To the editor,
An increasing number of investigations on the use of invasive and non-invasive brain stimulation for the treatment of depression have been performed in the last decade [2,4,5,9,10]. We recently showed that a simple and powerful technique of brain modulation – transcranial direct current stimulation (tDCS) – is effective to reduce depressive symptoms [1,3]. In tDCS, low amplitude direct currents are injected into the brain via scalp electrodes [7,8,11]. As shown by recent modeling studies, a significant amount of electric current can reach the brain using appropriately large electrodes and suitable placements [6,11]. An important question is whether the antidepressant effects induced by tDCS are similar to those of standard medical treatment. We compared the findings of a parallel-group, randomized, double-blind clinical trial investigating the effects of active vs. sham tDCS with an open-label trial in which patients with similar clinical characteristics received fluoxetine, a frequently prescribed serotonin reuptake inhibitor.
Forty-two patients with major depression participated in the study (28 females, mean age of 49.4 (±7.4) years (mean ± SD)). Diagnosis of unipolar major depressive disorder was confirmed by a licensed, senior clinical psychiatrist (S.P.R.) using the structured clinical interview for DSM-IV axis I disorders. Patients were required to be off medications (antidepressants) for 2 months prior to the trial. Exclusion criteria were neurological disorders, any comorbid axis I disorders, or substance abuse within 3 months of study participation. Furthermore, patients with major depression and psychotic features or axis II disorders were excluded. Written, informed consent was obtained from all participants before inclusion in the study, which was approved by the local ethics committee. The study was performed at the Psychiatric Institute of the University of Sao Paulo.
We compared the results of patients who received active and sham tDCS in a randomized, double-blind study as described in detail elsewhere [Reference Boggio, Rigonatti, Myczkowski, Nitsche, Pascual-Leone and Fregni1]. In summary, the parameters of stimulation were: 2 mA of intensity for 20 min for 10 days (anodal electrode on the left dorsolateral prefrontal cortex and cathode electrode on the contralateral supraorbital area). All patients were assessed at baseline and after 2, 4, and 6 weeks after the onset of treatment (tDCS or fluoxetine). Rating scales included the Beck Depression Inventory (BDI) and the 21-item Hamilton Depression Rating Scale (HDRS). In addition, we measured general cognitive performance using the mini-mental state examination (MMSE). Rating was performed by a trained and experienced psychologist (M.L.M.). We used a mixed linear model to analyze mood changes throughout the trial. We modeled mood change (as indexed by BDI and HDRS) using the covariates of time, group and interaction term between group and time. Because the fluoxetine data were originated from an open arm of this study, we used the BDI as the primary outcome to decrease the influence of the unblinded rater.
Patients were moderately depressed (see characteristics in Table 1 – there were no significant differences in demographic and clinical characteristics among the three groups of treatment). For our primary outcome (BDI), the mixed model revealed a significant interaction term group vs. time (F 6,117 = 5.49, p = 0.0001). In addition, further models comparing two groups showed a significant difference between active and sham tDCS groups (F 3,87 = 7.29, p = 0.0002); but not between fluoxetine and active tDCS (F 3,120 = 0.72, p = 0.54). The comparison between fluoxetine and sham tDCS only reached a trend for a significant difference (F 3,57 = 2.21, p = 0.09) (see Fig. 1). Immediately after 2 weeks of treatment, depression was decreased by 43.1% (±30.9) in the active tDCS group and by 15.0% (±35.2) in the fluoxetine group; after 6 weeks, however, depression reduction was similar in both groups of treatment (36.2% (±38.9) and 38.1% (±36.9), respectively). The HDRS scores revealed similar results: a significant overall interaction term (F 6,117 = 11.05, p < 0.0001) and significant differences between active and sham tDCS groups (F 3,87 = 4.04, p = 0.01), and sham tDCS and fluoxetine (F 3,57 = 16.4, p < 0.0001); but not between fluoxetine and active tDCS (F 3,120 = 2.01, p = 0.11).
Table 1 Demographic and clinical characteristics

tDCS – transcranial direct current stimulation; DLPFC – dorsolateral prefrontal cortex; HDRS – Hamilton Depression Rating Scale; SD – standard deviation; ns – not significant.
a As indexed by the number of failed antidepressants.
b p-Value – one-way ANOVA for continuous variables and Fisher's exact test for categorical variables.
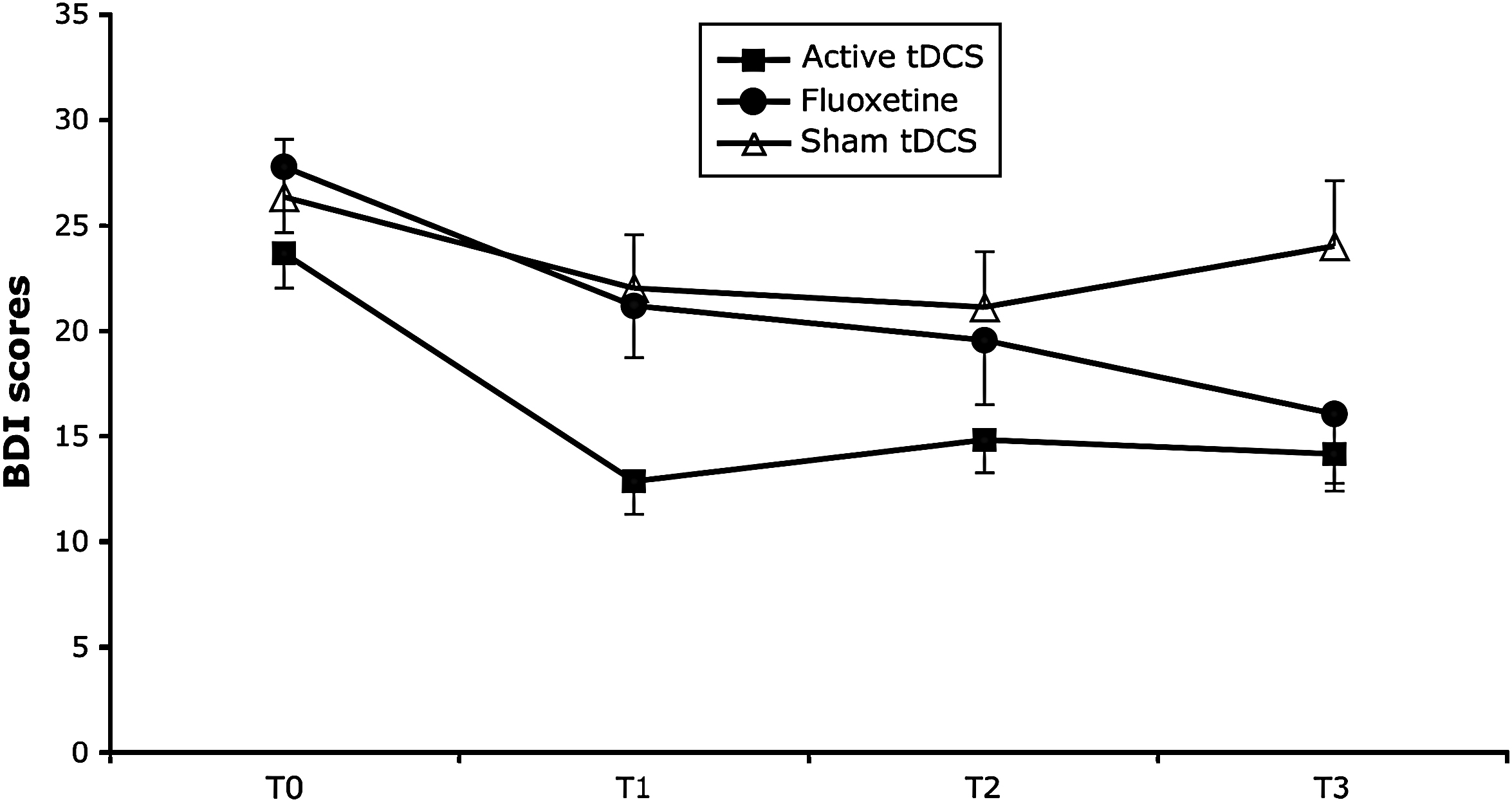
Fig. 1 Depression scores (as indexed by the Beck Depression Inventory (BDI)) change over time. BDI scores were assessed at baseline and after 2, 4 and 6 weeks of the treatment onset. Each point represents mean and error bars represent S.E.M.
The results of this study show that the antidepressant effects of non-invasive brain stimulation with tDCS are similar to those of a 6-week course of fluoxetine at a relatively small dose of 20 mg/day. However, the antidepressant benefit of tDCS appears to become significant faster than the benefit of fluoxetine.
We acknowledge that this study has several limitations. First, the comparison was made with different groups of patients that therefore could have had different baseline clinical characteristics; however, these were identical across these three groups regarding baseline clinical and demographic characteristics. Furthermore, these patients were recruited in the same service and during a similar period of time. Second, the fluoxetine trial was open-label, therefore a greater placebo response is possible. We therefore used the BDI as the main outcome to reduce the bias of the unblinded rater. In addition, the fact that the fluoxetine trial was open strengthens the findings of antidepressant efficacy of active tDCS, since tDCS was applied in a sham-controlled, double-blind fashion, and in a placebo-controlled trial fluoxetine might have been found to be less effective.
In summary, our findings encourage further prospective studies to explore the comparison between tDCS and antidepressants.
Acknowledgments
This work was supported by a research grant from FAPESP (05/00821-8). F.F. is supported by grants from NIH (DK071851-01) and the Harvard University David Rockefeller Center – Jorge Paulo Lemann Fellowship. A.P.-L. is supported by an NIH grant K24 RR018875. The authors are thankful to Barbara Bonetti for the invaluable help in the coordination of this study.





Comments
No Comments have been published for this article.