1. Introduction
Vitamin D has been known to play an essential role in regulating bone metabolism and maintaining calcium and phosphate homeostasis. The active form of vitamin D exerts its biological effects mainly by binding to the vitamin D receptor (VDR). VDR is expressed in most of the cells (Bouillon et al., Reference Bouillon, Carmeliet, Verlinden, van Etten, Verstuyf, Luderer, Lieben, Mathieu and Demay2008), which explains why VDR signaling has been implicated in a variety of physiological processes, including neuromuscular function. Indeed, the VDR has been found in the sensory and motor areas of the nervous system and muscles in both humans and rodent models (Bischoff et al., Reference Bischoff, Borchers, Gudat, Duermueller, Theiler, Stähelin and Dick2001; Eyles et al., Reference Eyles, Smith, Kinobe, Hewison and McGrath2005; Girgis et al., Reference Girgis, Mokbel, Cha, Houweling, Abboud, Fraser, Mason, Clifton-Bligh and Gunton2014; Prüfer et al., Reference Prüfer, Veenstra, Jirikowski and Kumar1999). In addition, many studies indicate that vitamin D is related to various neurological and neuromuscular disorders (Di Somma et al., Reference Di Somma, Scarano, Barrea, Zhukouskaya, Savastano, Mele, Scacchi, Aimaretti, Colao and Marzullo2017; Dodig et al., Reference Dodig, Tarnopolsky and Currie2017). VDR null mice display motor deficits and muscular impairments (Burne et al., Reference Burne, McGrath, Eyles and Mackay-Sim2005; Kalueff et al., Reference Kalueff, Lou, Laaksi and Tuohimaa2004). It has been suggested that Schwann cells and the neuromuscular junctions (NMJs) are a target of VDR signaling (Sakai et al., Reference Sakai, Suzuki, Tashiro, Tanaka, Takeda, Aizawa, Hirata, Yogo and Endo2015).
Zebrafish have become an effective animal model for studying neuromuscular functions since muscle activity can be assessed easily in the early stages of development, the first few days after fertilization (Sztal et al., Reference Sztal, Ruparelia, Williams and Bryson-Richardson2016). In zebrafish embryos, two paralogs for VDR genes (vdra and vdrb) have been identified (Kollitz et al., Reference Kollitz, Hawkins, Whitfield and Kullman2014; Lin et al., Reference Lin, Su, Tseng, Ding and Hwang2012). Although previous studies have demonstrated that VDR signaling in zebrafish regulates heart development (Han et al., Reference Han, Chen, Umansky, Oonk, Choi, Dickson, Ou, Cigliola, Yifa, Cao, Tornini, Cox, Tzahor and Poss2019; Kwon, Reference Kwon2016), ocular angiogenesis (Merrigan & Kennedy, Reference Merrigan and Kennedy2017), and hematopoiesis (Cortes et al., Reference Cortes, Chen, Stachura, Liu, Kwan, Wright, Vo, Theodore, Esain, Frost, Schlaeger, Goessling, Daley and North2016), its function in neuromuscular development is largely unknown.
2. Objective
The objective of the present study was to investigate whether loss of VDR affects neuromuscular activity in zebrafish embryo and larvae using touch-evoked response behavior analysis.
3. Methods
3.1. Animal care and use
The author asserts that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. Zebrafish (Danio rerio) were reared and maintained at 28.5°C in accordance with the Animal Care and Use Committee at Texas A&M University. Larvae were staged as hours postfertilization (hpf) or days postfertilization (dpf) according to the established guidelines (Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995).
3.2. The knockdown of VDR genes
The VDR gene knockdown experiment was carried out using antisense morpholino oligonucleotides (MOs) as described previously (Kwon, Reference Kwon2016; Lin et al., Reference Lin, Su, Tseng, Ding and Hwang2012). To knockdown vdra, a translation blocker (5′-AAC GGC ACT ATT TTC CGT AAG CAT C-3′) was used. To knockdown vdrb, a splice blocker (5′-TCC ATC ACT AGC AGA CGA GGG AAG A-3′) targeting the intron2-exon3 (I2E3) junction was used. Zebrafish embryos were co-injected with 5 ng of vdra MOs and 5 ng of vdrb MOs at the one-cell stage. All MOs used here were obtained from Gene Tools, LLC (Philomath, OR). The experiments were conducted at least three times.
3.3. Touch-evoked response behavior analysis
Touch-evoked zebrafish movements were recorded using a CCD camera mounted on a dissecting microscope at 40 hpf and 6 dpf. For touch responses at 40 hpf, tactile stimuli were generated by the manual dechorionation using fine forceps. For touch responses at 6 dpf, tactile stimuli were elicited by touching the tail region of the larvae with forceps. Images were captured, converted into a video file, and analyzed individual frames of time-lapse. To assess behavioral phenotypes, at least 10 embryos were examined in each group. The phenotypes described in this study were completely penetrant.
4. Results
To investigate the effects of the loss of VDRs on motor development, antisense MOs against vdra and vdrb (vdra/b MO) were co-injected into the one-cell embryo. During the hatching period, over 94% of the wild-type control embryos hatched by 3 dpf and 100% of them hatched on the next day (n = 17). It was noticeable that vdra/b MO-injection resulted in a reduction of hatching rate, 70% at 3 dpf. At 5 dpf, the hatching rate of vdra/b MO-injected larvae was still less than 81% (n = 31). At 40 hpf, 100% of control embryos showed rapid escape swimming behaviors in response to tactile stimuli generated in the manual dechorionation (Figure 1a–e; Supplementary Video S1). In contrast, 100% vdra/b morphants exhibited either a very weak or no muscle contraction and all of them failed to escape in response to touch during or after the dechorionation (Figure 1f–j; Supplementary Video S2).

Figure 1. Knockdown of VDRs impairs touch-evoked behaviors. Video frames showing touch-evoked escape response at 40 hpf of wild-type control (a–e) but not of vdra/b MO-injected (f–j) embryos. The time of the frame is shown in the top right portion. The mechanosensory stimulation during the manual dechorionation causes control embryos to escape rapidly and exit the field of view (d and e). In contrast, vdra/b MO-injected embryos do not exhibit any escape response and fail to exit the field of view at the same time frames (i and j). Scale bar = 500 μm.
To determine whether the impairment of touch-evoked escape in vdra/b morphants was due to the delayed onset of touch-responsiveness, the tactile response of zebrafish larvae at 6 dpf was examined. Upon touch at the tail, 100% of vdra/b-depleted larvae at 6 dpf displayed no obvious muscle contraction and none of them showed the escape response observed in 100% of control larvae (Figure 2; Supplementary Videos S3 and S4). vdra/b knockdown caused significantly reduced locomotor activity and perturbed development of the swim bladder. All of the vdra/b MO-injected larvae at 6 dpf had severe defects in free-swimming.
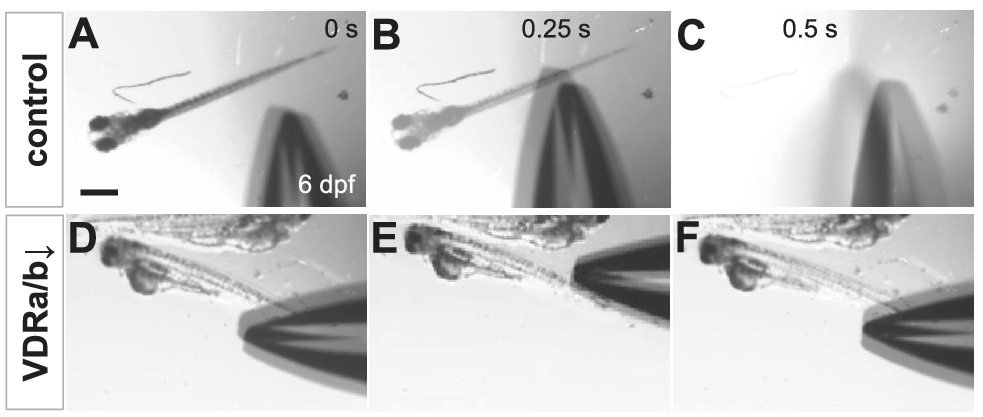
Figure 2. Loss-of-VDRs results in loss of touch-evoked escape swimming behaviors. Video frames showing touch-evoked response at 6 dpf of wild-type control (a–c) but not of vdra/b MO-injected (d–f) larvae. While the stimulation by forceps causes the wild-type larva to swim rapidly away and fully exit the field of view (c), vdra/b MO-injected larvae exhibit no touch-evoked response and remain in the same field of view (f). Scale bar = 500 μm.
5. Discussion
In the current study, VDR signaling was found to be involved in neuromuscular development and touch-evoked escape swimming behavior. In zebrafish embryos, spontaneous muscle contractions start to be noted around 17 hpf (Saint-Amant & Drapeau, Reference Saint-Amant and Drapeau1998). Even before hatching, the zebrafish embryos acquire the ability to respond to touch by 24–27 hpf (Carmean & Ribera, Reference Carmean and Ribera2010). The twitch contraction reaches the peak frequency, which is a driving force of releasing embryos from the chorion during the hatching period around 3 dpf. Delay in hatching found in vdra/b morphants could be due to the impaired muscle activity (Skobo et al., Reference Skobo, Benato, Grumati, Meneghetti, Cianfanelli, Castagnaro, Chrisam, Di Bartolomeo, Bonaldo, Cecconi and Dalla Valle2014).
Together with the muscular and motor impairments observed in VDR knockout mice (Burne et al., Reference Burne, McGrath, Eyles and Mackay-Sim2005; Kalueff et al., Reference Kalueff, Lou, Laaksi and Tuohimaa2004), these findings indicate that VDRs play an essential role in neuromuscular activity and locomotor behavior. The specific mechanism remains to be elucidated, but it may be explained by the effects of vitamin D on calcium homeostasis or maintenance of peripheral nerve axons and acetylcholine receptor clusters in NMJs (Lin et al., Reference Lin, Su, Tseng, Ding and Hwang2012; Sakai et al., Reference Sakai, Suzuki, Tashiro, Tanaka, Takeda, Aizawa, Hirata, Yogo and Endo2015).
6. Conclusion
This study demonstrates that knockdown of VDRs causes impairment of muscle performance and elimination of touch-evoked escape swimming response in zebrafish embryos and larvae. Thus, the VDR signaling is required for the correct neuromuscular activity during early development. These results suggest the role of VDR signaling in locomotor behavior appears to be well-conserved between mammals and fish.
Acknowledgments
The author wishes to thank Bruce B. Riley (Texas A&M University) for providing zebrafish lab facilities and valuable comments.
Funding Statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
The author declares no conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available as Supplementary Material.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/exp.2021.22.





Comments
Comments to the Author: This is a concise and interesting work. I watched the videos and they clearly shows the difference in the tactile response. The difference is dramatic. I think this would be a beneficial finding for zebrafish researchers.