Introduction
Managing antipsychotic-induced weight gain (AIWG) is challenging for patients, clinicians, and policy makers alike. This is partly due to extensive interindividual variability in anthropometric outcomes following antipsychotic commencement. Although antipsychotic choice is an established differentiator of risk [Reference Bak, Fransen, Janssen, van Os and Drukker1], genetic and nongenetic prognostic factors have also been studied for their influence on AIWG prognosis [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2–Reference Reynolds4]. A systematic review of pharmacogenomic associations of AIWG concluded that effect sizes of individual gene variants were too small to fulfill the promise of personalized medicine, and that future studies should explore the effects of combining genetic markers alongside clinical variables to improve prediction [Reference Zhang, Lencz, Zhang, Nitta, Maayan and John3]. However, no similar review of non-genetic prognostic factors has been undertaken to inform such work. Though often ignored amidst the drive for more complex genetic measures, many simple and routinely collected patient characteristics have been shown to influence the prognosis of medication side effects [Reference Wei, Chung-Yee Hui, Chang, Ho, Yang and Lin5, Reference Liao, Mao, Chen, Deng and Hsu6].
A range of pre-antipsychotic inititation biological (e.g., thyroid functioning, insulin resistance), clinical (e.g., positive and negative symptom burden), and sociodemographic (e.g., age, gender) variables have been evaluated for their influence on AIWG trajectory [Reference Gebhardt, Haberhausen, Heinzel-Gutenbrunner, Gebhardt, Remschmidt and Krieg7–Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11]. In the absence of a systematic appraisal of this research, the value of non-genetic prognostic factors in influencing AIWG prognosis is currently unknown. This includes the number of factors studied and the clinical utility and reliability of reported prognostic associations, for example, in influencing the stratified use of preventative AIWG interventions. Whether current research supports the use of prognostic factors to identify those at the highest risk of AIWG before or upon starting antipsychotic treatment is a question of significant importance to both practitioners and patients given the limited availability of either non-pharmacological or pharmacological management options to reverse AIWG[Reference Vancampfort, Firth, Correll, Solmi, Siskind and De Hert12, Reference Lee, Abraham and Cleaver13]. Whether the value of current non-genetic prognostic factor research is primarily in informing future research, for example, prognostic model development, needs to be addressed. Consequently, non-genetic prognostic factors represent a potentially underexploited resource within AIWG prognostication.
Objectives
The aim of this review was to identify, synthesize, and appraise research evaluating non-genetic prognostic factors and their association with anthropometric outcomes following antipsychotic initiation. The research question addressed was as follows: Among antipsychotic-naïve adults with a first episode of psychosis, are there non-genetic prognostic factors that reliably influence weight and associated outcomes following antipsychotic commencement? Review objectives were as follows:
-
(i) Identify what non-genetic prognostic factors have been investigated for their role in AIWG prognosis.
-
(ii) Determine the direction, strength, and quality of all prognostic factor-outcome associations.
-
(iii) Explore the clinical utility of any significant and reliable prognostic factor-outcome associations.
For the purposes of this review, a non-genetic prognostic factor-outcome association is defined as any association that does not include the study of a gene variant and its relationship with AIWG prognosis. This includes, but is not limited to, measurement of clinical, sociodemographic, or biological variables and their potential role as prognostic factors.
Methods
The protocol was registered on PROSPERO (CRD42021258148) and published separately [Reference Fitzgerald, Crowley, Byrne, O’Connell, Ensor and Dhubhlaing14]. A detailed outline of review methods is contained within the protocol and a brief description only is provided here. Protocol deviations and a copy of the completed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for this review are contained in the Supplementary Material [15].
Eligibility criteria
A summary of the modified PICOTS approach recommended for systematic reviews of prognostic factors applied to this review is outlined in Table 1 [Reference Riley, Moons, Snell, Ensor, Hooft and Altman16].
Table 1. Modified PICOTS criteria applied to this review

We included both randomized controlled trials (RCTs) and prospective non-randomized studies (NRS) that had a clear inception point. We excluded retrospective or cross-sectional studies to increase evidence certainty [Reference Riley and Moons17]. We accepted studies where the prognostic factor effect size was unadjusted or adjusted for other known prognostic factors. We excluded studies that looked solely at antipsychotic class/subclass or gut microbiome variants as potential prognostic factors. We included studies that were classified as exploratory or confirmatory in design. Confirmatory studies are designed to test the independence of a prognostic factor association and thus, provide more conclusive evidence compared to exploratory studies [Reference Riley, Moons, Snell, Ensor, Hooft and Altman16]. We classified studies as exploratory or confirmatory according to the author’s objectives and approach to study design and analysis. Strict inclusion criteria were used to limit heterogeneity across studies and facilitate a more meaningful interpretation of synthesized results.
Search strategy
We conducted focused and broad electronic searches using indexed and free-text words and phrases relating to prognosis, adults with psychosis, and antipsychotic-induced anthropometric changes. PubMed, CENTRAL, PsycINFO, and Embase were initially searched from inception until November 30, 2021. Reference searching, forward citation searching, searching of trial registers, and contacting content experts were undertaken between January 21 and September 30, 2022. The electronic database search was repeated before review completion to include articles published until May 7, 2023 to ensure newly published research was included in the review. Non-English language studies and grey literature were excluded.
Study selection
Rayyan (rayyan.ai) was used to screen the title and abstracts of all electronic searches. A pre-tested Microsoft Excel sheet was used for studies identified through other sources. Two reviewers independently screened all titles and abstracts (IF, LS, AB, and EC). Disagreements were resolved via consensus and discussion with a third independent reviewer, where required. Study authors were contacted to clarify queries on study conduct or design. The same process was repeated for all full-text articles retrieved. Where multiple studies appearing to use the same or overlapping participant data were identified, we classified the primary study as the publication presenting the most relevant or comprehensive data for our review question.
Data extraction and management
Data extraction was undertaken using a version of the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modeling Studies – Modified for Prognostic Factor Studies (CHARMS-PF) [Reference Riley, Moons, Snell, Ensor, Hooft and Altman16]. The modified checklist can be found in the study protocol [Reference Fitzgerald, Crowley, Byrne, O’Connell, Ensor and Dhubhlaing14]. We extracted all unadjusted and adjusted measures of association and variance estimates from each study. In the case of continuous outcomes, we extracted beta-coefficients and their standard errors (SE). We gave preference to extracting unstandardized beta coefficients (referred to as “b”) to facilitate result interpretation. Where unstandardized coefficients were unretrievable, the standardized beta coefficient (β) was extracted. Only unstandardized coefficients were eligible for meta-analysis. For the outcome risk of clinically significant weight gain (CSWG), we extracted odds ratios (OR) and SE. If these estimates were unavailable, we attempted to recover them using alternative available information provided. Before conversions were undertaken, we contacted authors to request missing results.
Risk of bias
Risk of bias was assessed independently by pairs of review authors (IF, EC, LS, JOC, and CNiD) using the Quality in Prognosis Studies (QUIPS) tool [Reference Hayden, van der Windt, Cartwright, Côté and Bombardier18]. Each domain was judged as being at high, moderate, or low risk of bias. An overall rating of bias to a given study is not recommended when using QUIPS [Reference Hayden, van der Windt, Cartwright, Côté and Bombardier18]. Disagreements between reviewers were resolved via consensus and recourse to a third author, where necessary. Attempts were made to contact study authors for information required to accurately complete assessments.
Data synthesis
Meta-analysis was conducted when usable data were available reporting the prognostic association between a factor and outcome in ≥ 3 studies deemed sufficiently homogenous. Only adequately adjusted results were considered suitable for meta-analysis [Reference Riley, Moons, Snell, Ensor, Hooft and Altman16]. We defined a minimum set of adjustment factors based on existing evidence of their association with weight trajectory in the general population (age, sex, ethnicity) [Reference Hruby and Hu19], and known influence of antipsychotic prescription on weight prognosis [Reference Bak, Fransen, Janssen, van Os and Drukker1]. We conducted all analyses via STATA (StataCorp version 17) with a random-effects generic inverse variance meta-analysis model. Restricted maximum likelihood estimation (REML) was used to fit all analyses, with 95% confidence intervals (CI) derived using the Hartung–Knapp Sidik–Jonkman (HKSJ) approach, to account for uncertainty in estimated variances [Reference Langan, Higgins, Jackson, Bowden, Veroniki and Kontopantelis20]. Where it was not appropriate to combine studies quantitatively, results were assessed qualitatively. Although our protocol outlined how we would assess for publication bias quantitatively and potential subgroup analyses, in both cases insufficient study numbers prohibited this.
Certainty of evidence
Evidence quality of each prognostic factor-outcome association was assessed using a Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach modified for prognostic factor research [Reference Huguet, Hayden, Stinson, McGrath, Chambers and Tougas21]. A quality rating in support of each prognostic factor-outcome association assessed across studies was assigned as high, moderate, low, or very low. Evidence quality was downgraded according to early phase of investigation, study limitations, inconsistency, indirectness, imprecision, and publication bias. Publication bias was assumed in the case of all factors unless a similar association between the factor and outcome had been repetitively assessed in independent studies. Evidence quality could be upgraded where evidence of a moderate–large effect size or exposure-response gradient was consistently demonstrated [Reference Huguet, Hayden, Stinson, McGrath, Chambers and Tougas21]. GRADE assessments were initially conducted by IF and reviewed independently by a second author (EC and LS). Disagreements were resolved via discussion and recourse to a third reviewer, where necessary.
Results
Study selection
After duplicate removal, database searching yielded 3845 articles for the title and abstract screening. Of 164 eligible for full-text review, 18 met the inclusion criteria. Of 123 assessed, a further 9 eligible studies were identified through other search methods. Studies involving apparent duplicate populations were identified in 10 cases. Additionally, in a small number of studies there was evidence of potentially overlapping populations, without clearly highlighting the availability of separate publications elsewhere. Twenty-seven studies were identified for final inclusion. Figure 1 contains a PRISMA flow diagram of the study selection process.

Figure 1. PRISMA flow diagram of study selection process.
Study characteristics
Table 2 contains a detailed overview of study characteristics. Seventy-two unique prognostic factors were assessed across studies involving 4426 participants. Across studies, 85% were prospective cohort studies, and the remaining retrospective analyses of RCTs. Study populations comprised both inpatient and outpatient cohorts. 13/27 (n = 3053) studies were classified as exploratory and 14/27 (n = 1373) confirmatory in design. Confirmatory studies generally involved comparatively smaller participant numbers (n < 100), except for two studies [Reference Chen, Li, Zhang, Zhu, Wu and Guan22, Reference Liu, Yu, Gao, Li, Guan and Thomas28]. Approximately 37% of studies were conducted in exclusively Chinese populations, although these were primarily exploratory. Change in body mass index (BMI) was the most studied outcome, followed by change in weight, risk of CSWG, and change in waist circumference, respectively. Median follow-up time was 12 weeks (IQR 18) and ranged from 4 weeks to 6 years. 46% of analyses were considered adequately adjusted for covariates. Inadequately adjusted assignment was most commonly due to lack of adjustment for varying antipsychotic prescription [Reference Zhang, Yao, Zhang, Chen, Sun and Yao33, Reference Verma, Liew, Subramaniam and Poon34, Reference Canal-Rivero, Ruiz-Veguilla, Labad, Ayesa-Arriola, Vázquez-Bourgon and Mayoral-van Son37, Reference Lago, Tomasik, van Rees, Rubey, Gonzalez-Vioque and Ramsey41, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43]. Only one study evaluating birth weight as a prognostic factor assessed for the presence of a non-linear relationship [Reference Garriga, Fernandez-Egea, Mallorqui, Serrano, Oliveira and Parellada40].
Table 2. Overview of study characteristics of all studies included in the review
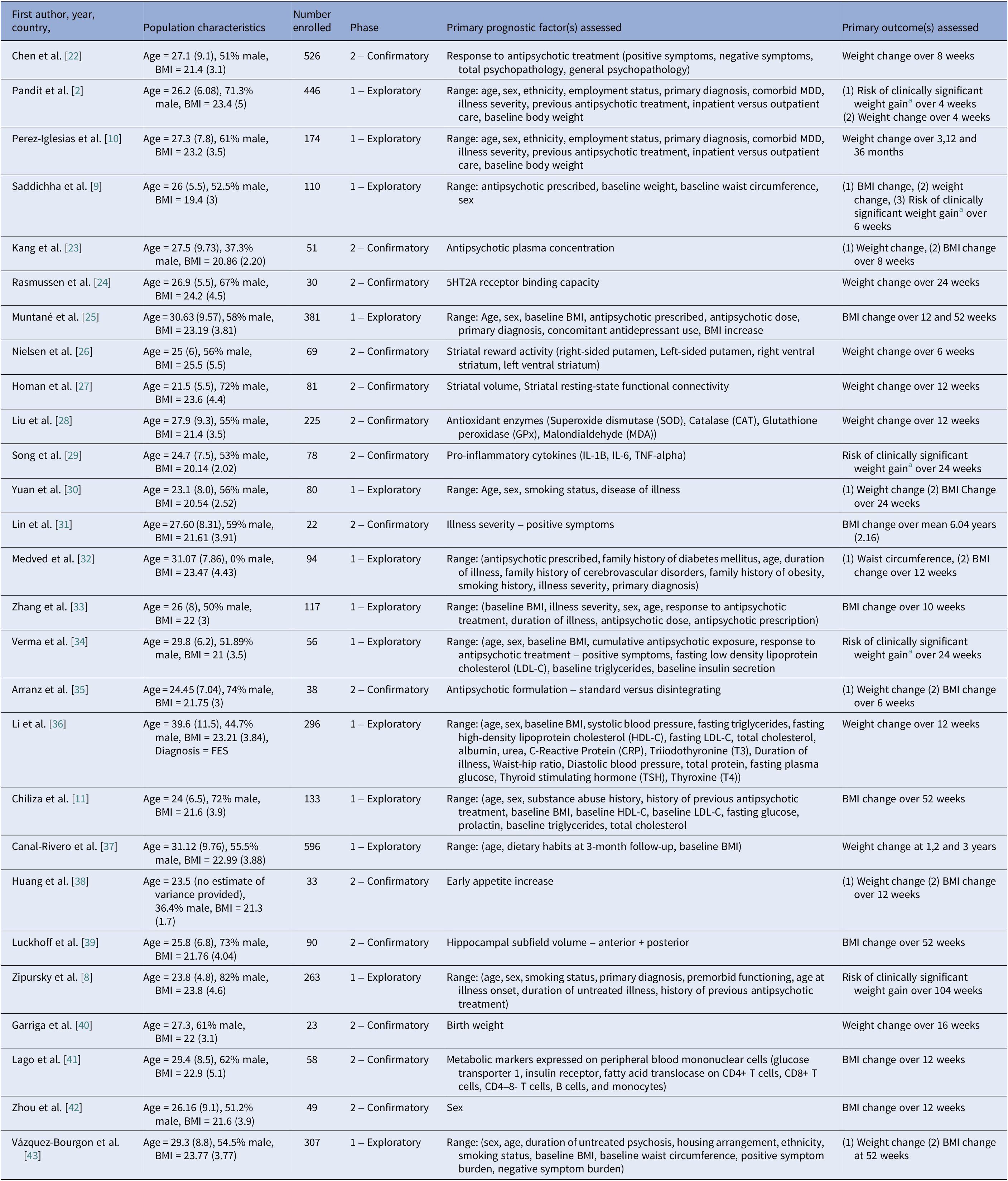
Abbreviations: BMI, body mass index; MDD, major depressive disorder.
a Clinically significant weight gain was defined in all studies as a 7% or greater increase in baseline body weight.
Prognostic factor characteristics
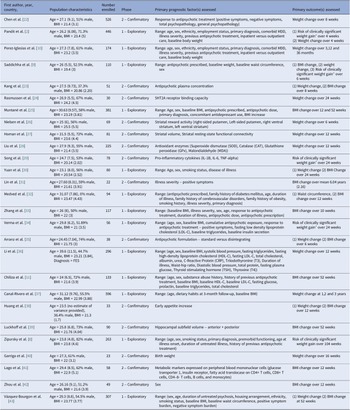
A detailed overview of all prognostic factors assessed and reported study estimates are contained within the Supplementary Material. Categories of factors assessed spanned clinical (e.g., psychopathology, comorbid psychiatric diagnoses), neurological (e.g., hippocampal volume, striatal functioning), biological (e.g., antioxidant enzymes, pro-inflammatory cytokines), social (e.g., smoking status), medical (e.g., co-medications), sociodemographic (e.g., age, ethnicity), anthropometric (e.g., pre-antipsychotic weight) and cardiometabolic (e.g., fasting plasma glucose) groupings. The most frequently studied prognostic factor-outcome associations can be found in Figure 2, although in many cases results were not of practical significance. Only moderate-quality results and/or results with potential significant practical impact will be discussed in additional detail in the discussion section. Of the 72 assessed, 65% of prognostic factors were evaluated in a single study. In only two studies was antipsychotic adherence accounted for through design or analysis [Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11, Reference Luckhoff, du Plessis, Kilian, Asmal, Scheffler and Phahladira39]. One study assessed the impact of diet and lifestyle as a prognostic factor [Reference Canal-Rivero, Ruiz-Veguilla, Labad, Ayesa-Arriola, Vázquez-Bourgon and Mayoral-van Son37]. No analysis adjusted for diet and lifestyle as a covariate.

Figure 2. Sankey chart outlining the most frequently studied prognostic factor-outcome associations. Thickness of each line connecting a prognostic factor with an outcome depends on the number of participants across studies examining the association. Each outcome studied is reflected in the diagram by a different color. Lines highlighted in pink specifically indicate moderate quality supporting evidence as per GRADE assessment. * Indicates moderate–large effect size demonstrated. ** Very low-quality supporting evidence for this prognostic factor-outcome association.
Findings from meta-analyses
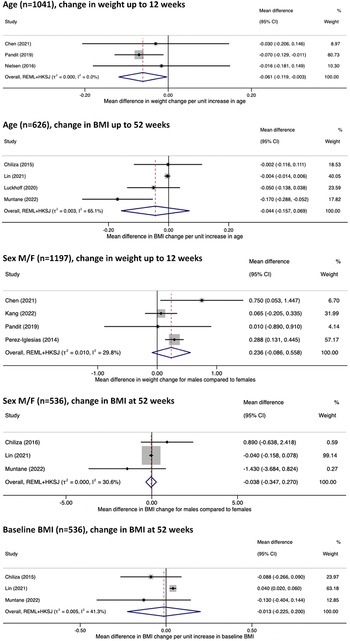
Prognostic factors eligible for meta-analysis were significantly limited by single study assessments, evaluation of different outcomes (BMI versus weight change) and at varying timelines, and heterogeneity of adjusted covariates. Inclusion in meta-analysis was also hampered by varying measurements of prognostic factors for example, antipsychotic prescription being classified by grouping or by antipsychotic prescribed [Reference Saddichha, Ameen and Akhtar9, Reference Perez-Iglesias, Crespo-Facorro, Martinez-Garcia, Ramirez-Bonilla, Alvarez-Jimenez and Pelayo-Teran10, Reference Chen, Li, Zhang, Zhu, Wu and Guan22, Reference Muntané, Vázquez-Bourgon, Sada, Martorell, Papiol and Bosch25]. Incomplete reporting of results was an additional barrier to meta-analysis [Reference Song, Fan, Li, Zhang, Gao and Zhao29]. Adjusted estimates of age, sex, and baseline BMI on change in weight and BMI were the only associations judged to be suitable for meta-analysis. The results are displayed in Figure 3. Confidence intervals were wide for all analyses, reflecting large uncertainty due to the small numbers of available study estimates for synthesis. Limited study estimates also led to greater uncertainty in the estimated heterogeneity (tau-squared), and confidence intervals were inflated using the HKSJ method to better account for this uncertainty [44].

Figure 3. Forest plot of adjusted estimates effects of age, sex, and pre-antipsychotic (baseline) BMI studied for their impact on change in BMI and weight at short- and long-term outcomes.
Risk of bias
All studies presented with a moderate risk of bias in at least one domain, and 37% with high risk in at least one domain. The most common section to receive a high risk of bias was statistical analysis and reporting, followed by study attrition and adjustment for other prognostic factors, respectively. Statistical pitfalls noted across studies included insufficient data presentation to assess the adequacy of the analytical approach and inappropriate or misleading modeling strategies for example, reliance on univariable analyses [Reference Saddichha, Ameen and Akhtar9, Reference Perez-Iglesias, Crespo-Facorro, Martinez-Garcia, Ramirez-Bonilla, Alvarez-Jimenez and Pelayo-Teran10, Reference Song, Fan, Li, Zhang, Gao and Zhao29, Reference Li, Gao, Lv, Zhang, Wang and Jiang36]. No study reported an a priori sample size calculation. Incomplete or selective reporting of analyses based on presence or absence of statistically significant results was another potential source of bias noted [Reference Liu, Yu, Gao, Li, Guan and Thomas28, Reference Zhang, Yao, Zhang, Chen, Sun and Yao33, Reference Canal-Rivero, Ruiz-Veguilla, Labad, Ayesa-Arriola, Vázquez-Bourgon and Mayoral-van Son37, Reference Garriga, Fernandez-Egea, Mallorqui, Serrano, Oliveira and Parellada40]. Inadequate adjustment for covariates limited result interpretation and aggregation [Reference Saddichha, Ameen and Akhtar9, Reference Homan, Argyelan, Fales, Barber and Szeszko27, Reference Medved, Kuzman, Jovanovic, Grubisin and Kuzman32, Reference Verma, Liew, Subramaniam and Poon34, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43]. In the case of attrition, sources of potential bias included inappropriate handling of missing data for example, complete case analysis [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Perez-Iglesias, Crespo-Facorro, Martinez-Garcia, Ramirez-Bonilla, Alvarez-Jimenez and Pelayo-Teran10, Reference Rasmussen, Ebdrup, Oranje, Pinborg, Knudsen and Glenthøj24, Reference Muntané, Vázquez-Bourgon, Sada, Martorell, Papiol and Bosch25, Reference Liu, Yu, Gao, Li, Guan and Thomas28, Reference Yuan, Zhang, Wang, Liu, Li and Kumar30, Reference Garriga, Fernandez-Egea, Mallorqui, Serrano, Oliveira and Parellada40, Reference Lago, Tomasik, van Rees, Rubey, Gonzalez-Vioque and Ramsey41]. Reasons for a moderate risk of bias assignment included concerns of selection bias, where in all but one study [Reference Saddichha, Ameen and Akhtar9], inadequate description or inappropriate recruitment methods were identified [Reference Riley and Moons17]. Unclear measurement properties of prognostic factors were also highlighted in several studies, including how factors were measured and included in analyses [Reference Nielsen, Rostrup, Wulff, Glenthøj and Ebdrup26, Reference Canal-Rivero, Ruiz-Veguilla, Labad, Ayesa-Arriola, Vázquez-Bourgon and Mayoral-van Son37, Reference Huang, Hei, Yang, Liu, Xiao and Long38]. Risk of-bias assessments were often complicated by poor quality reporting of study design, conduct, and particularly analysis methods and associated results. Complete risk of bias results are contained in the Supplementary Material.
GRADE assessment
Most prognostic factor-outcome associations presented with low-quality supporting evidence. Several had very low-quality evidence ratings. The highest quality rating assigned was moderate and was assigned in the case of:
-
• Age and BMI change (52 weeks)
-
• Age and weight change (0–12 weeks)
-
• Employment status and weight change/risk of CSWG (0–12 weeks)
-
• Trend of early BMI increase and change in BMI (52 weeks)
-
• Response to antipsychotic treatment and weight change (0–12 weeks)
-
• Antipsychotic plasma concentration and change in weight + BMI (0–12 weeks)
Common reasons for downgrading evidence quality included assessment in primarily exploratory studies and risk of bias concerns. Indirectness was identified in several studies where concerns of generalizability were identified [Reference Zipursky, Gu, Green, Perkins, Tohen and McEvoy8, Reference Homan, Argyelan, Fales, Barber and Szeszko27, Reference Garriga, Fernandez-Egea, Mallorqui, Serrano, Oliveira and Parellada40]. Evidence quality was rated up for employment status and trend of early BMI increase due to reported moderate–large effect sizes across studies. Studies published in the last 5 years generally displayed a higher quality of design, analysis, and reporting [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Chen, Li, Zhang, Zhu, Wu and Guan22, Reference Rasmussen, Ebdrup, Oranje, Pinborg, Knudsen and Glenthøj24]. Approximately 93% of confirmatory studies reported at least 1 statistically significant positive result, highly suggestive of publication bias and seen in other similar reviews [Reference Hayden, Wilson, Riley, Iles, Pincus and Ogilvie45]. Detailed results of all GRADE assessments can be found in the Supplementary Material.
Discussion
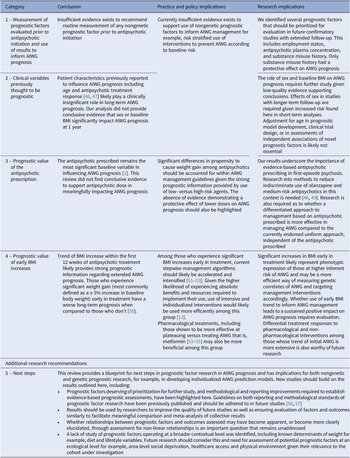
This review is the first systematic collation and exhaustive methodological evaluation of non-genetic prognostic factors studied prospectively for their influence on AIWG prognosis. The review focused on clarifying the current stance of evidence in the area and quantifying the clinical impact and associated reliability of reported prognostic associations. This review followed guidance from the Cochrane Prognosis Methods Group and used several quality design features to produce a transparent evidence summary and increase the strength of review recommendations for practice, policy, and research [Reference Riley, Moons, Snell, Ensor, Hooft and Altman16]. We also obtained several datasets where results were not previously published [Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43]. Although moderate quality evidence was found in support of a small number of factors, for many assessments, defects in study design, analysis, and reporting led to reduced confidence in reported estimates. Certainty in reported prognostic factor-outcome associations was often limited by small sample sizes, brief evaluation periods, and concerns of bias, including publication bias. Taken together, most prognostic factor-outcome associations highlighted in this review require further independent study to confirm results. Although reasons will be discussed further, review conclusions are summarized in Table 3.
Table 3. Summary of review conclusions

Prognostic factor-outcome associations with the greatest evidence certainty and results of highest practical importance, and from which conclusions in Table 3 were based on, will now be discussed. Factors with very low-quality supporting evidence will not be discussed. The value of sociodemographic and clinical variables previously thought to be prognostic including age, sex, baseline BMI and response to antipsychotic treatment will also be discussed [Reference Holt46, Reference Harrison, Gaughran, Murray, Lee, Cano and Dempster58]. Reasons for downgrading quality for each prognostic factor-outcome association and factors evaluated due to their potential role in AIWG etiology are discussed in the Supplementary Material.
Age
Most studies reported a small average negative effect of age on prognosis of weight increase [Reference Chen, Li, Zhang, Zhu, Wu and Guan22, Reference Nielsen, Rostrup, Wulff, Glenthøj and Ebdrup26, Reference Liu, Yu, Gao, Li, Guan and Thomas28], BMI increase [Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11, Reference Muntané, Vázquez-Bourgon, Sada, Martorell, Papiol and Bosch25, Reference Lin, Tseng, Tsai, Chi, Lee and Chen31, Reference Li, Gao, Lv, Zhang, Wang and Jiang36, Reference Luckhoff, du Plessis, Kilian, Asmal, Scheffler and Phahladira39, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43], and risk of CSWG [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Saddichha, Ameen and Akhtar9]. Results were similar in the case of varying follow-up durations [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Rasmussen, Ebdrup, Oranje, Pinborg, Knudsen and Glenthøj24, Reference Verma, Liew, Subramaniam and Poon34, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43], antipsychotic prescribed [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11, Reference Rasmussen, Ebdrup, Oranje, Pinborg, Knudsen and Glenthøj24], and study setting [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11, Reference Muntané, Vázquez-Bourgon, Sada, Martorell, Papiol and Bosch25, Reference Verma, Liew, Subramaniam and Poon34]. A similarly small average negative effect estimate was also seen independent of study quality, including risk of bias rating [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Verma, Liew, Subramaniam and Poon34, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43], although study precision in demonstrating a consistent negative effect was greater in better quality and larger studies [Reference Rasmussen, Ebdrup, Oranje, Pinborg, Knudsen and Glenthøj24, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43]. Meta-analysis results for change in weight and BMI were similar where reported effect sizes and the upper and lower limits of reported confidence intervals were compatible with no significant effect of age on AIWG prognosis. Evidence quality was moderate for adjusted estimates of BMI and weight change at all timepoints.
Sex
Sex was assessed in 11 exploratory and one confirmatory study. Results varied across studies, which may have been the result of heterogeneity in study design, particularly length of follow-up, and study quality [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2, Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11, Reference Verma, Liew, Subramaniam and Poon34, Reference Zhou, Hu, Gong, Zou and Yu42]. In studies assessed qualitatively, nonsignificant differences between sex were largely seen across short- [Reference Saddichha, Ameen and Akhtar9, Reference Yuan, Zhang, Wang, Liu, Li and Kumar30, Reference Zhang, Yao, Zhang, Chen, Sun and Yao33, Reference Verma, Liew, Subramaniam and Poon34, Reference Li, Gao, Lv, Zhang, Wang and Jiang36] and longer-term studies [Reference Zipursky, Gu, Green, Perkins, Tohen and McEvoy8, Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43], and in both unadjusted [Reference Saddichha, Ameen and Akhtar9, Reference Li, Gao, Lv, Zhang, Wang and Jiang36] and adjusted analyses [Reference Zipursky, Gu, Green, Perkins, Tohen and McEvoy8, Reference Yuan, Zhang, Wang, Liu, Li and Kumar30]. Meta-analysis of short-term studies found males to be at higher risk of AIWG, although the difference in effect size was not practically important. Increased risk among males was not seen in the meta-analysis of longer-term studies, although this analysis was limited by small study numbers and weight largely being assigned to a single study. For both outcomes quality was deemed low. Given the low-quality evidence, conclusions regarding sex’s role on AIWG prognosis are uncertain.
Early BMI increase
Baseline BMI was the most studied anthropometric measurement assessed for its impact on AIWG prognosis. Meta-analysis of studies with a follow-up of 12 months found no significant effect of baseline BMI on AIWG prognosis. However, evidence quality for baseline BMI as a prognostic factor was low for all outcomes, except for a change in waist circumference, where quality was very low. Thus, like sex, although a significant impact of baseline BMI was not seen here, results are inconclusive. Assessment of early anthropometric changes post antipsychotic initiation demonstrated significantly more prognostic promise. Several studies assessed the impact of early anthropometric changes on weight and BMI prognosis up to 52 weeks. One study (n = 381) assessed the value of 12-week BMI change on final BMI change at 12 months among participants prescribed varying antipsychotics and reported b = 0.89 (95% CI 0.73–1.05, p < 0.001) that is, every one-unit BMI increase at 12 weeks predicted an almost identical BMI increase at 1 year. Trend of early BMI increase explained approximately 30% variance in final BMI change – more than age, sex, baseline BMI, antipsychotic prescribed, and dosage combined [Reference Muntané, Vázquez-Bourgon, Sada, Martorell, Papiol and Bosch25]. In another study (n = 51), early BMI rate of change correlated almost perfectly with BMI and weight change at 8 weeks treatment, r = 0.988 and r = 0.992, p < 0.001, respectively [Reference Kang, Lu, Liu, Shao and Wu23]. In a 10-year follow-up, percentage weight increase at 12 months treatment had a significant prognostic effect on the odds of experiencing a 20% weight increase at 10 years [Reference Vázquez-Bourgon, Mayoral-van Son, Gómez-Revuelta, Juncal-Ruiz, Ortiz-García de la Foz and Tordesillas-Gutiérrez43]. Early BMI increase as a prognostic factor was judged to have moderate quality supporting evidence. Confirmatory evidence for the prognostic role of early anthropometric changes was also provided indirectly through other included studies. One study assessed the role of early appetite increase on AIWG prognosis and found that increased appetite at 4 weeks was positively associated with increased weight gain at 12 weeks, b = 0.67 (95% CI 0.31–1.03), p = 0.0003 [Reference Huang, Hei, Yang, Liu, Xiao and Long38]. At 12 weeks, participants with an earlier appetite increase (0–4 versus 4–8 weeks treatment) showed significantly greater weight gain, mean difference = 2.67 kg (95% CI 1.20–4.15), p < 0.0001 [Reference Huang, Hei, Yang, Liu, Xiao and Long38]. Quality for early appetite increase as a prognostic factor was judged to be low. Previous research in non-antipsychotic-naïve cohorts (n = 351) found that 5% weight increase at 1-month treatment predicted weight gain of 15% at 3 months (sensitivity 67%, specificity 88%, p = 0.001). Among those who gained <5% at 1 month, average weight gain at 3 months was significantly lower when compared to the ≥5% group, 2.4% versus 8.1%, p = 0.0005 [Reference Vandenberghe, Gholam-Rezaee, Saigí-Morgui, Delacrétaz, Choong and Solida-Tozzi50].
Antipsychotic dose
Exploratory studies assessing the prognostic value of antipsychotic dose on weight and BMI change were identified [Reference Kang, Lu, Liu, Shao and Wu23, Reference Rasmussen, Ebdrup, Oranje, Pinborg, Knudsen and Glenthøj24, Reference Zhang, Yao, Zhang, Chen, Sun and Yao33]. In the only study with numerical results [Reference Muntané, Vázquez-Bourgon, Sada, Martorell, Papiol and Bosch25], adjusted analysis found insignificant effect sizes of <0.01 on BMI change at 3 (p = 0.19) and 12 months (p = 0.24). Evidence quality was low and very low for impact on change in BMI and weight, respectively. One confirmatory study (n = 51) in China assessed the role of 4-week olanzapine plasma concentration in influencing weight and BMI change at 8 weeks and reported β = 0.376 (95% CI 0.08–0.67), p = 0.013 for weight change and β = 0.354 (95% CI 0.06–0.65), p = 0.019 for BMI change, suggesting a potential modest role of antipsychotic plasma concentration as a prognostic factor [Reference Kang, Lu, Liu, Shao and Wu23]. Evidence quality was moderate for antipsychotic plasma concentration as a prognostic factor. A previous meta-analysis assessing the impact of antipsychotic dose on weight trajectory found hyperbolic dose curves for most antipsychotics, that is, initial dose-related weight increases, with a subsequent plateau at higher doses. However, for most assessments, average weight differences between higher and lower doses did not differ to a clinically significant extent, although studies were of short duration [Reference Wu, Siafis, Hamza, Schneider-Thoma, Davis and Salanti59]. A separate review highlighting dose reduction as ineffective in significantly reversing AIWG supports the hypothesis of AIWG being somewhat dose-independent [Reference Speyer, Westergaard, Albert, Karlsen, Stürup and Nordentoft60], although the absence of linear relationships may mean dose reductions are more effective within certain ranges. Taken together, additional research is required to confirm whether antipsychotic dosing meaningfully impacts AIWG prognosis. Future studies should be designed specifically to test this hypothesis and should assess the prognostic value of plasma concentration rather than the dose to account for varying pharmacokinetics, account for potential trend differences between antipsychotics, evaluate the presence of non-linear relationships and assess dose ranges and timelines reflective of clinical practice.
Response to antipsychotic treatment
A positive response to antipsychotic treatment has previously been associated with negatively impacting AIWG prognosis, although the clinical significance of the relationship has been unclear [Reference Raben, Marshe, Chintoh, Gorbovskaya, Müller and Hahn47]. A confirmatory study (n = 529) in China assessed the association between AIWG and antipsychotic response measurements and reported weak correlations between weight gain and reduction in Positive and Negative Syndrome Scale (PANSS) positive, negative, and total subscores at 8 weeks. Adjusted analysis found a clinically insignificant positive association between total PANSS reduction and AIWG, β=0.03 (95% CI 0.01–0.05), p = 0.007 [Reference Chen, Li, Zhang, Zhu, Wu and Guan22]. In a separate study (n = 56), percentage change in PANSS positive subscale was not associated with substantial risk of CSWG at 6 months, OR = 1.0 (95% CI 0.9–1.2), p = 0.77 [Reference Verma, Liew, Subramaniam and Poon34]. Evidence quality was moderate for weight change and low for all other outcomes. Results here signify that treatment response is not a practically useful prognostic marker, although repeat assessments in additional ethnic groups are required to confirm generalizability of findings.
Moderate–large effect sizes
We identified a small number of prognostic factors with a reported moderate–large effect size. In one study (n = 446) without serious limitations, unemployment was associated with an increased risk of CSWG at 4 weeks treatment, OR = 2.83 (95% CI 1.50–5.36), p = 0.001. A significant impact on weight change was also reported [Reference Pandit, Cianci, Ter Hark, Winter-van Rossum, Ebdrup and Broberg2]. Evidence quality for unemployment as a prognostic factor was moderate. AIWG is mediated primarily through increased appetite and food cravings [Reference Holt46]. Unemployment may impact AIWG prognosis through increased consumption of higher calorie foods typically more readily consumed by those on lower incomes, as well as reduced access to lifestyle interventions shown to attenuate AIWG [Reference Vancampfort, Firth, Correll, Solmi, Siskind and De Hert12]. In another study [Reference Chiliza, Asmal, Oosthuizen, van Niekerk, Erasmus and Kidd11], a positive history of substance misuse was associated with a protective effect on BMI increase at 52 weeks treatment, b = −2.25 (95% CI −3.66–(−0.84)), p = 0.002. Substance abuse history may be a proxy for other prognostic factors, for example, poor self-care, or may provide supporting evidence for the striatum’s role in AIWG etiology, as previously demonstrated [Reference Nielsen, Rostrup, Wulff, Glenthøj and Ebdrup26, Reference Homan, Argyelan, Fales, Barber and Szeszko27]. However, the evidence quality for this prognostic factor was low. Given the practical implications of the reported range of effect sizes for both patients and clinicians, valid mechanisms underlying potential associations, and rates of occurrence in the population of interest, their independent prognostic value deserves further experiment in confirmatory studies.
Limitations
Review limitations include the exclusion of non-English language papers. Although a previous evidence review has suggested otherwise [Reference Nussbaumer-Streit, Klerings, Dobrescu, Persad, Stevens and Garritty61], there is potential that this decision may have introduced bias if statistically or clinically significant results were more likely to have been published in an English language journal. All attempts were made to avoid the inclusion of overlapping participant populations. However, in a minority of cases where clarification was not received, review authors decided on the likelihood that overlapping populations were present. This may mean that some studies were included or excluded inappropriately. As highlighted in similar reviews [Reference Garriga, Fernandez-Egea, Mallorqui, Serrano, Oliveira and Parellada40], primary studies may have been missed during searching due to lack of standardized indexing of prognostic factor studies. Exclusion of cross-sectional and retrospective study designs inherently increased evidence certainty underlying prognostic factor-outcome assessments but may have excluded studies that identified candidate factors deserving of further study. Individual participant data meta-analysis may have addressed some of the limitations identified in this review for example, publication bias or inappropriate statistical analyses [Reference Riley, Moons, Snell, Ensor, Hooft and Altman16], but was beyond the scope of this review.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1192/j.eurpsy.2023.2417.
Data availability statement
No new datasets were generated via this study. The dataset that was reanalyzed is available here: http://doi.org/10.17632/b2h5gr9m3c.1. Study estimates that were usable and extracted for all relevant outcomes are outlined in the Supplementary material. Data extraction forms can be found in the published study protocol.
Acknowledgments
We are grateful to the authors of studies included in this review who provided additional datasets not previously published and those who answered additional questions pertaining to study design, analysis, and reporting to allow us to complete more accurate risk of bias and GRADE assessments. Specifically, we would like to thank Dr Mette Ø Nielsen, Dr Bjørn H. Ebdrup, Dr Gerard Muntane, and Dr Bonga Chiliza.
Author contribution
I.F. conceived the review. I.F., L.J.S., A.B., and E.K.C. conducted the literature search. I.F. and J.E. extracted data and J.E. conducted the analyses. I.F., L.J.S., J.O.C., C.N.D., S.O.D., and E.K.C. conducted quality assessments. I.F. drafted the manuscript with L.J.S. and E.K.C. All authors contributed to the interpretation of findings and subsequent edits of the manuscript. L.J.S. and E.K.C provided overall supervision to the project. I.F. is the guarantor.
Financial support
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interest
The authors declare none.




Comments
No Comments have been published for this article.