Media summary: Infection-associated depression correlates with Chlamydia trachomatis more strongly than general inflammatory responses.
Introduction
The evolutionary reasons for the widespread presence of clinical depression are unclear. One line of evolutionary reasoning suggests that depression may cause individuals to shift away from unattainable goals (Nesse Reference Nesse2019). This explanation, however, seems inadequate for prolonged, incapacitating depression which should be purged by natural selection (Coyne Reference Coyne2010; Nesse Reference Nesse2019). The adaptive argument for short-term depression, however, may help explain the presence of adaptive neurological circuitry which then could function counter-productively when depression is prolonged and intractable. One hypothesis within this category of explanations invokes mismatches between modern and ancestral environments. Modern environments may have situational traps, such as economic immobility, which could keep depressed individuals from changing their living circumstances and thereby rebound from low mood (Nesse Reference Nesse2019). An alternative mismatch hypothesis proposes that the neocortex is particularly vulnerable to damaging environmental agents and that clinical depression results from the elevated presence of such hazards in modern relative to ancestral environments (Galecki and Talarowska Reference Galecki and Talarowska2017).
An alternative category of explanation invokes infection. Depression has been linked to innate immunological responses to infection and therefore could be interpreted as part of a mechanism that may facilitate recovery, for example, by encouraging rest, or may prevent additional infections in the depressed individual or kin (Anders et al., Reference Anders, Tanaka and Kinney2013; Kinney and Tanaka Reference Kinney and Tanaka2009; Nesse Reference Nesse2019; Raison and Miller Reference Raison and Miller2013b). As is the case with adaptive explanations of short-term depression that rely on goal-shifting, these hypotheses of infection-induced depression do not account well for prolonged, incapacitating depression. It is presumed that the fitness costs of prolonged, incapacitating depression must be incurred in order to obtain the even greater protective benefits of depression.
Clinical depression is distinguished from temporarily suppressed mood largely on the basis of its persistence for at least two weeks (American Psychiatric Association 2013). Accordingly, low mood associated with acute infectious disease is generally considered to be a manifestation of the disease rather than clinical depression. Persistent infections, however, might contribute to prolonged and debilitating depression that has not yet been attributed to infection. Evolutionary considerations emphasize that manifestations of infectious disease could benefit the host, parasite or neither (Ewald Reference Ewald1980). The reasoning leading to this conclusion is based on the coevolutionary instability that is associated with the evolutionary conflicts of interest between parasite and host. In this context, persistent, debilitating depression is not presumed to be an adaptation of the host or the infectious agent. Rather, an adaptive basis for depression may be manifested with an increased intensity or persistence that is beneficial to neither host nor pathogen but results instead from a disruption of the normal regulation of depression, a side effect of the coevolutionary arms race between particular parasites and their hosts.
Recent research has focused on the possibility that inflammation may play a causal role. Depression has been correlated with levels of inflammatory proteins (Khandaker et al. Reference Khandaker, Pearson, Zammit, Lewis and Jones2014; Pasco et al. Reference Pasco, Jacka, Williams, Henry, Nicholson, Kotowicz and Berk2010a, Reference Pasco, Nicholson, Williams, Jacka, Henry, Kotowicz, Schneider, Leonard and Berk2010b) and autoimmunity (Benros et al. Reference Benros, Waltoft, Nordentoft, Ostergaard, Eaton, Krogh and Mortensen2013), but anti-inflammatory treatment has ameliorated depression inconsistently across studies (Baune Reference Baune2017; Cubala and Landowski Reference Cubala and Landowski2014; Eyre et al. Reference Eyre, Air, Proctor, Rositano and Baune2015; O. Kohler et al. Reference Kohler, Benros, Nordentoft, Farkouh, Iyengar, Mors and Krogh2014, Reference Kohler, Krogh, Mors and Benros2016a) and among patients within studies that have different levels of inflammation (Raison et al. Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013).
Infections have been suggested as instigators of immunological responses that could lead to depression (Benros et al. Reference Benros, Waltoft, Nordentoft, Ostergaard, Eaton, Krogh and Mortensen2013; Canli Reference Canli2014; Doyle et al. Reference Doyle, Swain, Ewald, Cook and Ewald2015; Maes et al. Reference Maes, Yirmyia, Noraberg, Brene, Hibbeln, Perini, Kubera, Bob, Lerer and Maj2009; Miller et al. Reference Miller, Maletic and Raison2009; Raison and Miller Reference Raison and Miller2013b). Several pathogens that cause persistent infections have been associated with depression. Human papillomavirus (HPV) has been associated with depressive symptoms in human immunodeficiency virus (HIV)-infected patients (Dodd et al. Reference Dodd, Pereira, Marion, Andrasik, Rose, Simon, Fletcher, Lucci, Maher, O'Sullivan, Efantis-Potter and Antoni2009; Lopez et al. Reference Lopez, Antoni, Pereira, Seay, Whitehead, Potter, O'Sullivan and Fletcher2013). Chlamydia trachomatis has been associated with depression in women tested during their annual check-ups (Doyle et al. Reference Doyle, Swain, Ewald, Cook and Ewald2015). A meta-analysis (Wang et al. Reference Wang, Zhang, Lei, Liu, Zhou, Liu, Wang, Yang, Zhang, Fan and Xie2014) found significant associations of depression with several persistent pathogens but did not account for correlations among these pathogens.
Although associations between infection and depression are often interpreted as occurring through the intervening variable of inflammation (Benros et al. Reference Benros, Waltoft, Nordentoft, Ostergaard, Eaton, Krogh and Mortensen2013; Miller et al. Reference Miller, Maletic and Raison2009; Raison and Miller Reference Raison and Miller2013b), several findings emphasize the need to consider more specific effects of infectious agents. In a low- to middle-income population in Detroit, cytomegalovirus was correlated with depression, but inflammatory markers were not (Simanek et al. Reference Simanek, Cheng, Yolken, Uddin, Galea and Aiello2014). Similarly, cytomegalovirus was correlated with depression in elderly Latinos in northern California, but inflammatory markers and several other persistent pathogens (herpes simplex virus, varicella zoster virus, Helicobacter pylori and Toxoplasma gondii) were not (Simanek et al. Reference Simanek, Zheng, Yolken, Haan and Aiello2018b). In a nationwide US study cytomegalovirus and H. pylori were associated with depression in women, but a marker of inflammation (C-reactive protein) was not (Simanek et al. Reference Simanek, Parry and Dowd2018a). In the meta-analysis mentioned above (Wang et al. Reference Wang, Zhang, Lei, Liu, Zhou, Liu, Wang, Yang, Zhang, Fan and Xie2014), an association of CMV with depression fell just short of statistical significance, but this analysis did not include any of the more recent studies by Simanek and colleagues cited above.
The variation in the association of depression with both inflammation and infection is consistent with the looseness of the associations of depression with indicators of inflammation and the caveat that depression should not be categorized simply as an inflammatory disorder (Raison and Miller Reference Raison and Miller2013a). Associations of depression with specific infectious agents are difficult to interpret because different infections may occur in the same patient, particularly if they are transmitted by the same route. Sexual transmission, in particular, may favor co-occurrence because sexually transmitted infections are often inapparent and nearly always persistent. The contribution of a sexually transmitted pathogen to depression may therefore be inconspicuous because of asymptomatic infection and ambiguous because it could co-occur with other sexually transmitted pathogens that do not contribute to depression.
Inflammatory responses are complex and variable. Joint consideration of infection and inflammation raises the possibility that some pathogens may affect aspects of immunological responses in ways that lead to depression, whereas others may not. It was hypothesized (Doyle et al. Reference Doyle, Swain, Ewald, Cook and Ewald2015) that the association of depression with C. trachomatis resulted from persistent restriction of tryptophan, which the body invokes in association with inflammation as an adaptation to defend against infection (Olive and Sassetti Reference Olive and Sassetti2016; Schmidt and Schultze Reference Schmidt and Schultze2014). This restriction may be chronically stimulated by C. trachomatis because this bacterium synthesizes its own tryptophan (Aiyar et al. Reference Aiyar, Quayle, Buckner, Sherchand, Chang, Zea, Martin and Belland2014) and thus may survive in the presence of tryptophan restriction. Tryptophan is the precursor of serotonin, which plays a role in modulating mood (Fakhoury Reference Fakhoury2016; S. Kohler et al. Reference Kohler, Cierpinsky, Kronenberg and Adli2016b; Kupfer et al. Reference Kupfer, Frank and Phillips2012); restricted tryptophan levels may therefore result in chronically depleted serotonin and hence low mood (Akers and Tan Reference Akers and Tan2006).
The study by Doyle et al. (Reference Doyle, Swain, Ewald, Cook and Ewald2015), which correlated Chlamydia trachomatis with depression, did not find significant associations with the other sexually transmitted pathogens that were evaluated: Neisseria gonorrhoeae, Treponema pallidum, Candida albicans, Trichomonas vaginalis, HPV and HIV. The study, however, was based on only 500 women, and sample sizes for C. trachomatis were greater than for most of the other tested pathogens (Doyle et al. Reference Doyle, Swain, Ewald, Cook and Ewald2015). A larger study population is needed to determine whether sexually transmitted pathogens other than C. trachomatis are associated with depression. One particularly relevant uncertainty pertains to T. vaginalis; it was associated with a doubling of the risk of depression, but this numerical association was not statistically significant (Doyle et al. Reference Doyle, Swain, Ewald, Cook and Ewald2015). Resolution of these uncertainties bears on whether efforts to prevent infection-induced depression should focus on particular pathogens or inflammation in general.
To address this issue we conducted a study using data from the Kentucky Women's Health Registry at the University of Kentucky's Center for the Advancement of Women's Health, which includes health-related information from over 17,000 women. We focus on women to build on the previous study of women (Doyle et al. Reference Doyle, Swain, Ewald, Cook and Ewald2015) and because effects of infection on depression might be stronger in women than in men as a result of the reliance on tryptophan restriction as part of elevated innate immunity during the luteal phase of the menstrual cycle (Hrboticky Reference Hrboticky, Leiter and Anderson1989; Doyle Reference Doyle, Swain, Ewald, Cook and Ewald2015). At that time tryptophan restriction can occur in response to estrogen (through effects on dendritic cells and macrophages; Xiao et al. Reference Xiao, Liu and Link2004) even though other aspects of immune function are suppressed.
Methods
Reproductive-aged women 18–40 years of age were selected as subjects from the Kentucky Women's Health Registry (currently ‘Wellness, Health and You’). Participants were recruited from throughout Kentucky using brochures at the University of Kentucky health care clinics, state and county health departments, county agricultural extension offices, offices of private physicians, women's professional organizations, homemaker organizations and health-related events. Participants completed a registry questionnaire electronically or on paper after providing consent. All information was self-reported.
Although depression can be manifested by a variety of indicators, a duration of at least two weeks is a generally agreed upon threshold for distinguishing clinical depression from transient depression (https://www.psychiatry.org/patients-families/depression/what-is-depression). We therefore included in the depressed category anyone who responded that they had been ‘down, depressed or hopeless’ for a duration of at least two weeks at some point in their life (question U2a in Appendix 1). Participants were queried for pathogens and infectious conditions, socioeconomic and demographic status, drug use and lifestyle variables (Table 1). The timing of depression and infection was not noted in the directory. Our results therefore correlate depression with any of these variables without reference to the order of occurrence. Demographic details are presented in Table 2.
Table 1. Information gathered from the registry

Table 2. Descriptive characteristics of the study population

Immune suppression and state of infection often vary with the menstrual cycle (Doyle et al. Reference Doyle, Ewald and Ewald2007). Subjects were therefore excluded from the study if they reported menstrual disruption or cessation, were using hormonal birth control or hormone replacement therapy, were pregnant or postpartum, or had undergone an ovarectomy or hysterectomy (the last two were not distinguished in the registry).
For bivariate comparisons, chi-squared tests were performed. Boneferroni corrections were used to account for multiple comparisons. Although the correlations between the individual variables and reports of repression provide useful descriptive information, the possibility for correlations among the included pathogens, individual demographics and risk behavior indicator variables necessitate a multivariate analysis. For a multiple regression analysis we estimated the probability of an individual reporting depression of at least two weeks duration using a linear probability model (LPM) in which depression was considered as a function of participant characteristics and infection variables. In this analysis we used three separate models, focusing first on infection variables alone, then adding sequentially demographic and behavioral characteristics to better isolate the independent relationships between specific pathogens and depression.
The models estimating the probability of an individual reporting depression and the explanatory variables can be written as follows:
where ϕ i represents a vector of binary indicators for whether individual i reported experiencing each of the infection variables (Table 4 rows 1–6), λi is a vector of indicators for participant characteristics of individual i (Table 4 rows 7–18) and ψi is a vector of binary indicators for risk behaviors (Table 4, rows 19–29). Unique coefficients for each of the infections β 1, each demographic characteristic β 2 and each risk behavior β 3 are reported in Table 4. The β 0 represents the intercept, and ε i represents a heteroskedasticity-robust individual error term. Analyses were conducted using the standard Stata packages logit, probit and regress for multiple logistic regression, probit regression and linear probability models, respectively.
This study was approved by the Institutional Review Boards at Bellarmine University (approval number 0313-3) and the University of Kentucky.
Results
Depression
A total of 1510 women met the inclusion criteria and provided information on mental health; 74% of them reported depression. Bivariate tests showed statistically significant positive associations of depression with Chlamydia trachomatis, Trichomonas vaginalis, abnormal pap smears, HPV, herpes simplex infections, endometriosis, infertility, frequent yeast infections and unspecified vaginal infections (Table 3). The sample size was sufficient to detect a statistically significant difference (at p < 0.002 for a 2% difference in positivity) for each pathogen except for HIV and T. pallidum, for which there were only one and zero positive subjects, respectively.
Table 3. Bivariate associations between depression and sexually transmitted pathogens

Note: Numbers refer to the total number of subjects that tested positive for the pathogen. Percentages in parentheses refer to the percentage of all subjects in the category that were positive for the specified pathogen. Too few reported positivity for T. pallidum and HIV to meet minimum sample sizes for statistical testing.
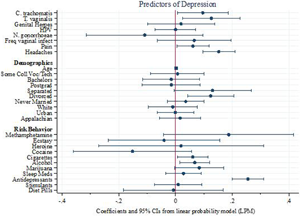
The only pathogens that were significantly correlated with depression in the multiple regression analysis were C. trachomatis and T. vaginalis. Among the demographic variables, the only significant correlates of depression were use of antidepressants, being divorced and smoking cigarettes or marijuana (Figure 1). Results are robust to alternative model specifications and standard logistic diagnostic tests for omission of high-leverage, standardized residuals and deviance.

Figure 1. Linear probability model predicting reports of depression. Points represent coefficients from the most inclusive linear probability model (Table 4, column 3). Bars represent 95% confidence intervals around each point. The first eight rows correspond to infection variables and two variables (pain and headaches) for which infection may be a contributing factor (Doyle et al., Reference Doyle, Swain, Ewald, Cook and Ewald2015).
We report results from heteroskedastic robust LPM for ease of interpretation, although p-values and other patterns are consistent across alternative modeling strategies, including probit and logistic regression models (Appendices 2 and 3, respectively).
Table 4 presents the results of the three LPM models indicating that the inclusion of more covariates highlights specific pathogens as substantively important predictors of reported depression. While several reported infection variables (HPV, frequent vaginal infections, C. trachomatis and T. vaginalis) are significant predictors in the model that account only for other infections (Table 4, column 1), the only infection variables that were significantly correlated with depression in the multiple regression analyses that account for the full set of demographic and behavioral covariates (Table 4, columns 2 and 3) are C. trachomatis and T. vaginalis, with magnitudes similar to those for headaches and pain. All else equal, each of these pathogens is associated with roughly a 10-percentage point increase (0.09 and 0.12, respectively) in the likelihood of reporting depression. Among the demographic and risk behavior variables, the only significant correlates of depression are use of antidepressants, alcohol, being divorced and smoking cigarettes. Associations of separation and marijuana usage are suggestive (p < 0.10) but not statistically significant. Figure 1 visually depicts the results of the most inclusive model (Table 4 column 3).
Table 4. Linear probability models for variables associated with depression

Note: 95% confidence intervals based on Huber–White robust standard errors in brackets below coefficients from three separate LPMs. In column 1 depression is regressed on the six infection variables. Column 2 adds controls for headaches, pain, and demographic characteristics. Column 3 adds controls for risk behavior indicators. †p < 0.1; *p < 0.05; **p < 0.01.
The previous study investigating associations between depression and sexually transmitted pathogens (Doyle et al. Reference Doyle, Swain, Ewald, Cook and Ewald2015) reported a significant association of depression with C. trachomatis and a non-significant association with T. vaginalis. Combined probability tests evaluating the overall findings of that study and the present one using the same (i.e. logistic) statistical model revealed a statistically significant association for C. trachomatis (p < 0.001, χ 2 = 22.87, d.f. = 4) and T. vaginalis (p < 0.05, χ 2 = 9.79, d.f. = 4), but not for any of the other pathogens. Depression occurred more frequently in women who reported infections with C. trachomatis and T. vaginalis (89.5%) than with either C. trachomatis (57.5%) or T. vaginalis (45.8%) alone (p < 0.025, χ 2 = 11.62, d.f. = 4).
Discussion
Our results show that several sexually transmitted pathogens were significantly associated with depression by bivariate analyses; however, only two of the tested pathogens – Chlamydia trachomatis and Trichomonas vaginalis – were significantly associated with depression after correlations among infections and pathogens were accounted for by multiple regression analysis. C. trachomatis, T. vaginalis and the pathogens that were not significantly associated with depression generate inflammation (Georgescu et al. Reference Georgescu, Mitran, Mitran, Caruntu, Sarbu, Matei, Nicolae, Tocut, Popa and Tampa2018; Hafner et al. Reference Hafner, Wilson and Timms2014; Hube et al. Reference Hube, Hay, Brasch, Veraldi and Schaller2015; Stevens and Criss Reference Stevens and Criss2018; Thurman and Doncel Reference Thurman and Doncel2011). The results therefore support the hypothesis that depression results from specific effects of particular pathogens rather than from a general inflammatory response associated with infection.
The lack of association between N. gonorrhoeae and depression is noteworthy because the pathologies of N. gonorrhoeae and C. trachomatis are similar. Each species can cause purulent discharges, pelvic inflammatory disease, oviduct inflammation and scarring, infertility and ectopic pregnancy (Hafner et al. Reference Hafner, Wilson and Timms2014; Stevens and Criss Reference Stevens and Criss2018). If the depression associated with C. trachomatis resulted from the awareness of the presence of these manifestations, then N. gonorrhoeae should have been similarly associated with depression. This difference also bears on the lack of information in the database concerning the timing of knowledge about C. trachomatis infection relative to the timing of depression by weakening the possibility that its association with depression resulted from an effect of knowledge about a urethritis-associated sexually transmitted disease.
The inflammation associated with C. trachomatis and N. gonorrhoeae involves elevation of interferon gamma and activity of neutrophils (Hafner et al. Reference Hafner, Wilson and Timms2014; Stevens and Criss Reference Stevens and Criss2018). Interferon gamma induces expression of indolamine 2,3-dioxygenase, which degrades tryptophan and thereby contributes to tryptophan restriction (Chen Reference Chen2011; Ziklo et al. Reference Ziklo, Vidgen, Taing, Huston and Timms2018). The difference between N. gonorrheae and C. trachomatis with respect to depression therefore cannot be attributed to the absence of this trigger for tryptophan restriction. Correlations between cytokines and depression are, however, complex, defying simple cause/effect explanations (Geisler et al. Reference Geisler, Sperner-Unterweger, Fuchs and Gostner2018). IDO1 and hence tryptophan can be controlled by different mechanisms, and C. trachomatis can elevate IDO1 independently of interferon gamma (Ziklo et al. Reference Ziklo, Huston, Taing and Timms2019).
Upon tryptophan restriction in vitro, genital serovars of C. trachomatis enter a quiescent persistent phase (Aiyar et al. Reference Aiyar, Quayle, Buckner, Sherchand, Chang, Zea, Martin and Belland2014). When indole is present, the tryptophan synthase of C. trachomatis generates tryptophan from the indole, allowing C. trachomatis to emerge from this quiescent phase to multiply and spread in the presence of tryptophan restriction (Aiyar et al. Reference Aiyar, Quayle, Buckner, Sherchand, Chang, Zea, Martin and Belland2014). Inflammation associated with N. gonorrhoeae is not known to be associated with resistance to tryptophan restriction. The ability of C. trachomatis to persist in the presence of tryptophan restriction accords with the possibility that tryptophan restriction may be less effective in controlling C. trachomatis than N. gonorrhoeae and thus C. trachomatis may be associated with more persistent tryptophan restriction and, consequently, depression.
The hypothesized mechanism for an effect of C. trachomatis on depression presumes that tryptophan restriction at the sites of infection lowers tryptophan levels in the blood sufficiently to reduce serotonin synthesis in the brain. Lowered plasma concentrations of tryptophan are thought to reduce tryptophan in the brain as a result of the competition between amino acids at the blood–brain barrier (Fernstrom and Wurtman Reference Fernstrom and Wurtman1997; Pardridge Reference Pardridge1979; Schiepers et al. Reference Schiepers, Wichers and Maes2005). As a result of this competition, even a small reduction in systemic tryptophan might lower serotonin synthesis in the brain (Fernstrom and Wurtman Reference Fernstrom and Wurtman1997). The greater the tryptophan sink is, however, the greater the potential for a reduction in serotonin synthesis. Serum concentrations of tryptophan average about 25% lower in depressed subjects than in subjects with normal mood (Cowen et al. Reference Cowen, Parry-Billings and Newsholme1989).
Persistent, systemic C. trachomatis infections should tend to generate a more substantial tryptophan sink and thus lower mood than infections that are restricted to the urogenital tissue. Reiter's syndrome is the main recognized category of systemic C. trachomatis disease. It is a persistent autoimmune disease that encompasses reactive arthritis and uveitis, with C. trachomatis being found in the joints and the conjunctiva of the eye, respectively (Haller-Schober and El-Shabrawi Reference Haller-Schober and El-Shabrawi2002; Rihl et al. Reference Rihl, Kohler, Klos and Zeidler2006). C. trachomatis reaches the joints via infected monocytes or macrophages (Rihl et al. Reference Rihl, Kohler, Klos and Zeidler2006). Infection of the eye occurs mainly through autoinoculation by urogenitally contaminated hands.
Integration of genetic associations of Reiter's syndrome provides a more comprehensive framework for evaluating the contribution of C. trachomatis to depression. The HLA-B27 allele is present in about 75% of subjects with sexually acquired Reiter's syndrome, which is caused mostly by C. trachomatis; the allele is present in about 90% of individuals with chronic disease (Baguley and Greenhouse Reference Baguley and Greenhouse2003). Pathological effects of HLA-B27 appear to occur in response to microbes: HLA-B27 transgenic rats have inflammatory joint disease except when they are germ-free (Taurog et al. Reference Taurog, Richardson, Croft, Simmons, Zhou, Fernandez-Sueiro, Balish and Hammer1994). Depression has been found to be more common in uveitis patients who were HLA-B27 positive, with about half of these subjects being mildly or clinically depressed according to Beck Depression Inventory tests (scores ≥ 9; Maca et al. Reference Maca, Schiesser, Sobala, Gruber, Pakesch, Prause and Barisani-Asenbauer2011). These findings accord with the hypothesis that persistent, systemic C. trachomatis infections may particularly associated with depression. We expect that C. trachomatis-infected reactive arthritis patients would also be prone to depression, but to our knowledge this possibility has not been investigated.
The mechanistic reason for the association between T. vaginalis and depression is unclear, but T. vaginalis synthesizes indole which may be used by C. trachomatis as a substrate for synthesis of tryptophan (Aiyar et al. Reference Aiyar, Quayle, Buckner, Sherchand, Chang, Zea, Martin and Belland2014; Lloyd et al.n Reference Lloyd, Lauritsen and Degn1991; Zubacova et al. Reference Zubacova, Krylov and Tachezy2011) and thus foster persistence of C. trachomatis (Ziklo et al. Reference Ziklo, Huston, Taing, Katouli and Timms2016). The higher frequency of depression among women who reported having both C. trachomatis and T. vaginalis infections is consistent with an exacerbating effect of T. vaginalis on C. trachomatis.
Our test focused on sexually transmitted pathogens because they tend to be persistent, but the arguments should apply to other pathogens that can persist in the presence of tryptophan restriction as a result of an ability to synthesize their own tryptophan. The presence of tryptophan synthase is not necessarily an indicator of this ability – C. trachomatis is functional in the presence of tryptophan restriction by virtue of mutations in its tryptophan synthase operon (Somboonna et al. Reference Somboonna, Ziklo, Ferrin, Hyuk Suh and Dean2019), suggesting an evolutionary arms race between host abilities to silence tryptophan synthesis and the ability of C. trachomatis to circumvent this silencing. To our knowledge the only other pathogen for which tryptophan synthesis is known to be used as part of a persistence strategy is Mycobacterium tuberculosis, which upregulates tryptophan synthase upon infection of epithelial cells (Ryndak et al. Reference Ryndak, Singh, Peng and Laal2015) and uses tryptophan-embedded phagosomal membranes to prevent fusion of the phagosome with lytic vesicles in macrophages, thereby evading intracellular destruction (Ferrari et al. Reference Ferrari, Langen, Naito and Pieters1999; Meena and Rajni Reference Meena and Rajni2010). Deletion of a tryptophan synthase gene in M. tuberculosis reduced persistence within macrophages in vitro as well as in lungs and spleen in a murine model (Smith et al. Reference Smith, Parish, Stoker and Bancroft2001). As is the case with C. trachomatis, failure of tryptophan restriction to resolve M. tuberculosis infection may therefore lead to persistent tryptophan restriction and low mood. Accordingly, depression is a major manifestation of tuberculosis (Ige and Lasebikan Reference Ige and Lasebikan2011).
The recognition that particular pathogens are associated with depression bears on the interpretation of drug effects. Minocycline, for example, ameliorates depression, an association that has been interpreted to result from its direct anti-inflammatory effects (Rosenblat and McIntyre Reference Rosenblat and McIntyre2018). Minocycline is effective against C. trachomatis (Romanowski et al. Reference Romanowski, Talbot, Stadnyk, Kowalchuk and Bowie1993) and M. tuberculosis (Deshpande et al. Reference Deshpande, Pasipanodya, Srivastava, Martin, Athale, van Zyl, Antiabong, Koeuth, Lee, Dheda and Gumbo2019) and could therefore ameliorate depression by controlling these pathogens. Our results therefore emphasize the need to evaluate the extent to which any ameliorative effects of antimicrobials are due to suppression of pathogens relative to direct suppression of inflammation.
We think that the main value of our findings lies in indicating directions for future study. The correlational nature of our data do not allow us to assign cause and effect. Knowledge about the presence of C. trachomatis infection might have contributed to depression, even though the lack of an association of depression with N. gonorrhoeae indicates that knowledge about the presence of a C. trachomatis infection is insufficient to generate the observed association. The lack of adequate sample size for HIV and T. pallidum does not allow us to evaluate an association of these pathogens with depression. Each has been associated with depression but the psychological effects of knowledge of chronic syphilis or AIDS on mood has not been distinguished from direct effects of the pathogens on mood.
The absence of information on temporal sequence of depression and other variables in our database prevents an assessment of whether infection occurred before depression or vice versa. Women could have an infection without knowing it because infections are often asymptomatic (e.g. with N. gonorrhoeae and C. trachomatis) and may have erred in reporting depression or infectious conditions. These factors undoubtedly will create variability in the results but should not have generated the observed associations. The presence of an association of depression with C. trachomatis but the absence of such an association with N. gonorrhoeae serves as a control for these uncertainties, because the symptoms of these infections and the prevalence of asymptomatic relative to symptomatic infections are similar for these two pathogens. We note, however, that numbers of infected individuals were still relatively small, particularly for N. gonorrhoeae and T. vaginalis, a fact that could influence the outcome of statistical testing. Additional analyses with larger samples sizes and assessment of the temporal sequence of infection relative to depression will be useful.
Animal studies may provide a useful direction for evaluating cause and effect as well as the potential for generality of infectious causation of depression beyond humans. One limitation of animal models is the difficulty in knowing whether an animal is depressed. Social withdrawal could result from other phenomena, such as malaise, pain, distrust, fear or insecurity. One of the most reliable examples of infection-induced depression in another host species is sad horse disease, which is caused by borna disease virus and is associated with affect that corresponds to human depression (Tizard et al. Reference Tizard, Ball, Stoica and Payne2016). In rats, borna disease virus infections of the central nervous system are associated with elevated IDO, suggesting that they also probably produce reductions in tryptophan (Formisano et al. Reference Formisano, Hornig, Yaddanapudi, Vasishtha, Parsons, Briese, Lipkin and Williams2017).
Animal models may also be helpful for evaluating alternative mechanisms. Mice infected with Bacille Calmette Guérin (= BCG, a strain derived from M. tuberculosis bovis) appear chronically depressed. BCG induced cytokines (TNF alpha and interferon gamma), which led to tryptophan catabolism via IDO. Accordingly, pre-treatment with an IDO inhibitor blocked BCG-induced depression, and IDO-deficient mice were resistant to BCG induction of depression even though they produced inflammatory cytokines associated with BCD infection (Moreau et al. Reference Moreau, Lestage, Verrier, Mormede, Kelley, Dantzer and Castanon2005; O'Connor et al. Reference O'Connor, Lawson, Andre, Briley, Szegedi, Lestage, Castanon, Herkenham, Dantzer and Kelley2009). These findings support a contribution of IDO activity to depression that is distinct from other effects of inflammatory cytokines.
Our results suggest that research needs to look beyond inflammation per se to processes associated with specific aspects of the inflammatory response, such as tryptophan restriction, and the effects of particular pathogens. The set of pathogens considered in this study was limited to those reported to the health registry. Our data set did not include, for example, cytomegalovirus infection, which causes persistent infections and has been correlated with depression in studies that did not find associations between depression and general markers of inflammation (Simanek et al. Reference Simanek, Cheng, Yolken, Uddin, Galea and Aiello2014; Simanek et al. Reference Simanek, Parry and Dowd2018a, b). Cytomegalovirus has been associated with tryptophan degradation in vivo (Sadeghi et al. Reference Sadeghi, Lahdou, Daniel, Schnitzler, Fusch, Schefold, Zeier, Iancu, Opelz and Terness2012). Similarly, H. pylori, which has been associated with depression in women (Simanek et al. Reference Simanek, Parry and Dowd2018a), has also been associated with elevated levels of the tryptophan-degrading enzyme, IDO1 (Larussa et al. Reference Larussa, Leone, Suraci, Nazionale, Procopio, Conforti, Abenavoli, Hribal, Imeneo and Luzza2015). Each of these two pathogens can cause infections that may persist for decades and may therefore be associated with chronic lowering of tryptophan levels resulting in depressed mood.
The associations of particular persistent infectious agents (C. trachomatis, M. tuberculosis, H. pylori and cytomegalovirus) with depression, tryptophan depletion and resistance to tryptophan depletion support the hypothesis that at least some depression may result from evolutionary arms races with particular pathogens. The presence of tryptophan restriction by IDO1 in rodents suggests a deep evolutionary presence of this adaptation in placental mammals. More broadly, molecular phylogenies indicate that IDO1 and its high affinity for tryptophan occurs in monotremes, marsupials and placentals, but not in birds, amphibians or fish. This pattern suggests that IDO1 evolved its tryptophan catabolic functions in monotremes from an IDO2-like molecule (Yuasa et al. Reference Yuasa, Takubo, Takahashi, Hasegawa, Noma and Suzuki2007; Yuasa et al. Reference Yuasa, Ball, Ho, Austin, Whittington, Belov, Maghzal, Jermiin and Hunt2009). This pattern suggests that tryptophan restriction has evolved as a mammalian adaptation for regulating tryptophan and potentially as defense against pathogens before the divergence of these three groups of mammals. A short-term depressive effect on mood may have been a component of this defense by encouraging rest and recovery. Selection on pathogens to persist, however, apparently has led to persistent tryptophan restriction as an ineffective by-product of the adaptive response that is ineffective for the particular pathogens that evolved the upper hand in the evolutionary arms race associated with this defense. This interpretation together with the findings reported in this paper draw attention to the need for research on infection, inflammation and depression that encompasses the entire spectrum of persistent infectious agents with attention to the possible effects that particular pathogens have on depression through induction of persistent tryptophan restriction.
Data availability statement
The data that support the findings of this study are available from The University of Kentucky's Wellness, Health and You data resource (https://www.wellnesshealthandyou.org), formerly the Kentucky Women's Health Registry. Restrictions apply to the availability of these data. A data use agreement was signed to allow use by the authors and thus are not publicly available from the authors.
Acknowledgements
This research was made possible by Wellness, Health and You (WHY) and the volunteers who graciously participated in this health survey. The primary investigators for the WHY survey are Heather Bush, PhD and Ann L. Coker, PhD. The WHY project is based at the University of Kentucky and has been supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR001998. The content of the database is solely the responsibility of the WHY investigators and does not necessarily represent the official views of the NIH. The authors thank Candace Brancato at WHY for extracting data and performing the first round of bivariate tests for the current study.
Author contributions
C.M.D., P.W.E. and H.A.S. generated the conceptual framework for the paper and reviewed the literature. H.S.E. originated and developed the connection between tryptophan restriction and depression. C.M.D arranged for access to the database and ran the final bivariate tests. C.M.D. and P.W.E contributed equally to manuscript preparation and conducted the combined probability analyses. W.A.S. performed the multivariate analysis and wrote the corresponding portions of the Methods and Results sections.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Publishing ethics
All authors declare that the manuscript is their own original work and does not duplicate another published work. The manuscript has been submitted only to this journal – it is not under consideration, accepted for publication or in press elsewhere. All listed authors know of and agree to the manuscript being submitted to the journal. The manuscript contains nothing that is abusive, defamatory, fraudulent, illegal, libelous or obscene.
Conflicts of interest
C.M.D., W.A.S., H.A.S., and P.W.E declare no conflict of interests.
Appendix 1: Questions from the Kentucky Women's Health Registry survey version 8.1 related to depression and infection

Appendix 2: Probit estimates of association with depression

Appendix 3: logit estimates of association with depression