Article contents
Chimeric antigen receptor engineered T cells and their application in the immunotherapy of solid tumours
Published online by Cambridge University Press: 28 January 2022
Abstract
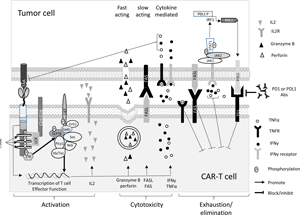
In this article, we reviewed the current literature studies and our understanding of the parameters that affect the chimeric antigen receptor T cells (CAR-T's) activation, effector function, in vivo persistence, and antitumour effects. These factors include T cell subsets and their differentiation stages, the components of chimeric antigen receptors (CAR) design, the expression promoters and delivery vectors, and the CAR-T production process. The CAR signalling and CAR-T activation were also studied in comparison to TCR. The last section of the review gave special consideration of CAR design for solid tumours, focusing on strategies to improve CAR-T tumour infiltration and survival in the hostile tumour microenvironment. With several hundred clinical trials undergoing worldwide, the pace of CAR-T immunotherapy moves from bench to bedside is unprecedented. We hope that the article will provide readers a clear and comprehensive view of this rapidly evolving field and will help scientists and physician to design effective CAR-Ts immunotherapy for solid tumours.
- Type
- Review
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 11
- Cited by


