast cancer risk. These data should be validated in a larger prospective cohort study setting.
This study was partially funded by the Israel Cancer Association through the Israeli Hereditary breast cancer consortium.
5. Conflict of interest statement
All authors declare that they have no conflict of interest regarding the data published.



