1. Introduction
1.1. Schizophrenia and depression
There is sound evidence pointing to a two-factorial structure of negative symptoms; the first factor being “diminished expression” with blunted expression of emotions and poverty of speech and the second being “avolition” with amotivation, anhedonia and social withdrawal [e.g. Reference Marder and Galderisi1]. Secondary negative symptoms are caused by positive symptoms, substance use, medication side effects and/or – particularly important here – depression [e.g. Reference Kirschner, Aleman and Kaiser2]. Negative and depressive symptoms can’t be differentiated easily, since there is considerable conceptual overlap. Mainly the avolition factor of negative symptoms encompasses symptoms that also belong to the main symptoms of depression: loss of interest, anhedonia, and reduced energy.
Comorbidity rates are high for schizophrenia and unipolar depression across stage and state of illness (acute psychotic episode: up to 60% comorbid major depressive episode (MDE); post-psychotic: moderate to severe MDE in 20% of chronic patients and 50% of first-episode patients); there seem to be shared aetiological aspects [Reference Upthegrove, Marwaha and Birchwood3]. Longitudinally, up to 80% of patients with schizophrenia experience an episode of major depression [Reference Upthegrove, Birchwood, Ross, Brunett, McCollum and Jones4]. Depression is the most important indicator for completed suicide in patients with schizophrenia [Reference Dutta, Murray, Allardyce, Jones and Boydell5]. Since both the treatment of depression in schizophrenia and of negative symptoms remains inadequate [e.g. Reference Lako, Taxis, Bruggeman, Knegtering, Burger and Wiersma6, Reference Fusar-Poli, Papanastasiou, Stahl, Rocchetti, Carpenter and Shergill7], it seems of particular importance to reliably delineate negative and depressive symptoms.
1.2. Expression, mood and subtypes of anhedonia might differentiate schizophrenia and depression
Emotional expression (i.e. speech, gestures and facial expressions) often is reduced in patients with schizophrenia compared to healthy controls and subjects with depression, which are nevertheless also showing expressional deficits [Reference Berenbaum and Oltmanns8–Reference Riehle, Mehl and Lincoln11]. Despite affective flattening, patients with schizophrenia often report unimpaired subjective experiences [e.g. Reference Kring and Moran12], whereas low, depressed mood is a main symptom of depression. Recent conceptualizations of anhedonia emphasize the importance of the (complex) reward system. Any reward system deficit (e.g. anticipatory or motivational anhedonia, disorganization) can hinder the individual from generating pleasurable experiences and could then present as (secondary) consummatory anhedonia [Reference Lambert, Da Silva, Ceniti, Rizvi, Foussias and Kennedy13]. There are findings indicating that patients with depression experience consummatory and anticipatory anhedonia whereas patients with schizophrenia mainly show a deficit in anticipatory pleasure [Reference Lambert, Da Silva, Ceniti, Rizvi, Foussias and Kennedy13–Reference Wu, Mata, Furman, Whitmer, Gotlib and Thompson15]. The emergence of anticipatory pleasure is more complex than the experience of consummatory pleasure and hints at motivational deficits in schizophrenia as opposed to deficits in experiencing emotions [Reference Foussias and Remington16].
1.3. Correlations of rating scales for depression and negative symptoms
Research on negative symptoms in the context of MDE seems scarce. Bottlender, Sato [Reference Bottlender, Sato, Groll, Jager, Kunze and Moller17] found that negative symptoms (measured with the Scale for the Assessment of Negative Symptoms (SANS)) were significantly associated with depressive symptoms (measured with the Montgomery-Asberg Depression Rating Scale) in MDE patients but not in patients with schizophrenia. This could be due to the SANS’ item content that encompasses a lot of symptoms also germane to the depressive domain (e.g. affective nonresponsivity, poverty of content of speech, increased latency of response) and symptoms that are not thought to be specific for negative symptoms anymore, i.e. attention/cognitive symptoms [e.g. Reference Marder and Galderisi1]. They found persisting negative symptoms to be indicative for schizophrenia and not MDE.
For schizophrenia patients, Park, Llerena [Reference Park, Llerena, McCarthy, Couture, Bennett and Blanchard18] found a weak correlation between observer-rated negative symptoms (CAINS) and the rater assessed Calgary Depression Scale for Schizophrenia (CDSS), Kring, Gur [Reference Kring, Gur, Blanchard, Horan and Reise19] found none. Engel, Fritzsche [Reference Engel, Fritzsche and Lincoln20] reported no significant association of CAINS and self-assessed BDI-II. Llerena, Park [Reference Llerena, Park, McCarthy, Couture, Bennett and Blanchard21] found no significant correlation of self-rated negative symptoms (MAP-SR) with the CDSS. Hartmann, Fritzsche [Reference Hartmann, Fritzsche and Lincoln22] reported no significant correlation between the BDI-II and PANSS-rated negative symptoms (however, it should be noted that two of the seven PANSS negative items assesses cognitive symptoms [e.g. 1]). Engel and Lincoln [Reference Engel and Lincoln23] reported a moderate and significant correlation of the MAP-SR with the BDI-II (r = 0.39). Overall, we found some – if scarce – evidence for overlap when measuring the two symptom domains in patients with schizophrenia. Concerning self- vs. observer-ratings, Engel and Lincoln [Reference Engel and Lincoln23] debated an underestimation of shared variance of negative and depressive symptoms when compared across sampling methods.
1.4. Objectives
We investigated if a) measures of negative symptoms and b) measures of depressive symptoms could differentiate between subjects with MDE, subjects with schizophrenia and healthy controls. We expect subjects with schizophrenia to show the greatest extent of negative symptoms and subjects with depression to show the greatest extent of depressive symptoms. Because of the overlap between negative symptoms and depression we expect subjects with schizophrenia to display more depressive symptoms and subjects with MDE to report more negative symptoms than healthy controls. We expect mainly the “expression” factor of negative symptoms and the assessments of depressive mood to reliably differentiate MDE and schizophrenia subjects.
1.5. Selection of instruments
To assess the scope of negative symptoms we used the Clinical Assessment Interview for Negative Symptoms (CAINS, Engel, Fritzsche [Reference Engel, Fritzsche and Lincoln20]) and the self-rating instrument Motivation and Pleasure Scale – Self-Report (MAP-SR, Engel and Lincoln [Reference Engel and Lincoln23]). The CAINS has been designed to assess negative symptoms according to the current conceptualization [Reference Marder and Galderisi1] and consists of two scales: “motivation and pleasure” (CAINS-MAP) and “expression” (CAINS-EXP). CAINS-EXP straightforwardly rater-assesses expressive deficits with four items. CAINS-MAP focuses on aspects of inner experience with its authors arguing that this is central for the emotional, social and motivational deficits and to be distinguished from behavior or functional outcome [Reference Kring, Gur, Blanchard, Horan and Reise19]. In the original validation study, the two factors correlate moderately (r = 0.24), show good internal consistency as well as test-retest reliability and interrater reliability. Convergent and discriminant validity (also to depressive symptoms) was established [Reference Kring, Gur, Blanchard, Horan and Reise19]. A further validation study found good psychometric properties for the German CAINS as well, with high internal consistency, a moderate correlation between the two factors (r = 0.44), good inter-rater agreement as well as convergent and discriminant validity; the latter also with depression [Reference Engel, Fritzsche and Lincoln20]. The MAP-SR assesses the “avolition” factor of negative symptoms as a self-report and is based on the CAINS’ “motivation and pleasure” scale. It taps social pleasure, recreational or work pleasure, feelings and motivations about close, caring relationships as well as motivation and effort to engage in activities. Llerena, Park [Reference Llerena, Park, McCarthy, Couture, Bennett and Blanchard21] found good internal consistency as well as convergent validity with the CAINS-MAP (r = 0.65) and social anhedonia (r = 0.48). For social performance, there was no significant correlation. Discriminant validity was established (i.a. for depression/anxiety). For the German MAP-SR, Engel and Lincoln [Reference Engel and Lincoln23] also found high internal consistency as well as mostly good convergent and discriminant validity. However, there was a moderate correlation with the BDI-II (r = 0.39).
To assess the scope of depressive symptoms, we used the Beck Depression Inventory (BDI, Hautzinger [Reference Hautzinger24]) as self-rating and the Hamilton Depression Scale (HAMD-17, Hamilton [Reference Hamilton25]) as rater assessment. Both tap emotional, cognitive, behavioral, and physical symptoms of depression. The BDI consists of 17 items and shows good validity, adequate test-retest reliability, and good inner consistency [Reference Beck, Steer and Carbin26]. For the German BDI high internal consistency and good convergent validity is reported [Reference Hautzinger24]. Concerning the observer rating, we expect greater discriminatory power from the HAMD’s 17-item version as opposed to HAMD-21, since this version doesn’t assess paranoia and depersonalization. The HAMD-17 is widely used and has good to adequate psychometric properties [e.g. Reference Bagby, Ryder, Schuller and Marshall27]. Because we expect items associated with mood to have the most discriminatory power, we researched established subscales with emphasis on mood for BDI and HAMD-17, respectively. The BDI subscale “cognitive/affective” consists of the first 14 items excluding somatic and functioning items [Reference Beck, Steer and Carbin26]. The Maier-Philipp Severity subscale of the HAMD-17 comprises the items assessing depressed mood, feelings of guilt, work and interests, retardation, agitation, and anxiety – psychic [Reference Maier and Philipp28].
2. Methods
2.1. Participants
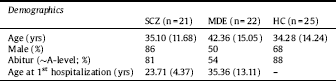
We included 21 participants with schizophrenia (SCZ), 22 participants with MDE and 25 healthy controls (HC). The patients were recruited from in-patient settings. Inclusion criteria were diagnosis of a psychotic disorder (SCZ) or major depressive episode (MDE) or no diagnosis (HC) according to DSM-IV (assessed with the German Brief Diagnostic Interview of Mental Disorders (Mini-DIPS)), age 18 to 65 years, sufficient German language skills, normal or corrected to normal vision and hearing as well as capability to give consent. To avoid overlap between the psychiatric groups we did not include subjects with schizoaffective disorder or a MDE with psychotic symptoms. Since we wanted to measure present symptoms, we excluded patients in remission (i.e. only met “life time” criteria in the diagnostic interview). Further exclusion criteria were substance dependence as leading clinical problem and intellectual disability. To keep the sample structure comparable, we included healthy controls that corresponded in age and gender to the recruited SCZ patients. Since depressed patients skew older and female and we prioritized a representative sample, we refrained from doing that with the MDE sample. Demographic and clinical characteristics of the samples can be found in Table 1.
2.2. Measures and procedure
The study protocol was approved by the ethics committee of the University of Tuebingen’s medical faculty. After obtaining informed consent, screened participants who met inclusion criteria were interviewed and asked to fill in questionnaires (duration approx. 1.5 h). Diagnosis was confirmed using the parts of the German Brief Diagnostic Interview of Mental Disorders (Mini-DIPS) that assess psychotic and affective disorders.
The German translation of the CAINS was kindly made available to us by the research group led by Tania Lincoln, Department of Clinical Psychology and Psychotherapy, University of Hamburg. The English version of the MAP-SR was translated into German by our research group and retranslated by an English native speaker. Differences to the original English versions were discussed among the translators and a consensus was agreed on.
2.3. Data analysis
For demographic data a rate of missings <10% was not reported. When calculating the self-ratings’ scale composites, a rate of 10% of missings was tolerated and replaced by the scale’s mean. Measures with more missing data were excluded from the analysis (one data point for MAP-SR and BDI, respectively). There were no missing data for the rater assessments. Scores for the MAP-SR were inverted so that larger scores indicate a greater extent of negative symptoms. With SPSS 25.0 we tested for normal distribution with the Shapiro-Wilk test. Since CAINS, MAP-SR, HAMD-17 and BDI and their subscales were all non-normally distributed in the control group, we used the non-parametrical Kruskal-Wallis-Test to assess the between-group effects. Post-hoc pairwise comparisons were performed using Dunn tests with Bonferroni correction.
3. Results
For the CAINS we found between-group differences in the scope of negative symptoms (H(2, N = 68) = 48.65, p <.001). Post-hoc analysis revealed significant differences between all groups: SCZ > MDE > HC, p < 0.05. MAP-SR also revealed differences between the groups (H(2, N = 68) = 25.77, p <.001). Here, post-hoc analysis showed SCZ = MDE > HC with p <.001 for MDE and controls and p =.007 for SCZ and controls. Because of the MAP-SR’s failure to differentiate SCZ and MDE, we further analyzed the CAINS’ two subscales and found that only its expression subscale significantly differentiated between subjects with depression and schizophrenia (SZC > MDE > HC). The group comparisons of the negative symptom scales can be found in Fig. 1.
Table 1 Sample demographics.
Concerning the scope of depressive symptoms we found between-group differences for BDI (H(2, N = 67) = 41.83, p < 0.001). Post-hoc analysis showed that all three groups differed significantly from each other: MDE > SCZ > HC, p < 0.05. For HAMD-17, there were significant differences as well (H(2, N = 68) = 54.14, p < 0.001). Post-hoc tests showed MDE > SCZ > HC, p < 0.05; see Fig. 2.
For the “mood associated” subscales of the measures for depressive symptoms, we found both to not significantly differentiate between depressed and schizophrenic subjects, while still showing between-group differences. HAMD-Maier-Philipp: H(2, N = 68) = 53.32, p < 0.001, MDE = SCZ > HC; BDI cognitive/affective: H(2, N = 67) = 37.12, p < 0.001, MDE = SCZ > HC. The “other” subscale of the HAMD17 as well as the somatic subscale of the BDI differentiated significantly between all groups. HAMD other: H(2, N = 68) = 43.74, p <.001, SZC > MDE > HC; BDI somatic H(2, N = 67) = 40.11, p <.001, MDE > SCZ > HC. The group comparisons of the depression measures’ subscales can be found in Fig. 3.
4. Discussion
We investigated if subjects with schizophrenia, subjects with MDE, and healthy controls differ in the scope of their negative and depressive symptoms as measured by self-ratings (BDI, MAP-SR) and observer assessments (HAMD-17, CAINS). All measures differentiated the psychiatric samples from the controls. The full rating scales of depressive symptoms (HAMD-17, BDI) and the rater assessment of negative symptoms (CAINS) – and specifically the expressive deficits (CAINS-EXP) – managed to discriminate between subjects with schizophrenia and those with MDE reliably; the self-rating of negative symptoms (MAP-SR) did not.
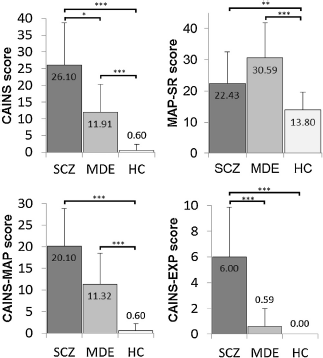
Fig. 1. Group comparisons of CAINS and MAP-SR, and CAINS-MAP and CAINS-EXP; means with standard deviations and significance markers.

Fig. 2. Group comparisons of HAMD-17 and BDI; means with standard deviations and significance markers.

Fig. 3. Group comparisons of HAMD-17 Maier-Philipp and “other” subscales, and BDI cognitive/affective and somatic subscales; means with standard deviations and significance markers.
Concerning the CAINS, its “expression” subscale (CAINS-EXP, assessing vocal prosody, gestures, facial expression, and speech) significantly differentiates schizophrenic and depressed subjects whereas the “avolition” subscale does not (although its means also are SCZ > MDE > HC). This differentiating effect of the CAINS-EXP is consistent with previous evidence that shows reduced facial expression of subjects with schizophrenia compared to subjects with depression (which are nevertheless also showing diminished expression); this also holds true for involuntary facial activity [Reference Berenbaum and Oltmanns8, Reference Tremeau, Malaspina, Duval, Correa and Hager-Budny9, Reference Gaebel and Wolwer29]. Gaebel and Wolwer [Reference Gaebel and Wolwer10] found diminished expression beyond acute psychotic episodes in schizophrenia patients; in subjects with depression this was primarily found when acutely depressed. One explanation for the MAP-SR’s failure to distinguish the psychiatric groups could be poor self-assessment by subjects with schizophrenia. There is evidence for deficits in self-assessment, with mainly positive symptoms and cognitive symptoms negatively affecting the ability to self-assess correctly [Reference Browne, Clarke, Gervin, Waddington, Larkin and O’Callaghan30–Reference Silberstein, Pinkham, Penn and Harvey32]. However, Hartmann, Fritzsche [Reference Hartmann, Fritzsche and Lincoln22] assessed patients with psychosis for depression with two observer ratings (CDSS and PANSS) and two self-rating scales (BDI and Symptom-Checklist Revised (SCL-90-R) and found self-ratings to correspond well with observer ratings. Since the CAINS “expression” subscale seems to play the decisive role, the MAP-SR’s failure to differentiate the psychiatric samples could be because it doesn’t measure expression. Moreover, in contrast to the means of the CAINS “motivation and pleasure” sub-scale (SCZ > MDE > HC), MDE-subjects reported greater symptom load on the MAP-SR than subjects with schizophrenia (MDE > SCZ > HC). The MAP-SR doesn’t seem to measure symptoms in the “motivation and pleasure” domain that are specific for anhedonia/avolition in schizophrenia. To differentiate between depressive symptoms and negative symptoms both instruments might not assess enough items that allow for a discrimination of subtypes of anhedonia (anticipatory vs. consummatory anhedonia) and mood symptoms (indifferent vs. depressed mood). Therefore, future research on instruments assessing the “amotivation” factor of negative symptoms should focus on a more detailed assessment of these symptom domains, if the aim is to differentiate reliably from depression. In conclusion, the findings of the current study indicate that the “expression” factor of negative symptoms as assessed with the CAINS-EXP can differentiate MDE and negative symptoms of schizophrenia, whereas both scales measuring the “amotivation” factor (CAINS-MAP and MAP-SR) do not allow for a specific distinction.
In this study, we used the BDI; the newer BDI-II additionally inquires agitation, worthlessness, loss of energy, and concentration difficulty and dropped body image change, work difficulty, weight loss, and somatic preoccupation. Interestingly, Hartmann, Fritzsche [Reference Hartmann, Fritzsche and Lincoln22] found that schizophrenia patients who self-reported fewer depressive symptoms than the clinicians observed, showed more negative symptoms, i.e. blunted affect and poor affective rapport. We also found slightly greater mean differences between schizophrenia patients and to MDE subjects in the self-assessment of depressive symptoms than in the observer rating. We supposed that while the HAMD-17 manages to differentiate patients with MDE and those with SCZ, there are some items that might reduce discriminatory power: four inquire physical symptoms, and three sleep problems. Patients with schizophrenia as well as clinically depressed patients both experience and report more physical symptoms [e.g. Reference Leucht, Burkard, Henderson, Maj and Sartorius33, Reference Greco, Eckert and Kroenke34]. Particularly sexual dysfunction is a common side effect of antipsychotics [e.g. Reference Baggaley35]. Moreover, 30 to 80% of schizophrenic patients also suffer from sleep disturbances [Reference Cohrs36]. Curiously, we could confirm this neither when analyzing the subscales of the HAMD-17 nor of the BDI. Focus on psychic symptoms and affective and cognitive symptoms of depression, respectively, did not increase discriminatory power. Conversely, the scales’ items that feature somatic and sleep symptoms seem to differentiate the psychiatric samples better. Maybe the subscales were still not specific enough for affective/mood symptoms. This warrants further research on the differential impact of the scales’ singular items. At this point, however, we propose utilizing the whole scales to help differentiate depressive from negative syndromes.
4.1. Limitations
Our psychiatric samples exhibited only mild to moderate negative and depressive symptoms respectively; a greater severity of symptoms might have shown the differences between the groups more clearly. Moreover, comparisons between SCZ and MDE groups are difficult since e.g. age of onset and gender ratio differ. However, a matching procedure would compromise representativity either of the schizophrenia or MDE sample. Furthermore, medication could be a confounding variable. Of the subjects with schizophrenia, 95% were on antipsychotic medication, 19% took at least one antidepressant. In the MDE group 31% of the subjects were on antipsychotics (usually in lower doses than the SCZ group), 91% on antidepressants. Medication-induced blunting could adversely affect the experience of pleasure in subjects with schizophrenia. These secondary negative symptoms could not be ruled out in the present study. Also, our raters were not blinded concerning the subject’s diagnosis – this could lead to over- or under-assessement of depressive and/or negative symptoms in concordance with diagnosis.
4.2. Conclusion
To differentiate negative symptoms and depression, clinicians might look for self-reported depressive symptoms and observable reduction in expression. The self-report of depressive symptoms is reliable and economical, but more importantly, there is evidence that the self-report might be more sensitive than rater-assessed depressive symptoms in schizophrenia patients [see also 22]. Reduced expression and moderate levels of depression point towards a negative syndrome, whereas relatively unimpaired expression and high scores of self-reported depressive symptoms are more likely to indicate a depressive syndrome.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgement
We thank Stefanie Ronge for her help in data acquisition and entry.







Comments
No Comments have been published for this article.