Introduction
Patients with severe mental illness, such as schizophrenia, have worse physical health and reduced life expectancy compared to the general population [Reference De Hert, Schreurs, Vancampfort and Van Winkel1]. Life expectancy of patients with schizophrenia is reduced 14.5 years [Reference Hjorthøj, Stürup, McGrath and Nordentoft2] and this excess mortality is mainly due to cardiovascular causes and obesity-related cancers [Reference De Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen and Asai3].
The development of cardiovascular diseases (CVD) and the excess cardiovascular mortality observed in these patients are associated with a number of modifiable cardiometabolic risk factors: abdominal obesity, high blood pressure, low level of high-density lipoprotein cholesterol, elevated triglycerides, and hyperglycaemia [Reference De Hert, Dekker, Wood, Kahl, Holt and Möller4, Reference Vancampfort, Wampers, Mitchell, Correll, De Herdt and Probst5]. This cluster of risk factors, considered as a whole, defines the so-called metabolic syndrome (MetS).
The association between schizophrenia and cardiometabolic risk factors is complex and determined by an inter-relationship between environmental factors (lifestyle and difficulty in accessing treatments), genetic vulnerability, and disease-related factors [Reference De Hert, Vancampfort, Correll, Mercken, Peuskens and Sweers6]; moreover, antipsychotic treatment represents an important contributor to risk of cardiometabolic dysfunction, particularly for certain drugs in vulnerable patients [Reference Stahl, Mignon and Meyer7, Reference Arango, Bobes, Aranda, Carmena, Garcia-Garcia and Rejas8].
Evidence of genetic determinants of the cardiometabolic risk in schizophrenia includes the identification of shared genetic traits between schizophrenia and diabetes mellitus or insulin resistance [Reference Hackinger, Prins, Mamakou, Zengini, Marouli and Brčić9, Reference Postolache, Del Bosque-Plata, Jabbour, Vergare, Wu and Gragnoli10], as well as an increased incidence of cardiometabolic risk factors in unaffected relatives of patients with schizophrenia and in antipsychotic-naïve patients [Reference Freyberg, Aslanoglou, Shah and Ballon11, Reference Moreno, Nuevo, Chatterji, Verdes, Arango and Ayuso-Mateos12].
In 2009, the European Psychiatric Association (EPA), together with the European Society of Cardiology and the European Association for the Study of Diabetes, published a position paper with the aim to reduce modifiable CVD risk factors, improve diabetes care, and overall health in patients with severe mental illnesses [Reference De Hert, Dekker, Wood, Kahl, Holt and Möller4].
In the following years, several meta-analyses and cohort studies have been published which highlighted the severity of cardiometabolic risk and diseases among patients with schizophrenia [Reference Correll, Robinson, Schooler, Brunette, Mueser and Rosenheck13, Reference Howell, Yarovova, Khwanda and Rosen14]. Guidelines on cardiometabolic risk management in these patients were also produced [Reference De Hert, Vancampfort, Correll, Mercken, Peuskens and Sweers6]. Moreover, in 2014 and 2018, European [Reference Rüther, Bobes, De Hert, Svensson, Mann and Batra15] and American guidances regarding tobacco dependence and strategies for smoking cessation [16] and European guidance for physical activities [Reference Stubbs, Vancampfort, Hallgren, Firth, Veronese and Solmi17] were published. However, all these recommendations have not sufficiently translated into clinical practice to a degree that is proportional to their importance.
A retrospective cohort analysis showed that screening of plasma lipid and glucose levels in patients taking antipsychotics remained low after the publication of specific monitoring guidelines, despite a significant increase with respect to the period preceding the guidelines publication [Reference Haupt, Rosenblatt, Kim, Baker, Whitehead and Newcomer18]. More recently, two different studies analyzed the adherence of clinicians to the guidelines on cardiovascular and metabolic monitoring and demonstrated poor monitoring of cardiac function [Reference Manchia, Firinu, Carpiniello and Pinna19] and suboptimal monitoring of metabolic risk in patients treated with antipsychotics [Reference Pereira, Budovich and Claudio-Saez20].
Finally, a recent meta-analysis of prospective studies in patients with psychiatric disorders on monitoring, diagnosis, control of risk factors, and treatment of CVD showed an association of schizophrenia with a low probability of having the smoking habit recorded, a diagnosis of hypertension, as well as treatment with antihypertensive and lipid-lowering drugs [Reference Ayerbe, Forgnone, Foguet-Boreu, González, Addo and Ayis21].
This gap needs to be addressed, given the stunning and unequivocal evidence of its priority.
The aim of this study was to evaluate the consensus level of a representative group of European psychiatrists on a series of statements regarding the assessment and the management of cardiometabolic risk factors in patients with schizophrenia.
Methods
A Steering Committee, consisting of re-known experts in the fields of diagnosis and treatment of schizophrenia (CA, MDH, AF, SG, PG, SL), diabetes and metabolic diseases Stefano Del Prato (SDP) and cardiovascular diseases (APM), Aldo Pietro Maggioni (APD) coming from different European countries, based on research evidence and clinical judgment selected the main topic of interest (cardiometabolic morbidity in patients with schizophrenia spectrum disorder) and then identified the following four main subtopics: (a) cardiometabolic risk factors in naïve and treated schizophrenic patients; (b) cardiometabolic risk factors related to antipsychotic treatment; (c) differences in the cardiometabolic profiles among antipsychotics; (d) management of cardiometabolic risk with a specific focus on tobacco use. Fourteen statements related to the above topics were then developed by the steering committee.
After further review the total number of statements were reduced to 12 (Table 1) and endorsed by all the members of the steering committee. The statements were then submitted to a validation panel (see Acknowledgment for panel members’ names) for revision/approval.
Table 1. Percentage of agreement on each statement of the Delphi questionnaire.
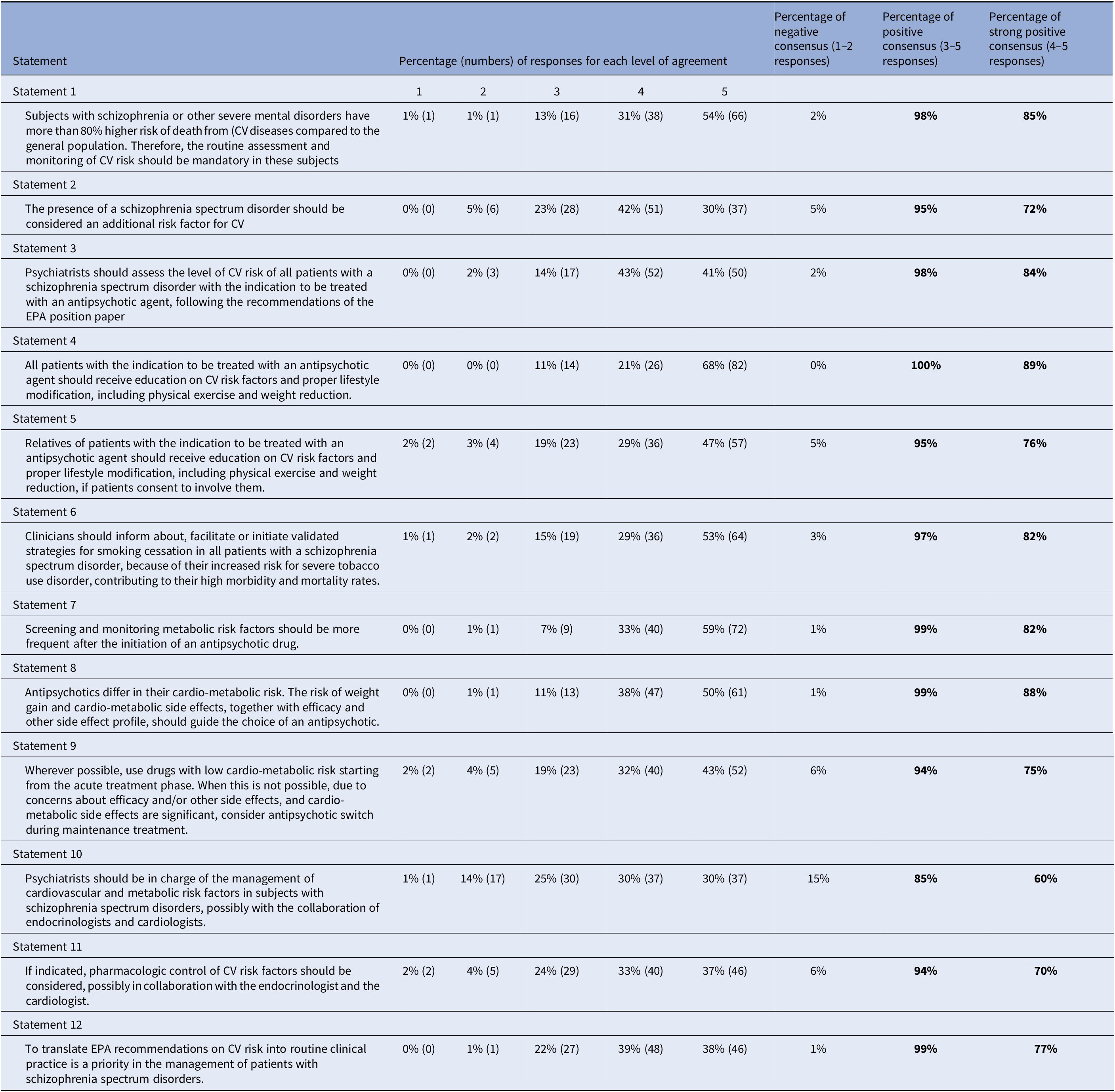
Note: 1 = Strongly disagree; 2 = Disagree; 3 = Agree; 4 = More than agree; 5 = Strongly agree.
Abbreviations: CV, cardiovascular; EPA, European Psychiatric Association.
After an in-depth discussion of the feedback received from the validation panel, the final questionnaire was approved.
The Delphi questionnaire initially developed in English was then translated into four other European languages (French, German, Italian, and Spanish), in order to ensure participants’ maximum understanding.
Delphi method and participants
The questionnaire was delivered to an expert panel of 191 European psychiatrists using a modified Delphi method [Reference Avella22].
Participants were experts in the treatment of schizophrenia, selected on the basis of their scientific production or from the members of the EPA Schizophrenia section and European College of Neuropsychopharmacology Schizophrenia network, from all geographical regions of Europe. As experts of the consensus topics and mostly active clinicians in their different countries/regions, they could have an impact on the practice of their colleagues in the real world in different ways; among others, their role in medical and psychiatric training, as well as in continuous medical education, especially for those from academia settings.
The panel of experts had to include at least 30% of females.
For each statement of the questionnaire, the expert had to express his/her level of agreement according to the following 5-point Likert scale: 1 = strongly disagree; 2 = disagree; 3 = agree; 4 = more than agree; 5 = strongly agree. In accordance with the Delphi standards, consensus is reached when the sum of items 1 and 2 (Disagree) or that of 3, 4, and 5 (Agree) reaches 66%. No consensus is reached, when the sum of the responses for a negative consensus (1 and 2) or a positive consensus (3, 4, and 5) is <66% [Reference Haupt, Rosenblatt, Kim, Baker, Whitehead and Newcomer18].
The panel of experts received an invitation email containing a brief introduction to the project with its objectives and process, and a link to the on-line questionnaire. The deadline for compiling the questionnaire was set after 14 days from the invitation and reminder emails were sent after 1 week, followed by reminder emails sent every 3 days. After deadline, with the aim of reaching the highest response rate, a one-week extension for filling in the Delphi questionnaire was granted.
No personal data were collected from the panel of experts, with the exception of the email addresses that were used only for sending invitation and reminder emails. All the data from the questionnaire were analyzed in an anonymous way.
Results
One-hundred twenty-two panelists from 27 European countries out of the selected 191 (64%) completed the Delphi questionnaire. Answers to the questionnaire are shown in Table 1.
Panelists’ agreement on statements was very high in the first round of the Delphi Study. Consensus was reached for all statements (Table 1), with a percentage of positive agreement higher than 85% on each statement, suggesting a shared view of European psychiatrists on the proposed topics. A strong agreement (responses 4 and 5) was reached with a percentage ≥ 70% on all statements except one, for which it was 61%. This latter statement concerned the central role of psychiatrists in the management of cardiovascular and metabolic risk factors in subjects with schizophrenia spectrum disorders.
Discussion
The aim of this study was to evaluate the consensus level of a representative group of European psychiatrists about a series of statements pertaining to the assessment and management of cardiometabolic risk factors in patients with schizophrenia spectrum disorders. The statements were primarily based on the recommendations reported in the EPA position paper and guidance, which were integrated with the most recent evidence.
The Delphi questionnaire was sent to 191 psychiatrists from all over European countries and, despite the COVID 19 emergency, the response rate was sufficiently high (64%).
Statements 1 and 2: Cardiometabolic Risk in Patients with Schizophrenia Spectrum Disorders
Ninety-eight and 95% of participants agreed that patient with schizophrenia spectrum disorders are at higher risk for CVD and related death and that schizophrenia should be considered a risk factor for CVD in itself, respectively.
These statements is supported by a high level of evidence. Patients with severe mental illnesses such as schizophrenia have a mortality risk increased by two or three fold compared to the general population [Reference De Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen and Asai3] with a 20% decrease in life expectancy [Reference Heald, Pendlebury, Anderson, Narayan, Guy and Gibson23]. The excess mortality is largely due to CVD; in fact, people with schizophrenia have nearly twice the normal risk of dying from CVD [Reference Heald, Pendlebury, Anderson, Narayan, Guy and Gibson23]. The development of CVD and the excess of mortality in these patients seem to be associated with a higher incidence of MetS [Reference Vancampfort, Firth, Schuch, Rosenbaum, Mugisha and Hallgren24–Reference Scuteri, Laurent, Cucca, Cockcroft, Cunha and Mañas26].
In people with schizophrenia, the prevalence of MetS is about 30% and the onset is 10 years earlier than in the general population [25]. Moreover, the prevalence of all the traits of the MetS is two or three times greater in the psychiatric population than in the general population [Reference De Hert, Schreurs, Vancampfort and Van Winkel1, Reference Vancampfort, Wampers, Mitchell, Correll, De Herdt and Probst5]. The higher incidence of MetS is generally related to several factors: genetic, behavioral (dietary factors, reduced physical activity, smoking habit), pharmacological (antipsychotics) [Reference Vancampfort, Wampers, Mitchell, Correll, De Herdt and Probst5], and reduced access to healthcare (partially justified by patients’ socioeconomic status).
As to lifestyle factors, there is a high prevalence of poor diet, smoking, and inadequate exercise among patients with severe psychiatric disorders. A cross-sectional study showed that many patients did not follow the recommendations for diet and daily exercise in spite of being aware of their unhealthy lifestyles. Thus, there is potential for interventions to improve lifestyle factors and, through this, reduce the risk of cardiometabolic disease [Reference Heald, Pendlebury, Anderson, Narayan, Guy and Gibson23].
Socioeconomic status and lifestyle do not completely explain the increased risk. Serious mental illnesses (such as schizophrenia spectrum disorders) and in general psychotic symptoms per se seem to have a strong association with several somatic illnesses [Reference Moreno, Nuevo, Chatterji, Verdes, Arango and Ayuso-Mateos12, Reference Nuevo, Chatterji, Fraguas, Verdes, Naidoo and Arango27]. This association may be explained by the existence of a pathophysiological link based on genetic, inflammatory, immunological, and/or metabolic mechanisms.
Data from drug-naïve patients with schizophrenia suggest that an intrinsic metabolic risk, leading to increased incidence of CVD, is associated with the disease, most likely due to a common genetic basis [Reference Freyberg, Aslanoglou, Shah and Ballon11]. Recent studies have demonstrated that schizophrenia and type 2 diabetes share genetic mechanisms that at least partially justify their concomitance [Reference Hackinger, Prins, Mamakou, Zengini, Marouli and Brčić9]. In addition, it has been reported that an inherited predisposition of patients with schizophrenia to psychoneuroendocrine dysfunction may confer increased risk of type 2 diabetes and MetS [Reference Postolache, Del Bosque-Plata, Jabbour, Vergare, Wu and Gragnoli10]. Finally, impaired glucose tolerance has been demonstrated in non-psychotic, first-degree relatives of patients with schizophrenia, further indicating an association between MetS and psychosis genetic risk [Reference Freyberg, Aslanoglou, Shah and Ballon11].
Statement 4, 5, 7: Cardiometabolic Risk Assessment and Management
More than 80% of participants strongly agreed (responses 4 and 5) that all patients with the indication of antipsychotic treatment and their relatives should be educated on CVD risk factors and proper lifestyle modification, including physical exercise, weight reduction, and smoking cessation.
As for the previous statements, a high level of evidence supports these statements. Psychiatric patients move less and adhere less to the prescriptions of correct physical activity; among these patients, people with schizophrenia were the least physically active [Reference Vancampfort, Firth, Schuch, Rosenbaum, Mugisha and Hallgren24]. The beneficial effect of a combination of healthy lifestyle behaviors for the primary prevention of MetS has been proven in the general population [Reference Garralda-Del-Villar, Carlos-Chillerón, Diaz-Gutierrez, Ruiz-Canela, Gea and Martinez-Gonzalez28].
The World Psychiatric Association (WPA) recommends that psychiatrists and other members of the multidisciplinary team educate and motivate patients with schizophrenia to improve their lifestyle through the use of behavioral interventions, including smoking cessation, dietary measures, and physical exercise [Reference De Hert, Vancampfort, Correll, Mercken, Peuskens and Sweers6].
The available guidelines [Reference De Hert, Vancampfort, Correll, Mercken, Peuskens and Sweers6, Reference De Hert, Cohen, Bobes, Cetkovich-Bakmas, Leucht and Ndetei29] recommended that, since patients with schizophrenia represent a high-risk group for developing cardiometabolic abnormalities, they should be routinely screened for CVD risk factors at all stages of the disorder.
The risk assessment must be carried out during the first visit and before the prescription of antipsychotic treatment. In patients without other metabolic risk factors treated with antipsychotics, the EPA position paper and WPA guidelines [Reference De Hert, Dekker, Wood, Kahl, Holt and Möller4, Reference De Hert, Cohen, Bobes, Cetkovich-Bakmas, Leucht and Ndetei29] recommend monitoring the CVD risk factors after 6 and 12 weeks and then every 12 months to assess the risk profile of the administered drug. If other risk factors are present, more frequent monitoring should be considered. Many of the MetS risk factors are modifiable. It is therefore recommended to treat any risk factor (hypertension, hypercholesterolemia, etc.), possibly involving the general practitioner. Although the primary prevention of cardiovascular risk factors in the general population has increased in the last decades [Reference Garralda-Del-Villar, Carlos-Chillerón, Diaz-Gutierrez, Ruiz-Canela, Gea and Martinez-Gonzalez28], more needs to be done in people with schizophrenia-spectrum disorders [Reference Morrato, Campagna, Brewer, Dickinson, DSK and Miller30]. As a matter of fact, only a few trials aimed at promoting healthy lifestyles and prevent CVD risk started in the last years in people with schizophrenia-spectrum disorders [Reference Westman, Eberhard, Gaughran, Lundin, Stenmark and Edman31, Reference Gaughran, Stahl, Patel, Ismail, Smith and Greenwood32]. As a reflection of the difficulties in implementation of preventive measures in these patients, a population-based register study demonstrated that the mortality from all causes and from diseases of the circulatory system declined faster for the general population than for patients with severe mental disorders [Reference Ösby, Westman, Hällgren and Gissler33].
Statement 6: Smoking and Mortality Risk
Eighty-two percent of the panelists strongly agreed (responses 4 and 5) that patients with schizophrenia spectrum disorders are at increased risk for severe tobacco use disorder and that this contributes to their high morbidity and mortality rates. For this reason, they agreed that clinicians should inform the patients about validated strategies for smoking cessation and facilitate or initiate them in these patients.
About 2/3 of patients with schizophrenia have a smoking habit (335), frequently start smoking at an earlier age and are heavier smokers than the general population [Reference McCreadie34, Reference de Leon and Diaz35]. These factors contribute to the high levels of morbidity in this population [Reference Kelly, McMahon, Wehring, Liu, Mackowick and Boggs36]. In particular, tobacco consumption is associated with cardiovascular risk, and mortality data in patients with schizophrenia demonstrated a 12-fold increase in the odds of cardiac related deaths in smokers as compared to non-smokers [Reference Kelly, McMahon, Wehring, Liu, Mackowick and Boggs36].
The smoking habit has an important genetic component (i.e., a high heritability) and a single gene explains 14% of the risk of being a smoker or a non-smoker [Reference Gorwood, Le Strat and Ramoz37]. This is the first case in psychiatry where a single gene accounts for such a high percentage of risk. Moreover, looking at the polygenic risk score in schizophrenia and nicotine dependence, a genetic overlap is observed between the two conditions [Reference Hartz, Horton, Oehlert, Carey, Agrawal and Bogdan38].
A study has demonstrated that the motivation to quit is as high among psychiatric patients as in the general population [Reference Rüther, Bobes, De Hert, Svensson, Mann and Batra15].
Moreover, looking at pharmacological therapies for smoking cessation, a meta-analysis of 28 RCTs calculated the risk ratio of smoking cessation at 3 months showing that the efficacy and persistence of the effect are the same in patients with schizophrenia than they are in the general population, with a very low number needed to treat [Reference Pearsall, Smith and Geddes39].
In conclusion, the evidence showed that patients with schizophrenia want to quit as much as the general population and that available treatments work as they do in the general population. Therefore, smoking cessation interventions must be promoted.
Statement 3, 10, 11: Coordination of Cardiometabolic Risk Assessment and Management
Ninety-eight percent of participants agreed that the psychiatrist should assess the CVD risk of patients with a schizophrenia spectrum disorder with the indication of an antipsychotic treatment but only 60% of them strongly agreed (responses 4 and 5) that psychiatrists should also be in charge of the management of cardiovascular and metabolic risk factors in subjects with schizophrenia spectrum disorders.
In this case, responses may have been influenced by the huge differences between the healthcare systems of the various European countries. As the EPA guidance recommend, the psychiatrist and the general practitioner should play an active role in the assessment and management of cardiovascular risk factors, as an integral part of the care of their psychiatric patients [Reference De Hert, Dekker, Wood, Kahl, Holt and Möller4]. Difficulties experienced by people with severe mental disorders in accessing general medical services contribute to reduced life expectancy (Lawrence & Kisely, 2010)[Reference Lawrence and Kisely57, Reference Ronaldson, Elton, Jayakumar, Jieman, Halvorsrud and Bhui40]. Several studies demonstrated underutilization of health care services, especially specialized care services, by psychiatric patients [Reference Ronaldson, Elton, Jayakumar, Jieman, Halvorsrud and Bhui40–Reference Bresee, Majumdar, Patten and Johnson42], particularly by patients with schizophrenia. To ensure standards of care and to avoid further stigmatization of these patients [Reference Corrigan, Mittal, Reaves, Haynes, Han and Morris43], psychiatrists should facilitate patients’ access to primary and specialized somatic health care services and collaborate with primary care physicians, endocrinologists, and cardiologists to ensure accurate follow-up of patients’ health care.
Statement 8, 9: Antipsychotics and Cardiometabolic Risk
Along with additional lifestyle and environmental factors, the combination of antipsychotic treatment and intrinsic risk factors leads to the serious metabolic dysfunctions described in these patients [Reference Freyberg, Aslanoglou, Shah and Ballon11].
Eighty-eight percent of participants widely agreed (responses 4 and 5) that the cardiometabolic profile of an antipsychotic should guide the choice of treatment, together with efficacy and other side effects profile. Seventy-five percent strongly agreed (responses 4 and 5) that wherever possible, drugs with low cardiometabolic risk should be preferred both in acute or, if not possible, in chronic treatment.
It is well known that antipsychotics negatively impact on MetS risk factors (e.g., they are linked to insulin resistance, diabetes/hyperglycemia, dyslipidemia, overweight/obesity) [Reference Newcomer44]. Most of them induce weight gain [Reference Postolache, Del Bosque-Plata, Jabbour, Vergare, Wu and Gragnoli10]. Although it is well known that not all antipsychotics have the same impact on MetS and on its traits [Reference De Hert, Schreurs, Vancampfort and Van Winkel1, Reference Leucht, Corves, Arbter, Engel, Li and Davis45].
Recent studies showed a marked difference among antipsychotics in terms of effects on weight [Reference Huhn, Nikolakopoulou, Schneider-Thoma, Krause, Samara and Peter46] and metabolic side-effects, with olanzapine and clozapine exhibiting the worst profiles and aripiprazole, cariprazine, lurasidone, and ziprasidone the most favorable ones [Reference Pillinger, McCutcheon, Vano, Mizuno, Arumuham and Hindley47]
Many mechanisms are implicated in the metabolic impact of the various antipsychotics, from their effect on histamine receptors, to an action on serotonin [Reference Freyberg, Aslanoglou, Shah and Ballon11, Reference Miron, Baroană, Popescu and Ionică48, Reference Mathews, Newcomer, Mathews, Fales, Pierce and Akers49], to an indirect effect of dopamine on prolactin release and consequently the regulation of estradiol, progesterone, and testosterone metabolism [Reference Baptista, Reyes and Hernández50].
From a molecular point of view, the side effects as well as the effectiveness of antipsychotics are linked to their specific binding profile with the receptors. Antipsychotics have different receptor affinity profiles and the individual molecules have different receptor affinities depending on the dosage; the binding profile of the drugs therefore depends on the level of the drugs itself in the plasma and brain [Reference Gareri, Segura-García, Manfredi, Bruni, Ciambrone and Cerminara51, Reference Correll, Lencz and Malhotra52].
The antipsychotic-induced metabolic abnormalities also show a high interindividual variability suggesting the presence of genetic determinants [Reference De Hert, Schreurs, Vancampfort and Van Winkel1, Reference Mulder, Franke, van der-Beek van der, Arends, Wilmink and Scheffer53, Reference Ellingrod, Miller, Taylor, Moline, Holman and Kerr54]. Based on the above evidence, the choice of an antipsychotic should be made on an individual basis, taking into consideration the safety profile of the different antipsychotics, accurately weighing the risk of major adverse effects, for example, intolerance to extrapyramidal side effects which increases the risk of tardive dyskinesia [Reference Misdrahi, Tessier, Daubigney, Meissner, Schurhoff and Boyer56], or presence of family risk factors for metabolic abnormalities which increases the risk of CVD.
In general, addressing medical comorbidity should be part of the routine care of patients and safety concerns should be one of the main drivers of antipsychotic prescriptions [Reference Meyer55].
Conclusions
European psychiatrists agreed that patients with schizophrenia spectrum disorders are at greater CVD risk. Lifestyle, socioeconomic level, and genetic background play a role in this risk.
In line with the EPA recommendations and the most recent evidence, the surveyed psychiatrists agreed on the importance of an early identification of CVD risks and illness, which should be coupled with an immediate implementation of appropriate risk management strategies. The development of lifestyle interventions to prevent and treat CVD in patients with schizophrenia spectrum disorders is paramount. The cardio-metabolic risk profile of each antipsychotic should be one of the main informants for treatment choice. In general, they have identified the central coordinating figure of this management in the psychiatrist, but the need for a team effort including the specialists as well as the general practitioner must be encouraged.
Not surprisingly, but yet very importantly, 99% of the surveyed psychiatrists agreed that translating the EPA recommendations on CVD risk into routine clinical practice is a priority in the management of patients with schizophrenia spectrum disorders.
Members of the Validation Panel
Paola Bucci (Italy), Alain Dervaux (France), Alkomiet Hasan (Germany), Stephan Heres (Germany), Antonio Vita (Italy), Florence Vorspan (France).
Participants in the Delphi Process
Katryn Abrahams (Belgium), Young Allan (UK), Mario Amore (Italy), Karow Ann (Germany), Daniil Aptalidis (Bulgaria), Massimo Ballerini (Italy), Josef Bäuml (Germany), István Bitter (Hungary), Julio Bobes (Spain), Laurynas Bukelskis (Lithuania), Marc Calmeyn (Belgium), Mary Cannon (Ireland), Kirtsen Catthorr (Belgium), Dan Cohen (Netherlands), Anna Comparelli (Italy), Eric Constant (Belgium), Valentina Corigliano (Italy), Christoph Correll (Germany), Eijnde D.op’t (Netherlands), Karlovic Dalibor (Croatia), Paola Dazzan (UK), Sergio De Filippis (Italy), Dirk De Wachter (Belgium), Jurgen Defruyt (Belgium), Sonia Dollfus (France), Elena Dragioti (Sweden), Caroline Dubertret (France), Bjørn Ebdrup (Denmark), Gamze Erzin (Turkey), Saana Eskelinen (FinlandI), Peter Falkai (Germany), Gerardo Favaretto (Italy), Emilio Fernandez-Egea (UK), Maria Luisa Figueira (Portugal), Slechten Floris (Netherlands), Petros Fotiadis (Greece), Kostas Fountoulakis (Greece), David Fraguas (Spain), Maria Paz Garcia-Portilla (Spain), Reynolds Gavin (UK), Inez Germeys (Belgium), Benoit Gillain (Belgium), Sladjana Gluscevic (Serbia), Xenia Gonda (Hungary), Guy Goodwin (UK), Euphrosyne Gouzoulis-Mayfrank (Germany), Iria Grande (Spain), Eric Hanh (Germany), Alex Hofer (Austria), Birgit Janssen (Germany), Marek Jarema (Poland), Natalia Jimeno (Spain), Peter Jones (UK), Stefan Kaiser (Switzerland), Frédéric Kochman (France), Anna Kuranova (Netherlands), Martin Lambert (Germany), Christophe Lançon (France), Berthold Langguth (Germany), Marion Leboyer (France), Dusica Lecic Tosevski (Serbia), Karolina Leopold (Germany), Jan Libiger (Czech R.), Pierre-Michel Llorca (France), Maria Paz Long (UK), Claudio Lucii (Italy), Bak Maarten (Netherlands), Piero Antonio Magnani (Italy), Jasmina Mallet (France), Nadja P Maric (Serbia), Andre Masson (Belgium), Celine Matton (Belgium), Mauro Mauri (Italy), Ingrid Melle (Norway), Thomas Messer (Germnany), Andreas Meyer-Lindenberg (Germany), Tim Moons (Belgium), Dieter Naber (Germany), Stefano Nassini (Italy), Alvydas Navickas (Lithuania), Mette Ødegaard Nielsen (Denmark), Philippe Nuss (UK), David Nutt (UK), Georgios Papazizis (Greece), Jozef Peuskens (Belgium), Stefan Priebe (UK), Goran Racetovic (Bosnia), Elmars Rancans (Latvia), Anita Riecher-Rössler (Switzerland), Martina Rojnic (Croatia), Janusz K Rybakowski (Poland), Gabriele Sachs (Austria), Myrto Samara (Greece), Jerzy Samochowiec (Poland), I San (Spain), Norman Sartorius (Switzerland), Nikolaos Smyrnis, (Greece), Daria Smirnova (Russia), Iris Sommer (Netherlands), Geerke Steegen (Belgium), Drozdstoj Stoyanov (Bulgaria), Jaana Suvisaari (Finland, Agata Szulc (Poland), Thi-Minh-Tam Ta (Germany), Pierre Thomas (Pierre), Jari Tiihonen (Finland), Sarah Tosato (Italy), Alp Üçok (Turkey), Marie Noelle Vacheron (France), Ruud Van Winkel (Belgium), Veronica Velasco Gonzalez (Spain), Helene Verdoux (France), Domagoj Vidovic (Croatia), Eduard Vieta (Spain), Vincenzo Villari (Italy), Jelena Vrublevska (Latvia), Olivera Vukocic (Serbia), Marcin Wojnar (Poland), Aysegul Yildiz (Turkey), Alessandro Zuddas (Italy).
Acknowledgment
The authors wish to thank Ethos srl for logistic support in conducting the Delphi study.
Financial Support
The work has been carried out thanks to an unrestricted grant from Angelini. CA was supported by the Spanish Ministry of Science and Innovation. Instituto de Salud Carlos III (SAM16PE07CP1, PI16/02012, PI19/024), co-financed by ERDF Funds from the European Commission, “A way of making Europe,” CIBERSAM. Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), European Union Structural Funds. European Union Seventh Framework Program under grant agreements FP7-4-HEALTH-2009-2.2.1-2-241909 (Project EU-GEI), FP7-HEALTH-2013-2.2.1-2-603196 (Project PSYSCAN) and European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement No 115916, Project PRISM, and grant agreement No 777394, Project AIMS-2-TRIALS), Fundación Familia Alonso and Fundación Alicia Koplowitz.
Conflict of Interest
S. Galderisi has been a consultant and/or advisor to or has received honoraria or expenses from: Millennium Pharmaceuticals, Innova Pharma-Recordati Group, Janssen Pharmaceutica NV, Sunovion Pharmaceuticals, Janssen-Cilag Polska, Gedeon-Richter-Recordati, Pierre Fabre, Otsuka, Lundbeck Italia and Angelini-Acraf outside the submitted work. A.P. Maggioni received personal fees for the participation in committees of studies sponsored by Bayer, Fresenius, Novartis outside the present work. C. Arango. has been a consultant to or has received honoraria or grants from Acadia, Angelini, Gedeon Richter, Janssen Cilag, Lundbeck, Minerva, Otsuka, Roche, Sage, Servier, Shire, Schering Plow, Sumitomo Dainippon Pharma, Sunovion and Takeda. P. Gorwood received during the last 5 years fees for presentations at congresses or participation in scientific boards from Alcediag-Alcen, Angelini, GSK, Janssen, Lundbeck, Otsuka, SAGE and Servier. A. Mucci acted as an independent consultant of Ethos srl in this project. She received honoraria, advisory board or consulting fees, outside the present work, from the following companies: Angelini- Acraf, Astra Zeneca, Bristol-Myers Squibb, Gedeon Richter Bulgaria, Innova-Pharma, Janssen Pharmaceuticals, Lundbeck, Otsuka, Pfizer and Pierre Fabre. M. de Hert has no competing interests.
Authorship Contributions
All the authors drafted the manuscript. All the authors contributed to formulating the questionnaire, analyzing the responses, and made critical revisions of the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, SG, upon reasonable request.




Comments
No Comments have been published for this article.