Introduction
Schizophrenia spectrum disorders are complex heterogeneous disorders characterized by positive and negative psychotic symptoms, accompanied by deviations in cognition and functioning. The underlying etiopathology involves a multitude of genetic factors [1] and several environmental exposures [Reference Stilo and Murray2] and their interactions [Reference Guloksuz, Pries, Delespaul, Kenis, Luykx and Lin3–Reference Zwicker, Denovan-Wright and Uher5]. These risk factors for schizophrenia spectrum disorders not only increase susceptibility to psychosis but may also influence prognosis and outcomes of schizophrenia spectrum disorders. In this regard, these factors may explain heterogeneous outcomes and varying illness trajectories observed across the psychosis spectrum [Reference Guloksuz and van Os6].
Several studies have investigated the role of environmental exposures underlying the heterogeneity of outcome in schizophrenia spectrum disorder. These studies have particularly focused on the impact of childhood adversities and cannabis use on various outcome measures in schizophrenia spectrum disorders. Childhood trauma was associated with a higher number of hospitalizations, lower overall functioning scores, and lower quality of life scores in patients with schizophrenia [Reference Baudin, Godin, Lajnef, Aouizerate, Berna and Brunel7]. Similarly, another study showed that patients who were exposed to sexual or physical abuse before the age of 11 years had poorer functioning levels compared to patients without childhood trauma [Reference Alameda, Ferrari, Baumann, Gholam-Rezaee and Do8]. A recent meta-analysis of 12 studies examining the association between childhood adversity and treatment outcomes in patients diagnosed with psychotic disorders indicates that childhood adversity is associated with poorer treatment outcomes (OR = 1.51, 95% CI: 1.08–2.10) [Reference Thomas, Höfler, Schäfer and Trautmann9]. These findings should come as no surprise given the wide-ranging impact of childhood trauma (i.e., sexual abuse) on mental and physical outcomes in the general population [Reference Hailes, Yu, Danese and Fazel10], as well as its relation with service engagement and medication adherence in patient populations [Reference Bloomfield, Yusuf, Srinivasan, Kelleher, Bell and Pitman11].
Studies focusing on cannabis use in patients with schizophrenia show that cannabis use is associated with decreased functioning, reduced service engagement, and poor treatment outcomes in schizophrenia spectrum disorder; and that reducing cannabis use improves global functioning scores in patients with psychosis [Reference Schimmelmann, Conus, Cotton, Kupferschmid, McGorry and Lambert12–Reference Major, Woolley, Rahaman and Joyce14]. Another study revealed that premorbid cannabis use was associated with more severe psychotic symptoms, earlier age of onset, and impaired functioning [Reference Ringen, Nesvåg, Helle, Lagerberg, Lange and Løberg15]. Notwithstanding the need for high-quality evidence from large cohorts, it appears from these observations that the assessment of lifetime exposures to environmental risk (or protective) factors may provide guidance for prediction of illness course and clinical and functional outcomes in schizophrenia spectrum disorder.
Recently, we have estimated a cumulative environmental exposure score for schizophrenia, the exposome score for schizophrenia (ES-SCZ), which takes into account the interdependency of environmental exposures [Reference Guloksuz, Rutten, Pries, ten Have, de Graaf and van Dorsselaer16] and therefore prevents overestimation of each exposure’s effect size for schizophrenia risk [Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17]. Our recent findings demonstrate that ES-SCZ has a good discriminative function (AUC = 84) for identifying schizophrenia, as well as for stratifying psychosis risk in the general population [Reference Pries, Erzin, van Os, Ten Have, de Graaf and van Dorsselaer18,Reference Guloksuz, Pries, Have, Graaf, Dorsselaer and Klingenberg19]. Furthermore, we have shown that ES-SCZ is associated with several psychiatric diagnoses and other medical outcomes in the general population [Reference Pries, Erzin, van Os, Ten Have, de Graaf and van Dorsselaer18]. In our longitudinal study of ES-SCZ in a 9-year population-based prospective cohort, we have demonstrated that ES-SCZ is associated with mental and physical health outcomes and moderates the impact of stressful life events on mental and physical well-being in the general population [Reference Pries, van Os, Ten Have, de Graaf, van Dorsselaer and Bak20]. These findings suggest that ES-SCZ, similar to polygenic risk score for schizophrenia (PRS-SCZ), can provide potential utility in the context of risk stratification and outcome prediction.
In this study, we therefore aimed to investigate whether ES-SCZ was associated with global functioning in patients with a schizophrenia spectrum disorder. To test and replicate our findings, we analyzed independent datasets derived from two studies that followed uniform assessment schedules: the European Network of National Networks Studying Gene–Environment Interactions in Schizophrenia (EUGEI) and the Genetic Risk and Outcome of Psychosis (GROUP). Furthermore, in light of our previous findings that showed a broader impact of ES-SCZ on mental and physical outcomes, we additionally analyzed the association of ES-SCZ with global functioning in siblings of these patients and healthy control groups, and subsequently compared those with that in patients with schizophrenia spectrum disorder.
Methods
Data on patients diagnosed with schizophrenia spectrum disorders, their unaffected siblings, and healthy controls were derived from the “vulnerability and severity” Work Package 6 (WP6) of the EUGEI [21] collected in Turkey, Spain, and Serbia. Data collection was carried out by psychiatrists, psychologists, or trained research assistants who followed on-site training sessions and annual online training modules to maintain high inter-rater reliability [21,Reference Korver, Quee, Boos, Simons and de Haan22]. The baseline wave of the GROUP study, collected in the Netherlands, was used for replication in an independent dataset. Both projects (EUGEI and GROUP) were approved by the Medical Ethics Committees of all participating sites and conducted in accordance with the Declaration of Helsinki. All respondents provided written informed consent, and, in the case of minors, such a consent was also obtained from parents or legal guardians. Details of the GROUP and EUGEI projects were provided elsewhere [21,Reference Korver, Quee, Boos, Simons and de Haan22].
Patients were diagnosed with schizophrenia spectrum disorders according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), which was further validated using the Operational Criteria Checklist for Psychotic and Affective Illness [Reference McGuffin, Farmer and Harvey23] in EUGEI-WP6, and the Schedules for Clinical Assessment in Neuropsychiatry [Reference Wing, Babor, Brugha, Burke, Cooper and Giel24] or the Comprehensive Assessment of Symptoms and History [Reference Andreasen, Flaum and Arndt25] in GROUP. The average duration of illness in EUGEI-WP6 since the first contact with mental health services was 9.9 years. Healthy controls who had no lifetime psychotic disorder history were collected from the same population as the patients. For all participants, a diagnosis of psychotic disorder due to another medical condition, a history of head injury with loss of consciousness, and an intelligence quotient <70 were exclusion criteria for all participants. The EUGEI-WP6 consisted of 1,261 patients, 1,282 unaffected siblings of these patients, and 1,525 healthy controls (Table 1). Only the patient population of the GROUP sample at baseline (n = 1,119) served as the replication dataset (Table 1) given that the Global Assessment of Functioning (GAF) was not applied to siblings and healthy controls in the GROUP study.
Table 1. Sample characteristics.

Abbreviations: EUGEI, the European Network of National Networks Studying Gene–Environment Interactions in Schizophrenia; ES-SCZ, exposome score for schizophrenia; GAF, Global Assessment of Functioning; GROUP, the Genetic Risk and Outcome of Psychosis.
a The patient population of the GROUP served as the replication dataset.
Measurements
Functioning scores
Functioning was assessed using the GAF scale [Reference Endicott, Spitzer, Fleiss and Cohen26,27]. The GAF is a well-known standard rating scale to measure social, occupational, and psychological functioning. The GAF score varies from 1 to 100, higher scores reflecting an increase in mental health and capability of coping and lower scores reflecting a decrease in mental health and capability of coping. The validity study of the GAF in the DSM-IV-TR has demonstrated that the GAF scale is valid for assessing the symptom and disability dimensions [Reference Pedersen and Karterud28,Reference Pedersen, Hagtvet and Karterud29].
Exposome score for schizophrenia
To estimate the cumulative environmental load, we calculated the ES-SCZ based on our formerly validated estimates [Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17]. Conforming to our previous studies [Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4,Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17], we constituted the ES-SCZ by summing log-odds weighted environmental exposures (each exposure defined as absent = “0” and present = “1”) including cannabis use, hearing impairment, winter-birth, and childhood adversity domains (emotional and physical neglect; emotional, physical, and sexual abuse; and bullying). For ease of interpretation, a constant of 2 is added to ES-SCZ. The assessments and definitions of environmental exposures conforming to previous analyses [Reference Guloksuz, Pries, Delespaul, Kenis, Luykx and Lin3,Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4,Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17] are provided below.
Childhood adversity was assessed using the Childhood Trauma Questionnaire Short Form (CTQ-SF) [Reference Bernstein, Stein, Newcomb, Walker, Pogge and Ahluvalia30]. This form consists of 28 items, rated on a 5-point Likert scale, measuring five domains of maltreatment (emotional and physical neglect; and emotional, physical, and sexual abuse). The psychometric characteristics of the translated versions (Spanish, Turkish, Dutch, and Serbian) of the CTQ have been comprehensively studied [Reference Şar, Akyüz, Kundakçı, Kızıltan and Doğan31–Reference Hernandez, Gallardo-Pujol, Pereda, Arntz, Bernstein and Gaviria33]. To dichotomize each childhood adversity domain (0 = “absent” and 1 = “present”), consistent with previous work in the EUGEI [Reference Guloksuz, Pries, Delespaul, Kenis, Luykx and Lin3,Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4,Reference Kraan, Velthorst, Themmen, Valmaggia, Kempton and McGuire34], we used the following cutoff scores for each domain: ≥9 for emotional abuse, ≥8 for physical abuse, ≥6 for sexual abuse, ≥10 for emotional neglect, and ≥8 for physical neglect.
Cannabis use was assessed using the Cannabis Experiences Questionnaire (modified version) [Reference Barkus, Stirling, Hopkins and Lewis35] in EUGEI-WP6 and the Composite International Diagnostic Interview (CIDI; L section) [Reference Robins, Wing, Wittchen, Helzer, Babor and Burke36] in GROUP. The Cannabis Experiences Questionnaire (0 = “none”; 1 = “only once or twice”; 2 = “a few times a year”; 3 = “a few times a month”; 4 = “once or more a week”; 5 = “everyday”) and CIDI (0 = “none”; 1 = “less than weekly”; 2 = “weekly”; 3 = “daily”) are Likert-type scales. Following previous work [Reference Guloksuz, Pries, Delespaul, Kenis, Luykx and Lin3,Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4,Reference van Winkel37–Reference Radhakrishnan, Guloksuz, Ten Have, de Graaf, van Dorsselaer and Gunther39], a binary regular cannabis use variable was constructed by using the cutoff value of one or more per week during the lifetime period most frequent use.
Conforming to previous studies exploring the association between the season of birth and schizophrenia spectrum disorder in the Northern hemisphere sites [Reference Davies, Welham, Chant, Torrey and McGrath40], the high-risk birth period was the winter solstice (December–March).
Hearing impairment in the last 12 months was assessed using a self-report evaluation (0 = “absent” and 1 = “present”) [Reference Guloksuz, Pries, Delespaul, Kenis, Luykx and Lin3].
The short version of Retrospective Bullying Questionnaire (RBQ) was used to evaluate the history of exposure to childhood bullying (emotional, psychological, or physical violence) before the age of 17 [Reference Hunter, Mora Merchán and Ortega Ruiz41,Reference Schäfer, Korn, Smith, Hunter, Mora‐Merchán and Singer42]. The RBQ measures the severity of the bullying experience as 0 = “none”, 1 = “some” (no physical injuries)”, 2 = “moderate” (minor injuries or transient emotional reactions)”, and 3 = “marked” (severe and frequent physical or psychological harm). By using the cutoff point ≥1, childhood bullying was dichotomized as 0 = “absent” and ≥1 = “present”, conforming to previous studies [Reference Guloksuz, Pries, Delespaul, Kenis, Luykx and Lin3,Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4,Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17].
Genetic data processing and polygenic risk score for schizophrenia
Samples of all individuals were genotyped at the Cardiff University Institute of Psychological Medicine and Clinical Neurology, using custom Illumina HumanCoreExome-24 BeadChip genotyping arrays containing probes for 570,038 genetic variants (Illumina, San Diego, CA). Genotype data were called using the Genome Studio package and transferred into PLINK format for further analysis. Quality control was conducted in PLINK v1.07 [Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira and Bender43] or with custom Perl scripts. Variants with call rate <98% were excluded from the dataset. Hardy–Weinberg equilibrium p-value was calculated separately in Turkish, Northern European, and Southern European samples. Variants with Hardy–Weinberg equilibrium p-value <1 × 10−6 in any of these three regions were excluded from the dataset. After quality control, 559,505 variants remained.
Samples with call rate <98% were excluded from the dataset. A linkage disequilibrium (LD) pruned set of variants was calculated using the --indep-pairwise command in PLINK (maximum r2 = 0.25; window size = 500 SNPs; window step size = 50 SNPs) and used for further analyses. Homozygosity F values were calculated using the --het command in PLINK, and outlier samples (F < −0.11 or F > 0.15) were excluded. The genotypic sex of samples was calculated from X chromosome data using the --check-sex command in PLINK, and samples with different genotypic sex to their database sex were excluded.
Identity-by-descent values were calculated for the sample in PLINK. Samples with one or more siblings among the genotyped samples according to the database but no identified genotypic siblings (defined as PI-HAT >0.35 and <0.65) were excluded. After these were removed from consideration, samples with two or more siblings in the database that were not supported by the genotypic data were also excluded.
After visually observing clustering of errors by genotyping chip, we decided to exclude chips with a high proportion of errors. All samples on chips with five or more sample exclusions due to heterozygosity or call rate (out of 12 possible samples) were excluded. All samples on chips with four or more sample exclusions due to sex or relative checks were also excluded, unless their identity was corroborated by concordance between database and genotype relatedness data with a sample on another chip.
Genetic ancestry principal components (PCs) were calculated by conducting a PC analysis (i.e., a dimensionality-reduction method) in PLINK using LD pruned variants after combining the dataset with the Thousand Genomes reference dataset. Due to the inherently multipopulation nature of the dataset and the variety of possible analyses, no exclusions were made to the whole dataset based on this analysis. Population effects were corrected for separately in individual analyses. After quality control, genotypes were imputed on the Michigan Imputation Server using the Haplotype Reference Consortium reference panel (version 1.1) and the programs Eagle for haplotype phasing and Minimac3 for imputation [Reference Das, Forer, Schönherr, Sidore, Locke and Kwong44,Reference Loh, Danecek, Palamara, Fuchsberger, Reshef and Finucane45]. After imputation, variants with an imputation r2 > 0.6, minor allele frequency (MAF) >0.1%, and call rate >99% were retained (8,277,535 variants). Best-guess genotypes were generated from genotype probabilities using PLINK. PRS-SCZ was constructed using summary statistics from the Psychiatric Genomics Consortium genome-wide association study, excluding samples present in the GROUP data [1]. Clumping was performed in imputed best-guess genotypes for each dataset using PLINK (maximum r2 = 0.2; window size = 500 kb; minimum MAF = 10%; minimum imputation information score = 0.7), and variants within regions of long-range LD around the genome (including the human major histocompatibility complex) were excluded [Reference Price, Weale, Patterson, Myers, Need and Shianna46]. PRS-SCZ was then constructed from best-guess genotypes using PLINK at 10 different p-value thresholds (PT = 1, 0.5, 0.3, 0.2, 0.1, 0.05, 0.01, 1 × 10−4, 1 × 10−6, 5 × 10−8). Consistent with previous research in the field [Reference Allardyce, Leonenko, Hamshere, Pardiñas, Forty and Knott47,Reference Sørensen, Debost, Agerbo, Benros, McGrath and Mortensen48], we used p = 0.05 for our primary analysis, as this threshold explained most variation in the phenotype in the Psychiatric Genomics Consortium analysis [1].
Statistical analyses
Stata software version 16.0 was used for the analysis [49]. Supplementary Table S1 reports missing data for the EUGEI and GROUP datasets, separately. The analyses were conducted on both multiple imputed data and raw data. Following the previous analyses in this dataset [Reference Guloksuz, Pries, Delespaul, Kenis, Luykx and Lin3,Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4], the multiple imputation chained equation [Reference Royston and White50] including all variables included in the analyses was applied with 20 imputations restricted to in-range values (relative efficiency ≥99%). Data were separately imputed for the subsamples with genetic information. ES-SCZ was calculated after imputing missing values of the environmental exposures (cannabis use, hearing impairment, winter-birth, and childhood adversity domains). All the analyses were run on multiple imputed data and pooled using Rubin’s rules [Reference Rubin51].
Analyses were stratified by the subgroups: patients, unaffected siblings, and healthy controls. The SUEST and LINCOM commands were applied to compare coefficient differences between groups. All analyses were a priori adjusted for age, sex, and education (1 = “No qualification,” 2 = “With qualification (secondary),” 3 = “Tertiary,” 4 = “Vocational,” and 5 = “University”). Analyses in the EUGEI dataset were additionally adjusted for country (Turkey, Spain, and Serbia). The sensitivity analyses adjusted for genetic vulnerability for schizophrenia (PRS-SCZ) were restricted to participants of white ethnic origin. For replication in the baseline data from patients of the GROUP study, we performed the same models except the adjustment for country as GROUP was a Dutch national study. For visualization, a scatter plot that displayed the relationship between ES-SCZ and GAF was constructed. Prediction lines and 95% confidence interval were retrieved and reported in Figure 1.

Figure 1. Linear prediction lines with 95% confidence interval of a scatter plot of the exposome score for schizophrenia (ES-SCZ; higher scores reflect an increase of environmental vulnerability) on the symptom and disability dimensions of the Global Assessment of Functioning (GAF; higher scores reflect an increase in functioning) scale per group (controls, siblings, and patients).
Results
Table 1 reports the demographic characteristics and the frequencies of exposure and outcome per dataset. Missing values per dataset are shown in Supplementary Table S1.
Association between ES-SCZ and GAF dimensions in the EUGEI dataset
Stratified analyses of patients, siblings, and healthy controls indicated that ES-SCZ was associated with the GAF dimensions in patients (symptom: B = −1.53 [95% CI −2.43; −0.64], p-value = 0.001; disability: B = −1.44 [95% CI −2.30; −0.58], p-value = 0.001), siblings (symptom: B = −3.07 [95% CI −3.75; −2.39], p-value < 0.001; disability: B = −2.52 [95% CI −3.19; −1.85], p-value < 0.001), and healthy controls (symptom: B = −1.50 [95% CI −1.98; −1.03], p-value < 0.001; disability: B = −1.31 [95% CI −1.75; −0.87], p-value < 0.001).
The degree of association between ES-SCZ and the GAF symptom dimension was the highest in unaffected siblings, followed by patients and controls: −3.07, −1.53, and −1.50, respectively. Group comparison showed that there were significant differences between controls and siblings (B = 1.57 [95% CI 0.49; 2.65], p-value = 0.004) and between siblings and patients (B = −1.54 [95% CI −2.70; −0.38], p-value = 0.010), whereas controls and patients were not statistically significantly different (B = 0.03 [95% CI −1.16; 1.23], p-value = 0.958).
The degree of association between ES-SCZ and the GAF disability dimension was the highest in siblings, followed by patients and controls: −2.52, −1.44, and −1.31, respectively. Group comparison showed that there were significant differences between controls and siblings (B = 1.21 [95% CI 0.22; 2.20], p-value = 0.017), whereas siblings and patients (B = −1.08 [95% CI −2.24; 0.08], p-value = 0.067), as well as controls and patients (B = 0.13 [95% CI −0.91; 1.17], p-value = 0.806) were not statistically significantly different.
The results remained the same after adjusting for PRS-SCZ and 10 PCs (Tables 2 and 3). Furthermore, the analyses using unimputed data confirmed the results (Tables 4 and 5 for analyses adjusted for genetic vulnerability), with the exception of the association between ES-SCZ and disability: the comparison between siblings and patients in the unadjusted unimputed data became significant. Furthermore, the comparison between controls and siblings as well as siblings and patients in the genetically adjusted analyses of the association between ES-SCZ and symptoms in the unimputed data became trend significant (Table 3). For visualization, Figure 1 shows the linear prediction lines from a scatter plot of ES-SCZ on GAF dimensions per group.
Table 2. Results of the models adjusted for PRS-SCZ from the imputed datasets.
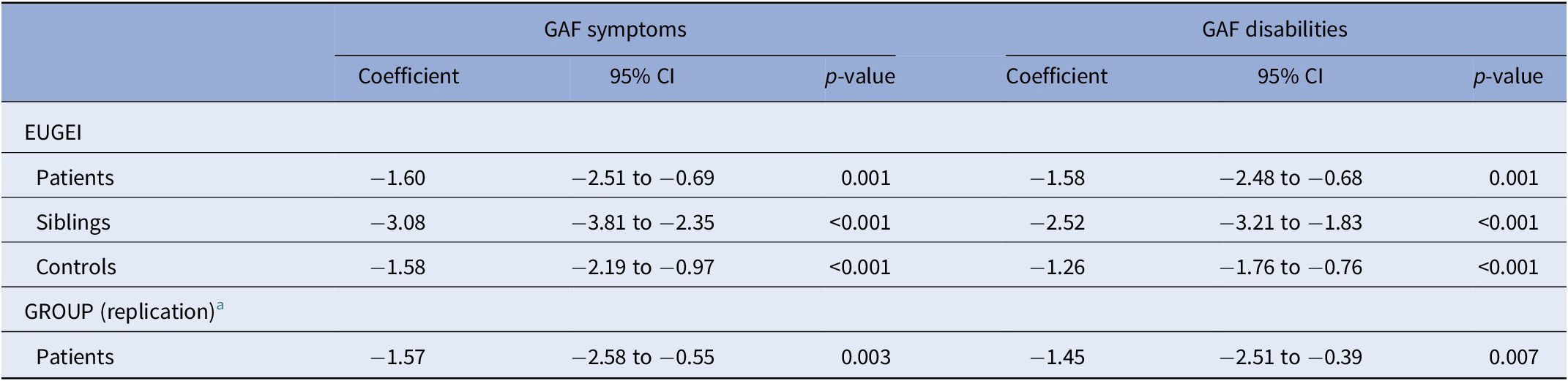
Notes: All analyses were adjusted for age, sex, and education (1 = “No qualification,” 2 = “With qualification (secondary),” 3 = “Tertiary,” 4 = “Vocational,” and 5 = “University”), PRS-SCZ, and 10 principal components. Analyses in the EUGEI were additionally adjusted for country (Turkey, Spain, and Serbia).
Abbreviations: CI, confidence interval; EUGEI, the European Network of National Networks Studying Gene–Environment Interactions in Schizophrenia; GAF, Global Assessment of Functioning; GROUP, the Genetic Risk and Outcome of Psychosis; PRS-SCZ, polygenic risk score for schizophrenia.
a The patient population of the GROUP served as the replication dataset.
Table 3. The comparison of the degree of the association between ES-SCZ and functioning across groups.

Notes: Comparisons between controls versus siblings, siblings versus patients, and controls versus patients.
Abbreviations: CI, confidence interval; ES-SCZ, exposome score for schizophrenia; GAF, Global Assessment of Functioning; PRS-SCZ, polygenic risk score for schizophrenia.
a Results of the models adjusted for PRS-SCZ from the imputed dataset.
b Results of the models from the unimputed dataset.
c Results of the models adjusted for PRS-SCZ from the unimputed dataset.
Table 4. Results of the models from the unimputed datasets.

Notes: All analyses were adjusted for age, sex, and education (1 = “No qualification,” 2 = “With qualification (secondary),” 3 = “Tertiary,” 4 = “Vocational,” and 5 = “University”). Analyses in the EUGEI were additionally adjusted for country (Turkey, Spain, and Serbia).
Abbreviations: CI, confidence interval; EUGEI, the European Network of National Networks Studying Gene–Environment Interactions in Schizophrenia; GAF, Global Assessment of Functioning; GROUP, the Genetic Risk and Outcome of Psychosis.
a The patient population of the GROUP served as the replication dataset.
Table 5. Results of the models adjusted for PRS-SCZ from the unimputed datasets.

Notes: All analyses were adjusted for age, sex, and education (1 = “No qualification,” 2 = “With qualification (secondary),” 3 = “Tertiary,” 4 = “Vocational,” and 5 = “University”), PRS-SCZ, and 10 principal components. Analyses in the EUGEI were additionally adjusted for country (Turkey, Spain, and Serbia).
Abbreviations: CI, confidence interval; EUGEI, the European Network of National Networks Studying Gene–Environment Interactions in Schizophrenia; GAF, Global Assessment of Functioning; GROUP, the Genetic Risk and Outcome of Psychosis; PRS-SCZ, polygenic risk score for schizophrenia.
a The patient population of the GROUP served as the replication dataset.
Replication of the association between ES-SCZ and GAF dimensions in the GROUP dataset
The investigation of the association between ES-SCZ and GAF dimensions in the GROUP dataset showed that ES-SCZ was associated with the GAF symptom dimension (B = −1.80 [95% CI −2.57; −1.03], p-value < 0.001) and the disability dimension (B = −1.63 [95% CI −2.40; −0.86], p-value < 0.001) in patients. The results remained the same when adjusting for genetic vulnerability for schizophrenia (Table 2). The analyses using unimputed data confirmed the association between ES-SCZ and the GAF symptom dimension (Tables 4 and 5).
Discussion
This study examined whether the cumulative environmental load for schizophrenia (ES-SCZ) was associated with global functioning in patients diagnosed with schizophrenia spectrum disorder, unaffected siblings, and healthy controls, respectively. We found that ES-SCZ was associated with the GAF symptom and disability dimensions in all three groups. These findings remained consistent in the models adjusted for PRS-SCZ. By analyzing an independent dataset with identical assessment measures, we replicated the results observed in the patient group. Furthermore, our secondary analysis revealed that the degree of associations of ES-SCZ with both the symptom and disability dimensions of the GAF were higher in unaffected siblings than in patients and healthy participants.
Utilizing the exposome score for schizophrenia
Our findings—replicated in two independent samples—indicate that ES-SCZ might be a marker for poor functioning in patients diagnosed with schizophrenia spectrum disorder. These findings suggest that ES-SCZ is not only linked to an increased risk for psychosis expression across the extended psychosis phenotype [Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4,Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17] but may also be related to the severity of functional disability. Furthermore, findings from the models adjusted for PRS-SCZ demonstrate that the associations between ES-SCZ and functioning outcomes are not reducible to the individual-level genomic risk score for schizophrenia that has previously been linked to poor outcome in schizophrenia [Reference Frank, Lang, Witt, Strohmaier, Rujescu and Cichon52–Reference Zhang, Robinson, Yu, Gallego, Fleischhacker and Kahn54]. Therefore, it appears that an outcome prediction model that supplements PRS-SCZ with ES-SCZ is likely to yield better predictive performance than a pure genetic prediction. As a potential marker of functioning, ES-SCZ can be used for the severity stratification in large-scale clinical trials and observational studies [Reference Guloksuz, Pries, Have, Graaf, Dorsselaer and Klingenberg19]. Furthermore, ES-SCZ can be integrated into clinical characterization [Reference Maj, van Os, De Hert, Gaebel, Galderisi and Green55] and future transdiagnostic staging models [Reference Shah, Scott, McGorry, Cross, Keshavan and Nelson56]. However, prospective controlled studies, ideally conducted in first episode psychosis (FEP) cohorts, are required to assess the prognostic performance of ESC-SCZ for predicting outcome in psychotic disorders. In the future, our research group, therefore, aims to systematically test the performance of ES-SCZ using prognostic modeling analyses in a pragmatic, multicenter, single-blind randomized controlled trial of FEP patients (the HAMLETT-OPHELIA study) that is scheduled to follow up 512 FEP patients over 40 years [Reference Begemann, Thompson, Veling, Gangadin, Geraets and van’t Hag57], with the primary aims of optimizing tailored treatment to inform early intervention strategies and personalized medicine efforts in FEP. The ultimate goal of this project would be to improve multilevel forecasting by integrating individual-level genomic and exposomic information with rich clinical, existential, and social data to entangle the complexity underlying outcome heterogeneity in psychosis.
Our findings also demonstrate that ES-SCZ may be beneficial for identifying poor functioning not only in patients diagnosed with schizophrenia spectrum disorder but also in their unaffected siblings and healthy control participants. These findings echo findings from previous research showing a temporal association of ES-SCZ with broad mental and physical health outcomes in a 9-year population-based prospective cohort [Reference Pries, van Os, Ten Have, de Graaf, van Dorsselaer and Bak20]. Previous research similarly showed that ES-SCZ was associated with a multitude of mental disorders, including depression, anxiety, and alcohol use disorders, traits such as neuroticism, as well as other medical outcomes such as asthma, migraine, and ulcers [Reference Pries, Erzin, van Os, Ten Have, de Graaf and van Dorsselaer18]. These results are indeed anticipated given that each environmental exposure as a constituent of ES-SCZ (e.g., childhood adversities, cannabis use, and childhood bullying) has been individually linked to poor mental and physical well-being in addition to multidimensional psychopathology [Reference Guloksuz, Rutten, Pries, ten Have, de Graaf and van Dorsselaer16,Reference Guloksuz, van Os and Rutten58–Reference Gur, Moore, Rosen, Barzilay, Roalf and Calkins60].
Finally, our secondary analysis showed that the degree of association between ES-SCZ and GAF functioning scores was greater in siblings than in patients and healthy controls. These results were consistent with previous findings of an investigation of PRS-SCZ in the EUGEI and GROUP datasets that revealed an association of PRS-SCZ with subthreshold psychosis phenotypes in unaffected siblings but not in healthy participants [Reference van Os, L-K, Delespaul, Kenis, Luykx and Lin61]. In siblings, who share genetic composition largely with patients, environment may be a more important factor for determining the level of functioning [Reference Marsman, Pries, Ten Have, de Graaf, van Dorsselaer and Bak62]. Furthermore, the differences found between unaffected siblings, healthy controls, and patients might be driven by specific patterns of gene–environment and environment–environment interactions. It is possible that unmeasured genetic and environmental vulnerability, which impact especially individuals with high ES-SCZ, might drive this stronger association in siblings. One of these unmeasured environmental vulnerabilities might be the fact that siblings grew up with a relative with mental health problems which can represent a strong adversity and make individuals more susceptible to the effects of environmental exposures later in life.
Strengths and limitations
The major strengths of our study were the use of two large independent datasets for test and replication; and the uniform measurement schedule and similar sampling strategy in both datasets. Furthermore, we used ES-SCZ that was previously constructed, validated, and demonstrated to perform better than other cumulative environmental scores in our study population [Reference Pries, Dal Ferro, van Os, Delespaul, Kenis and Lin4,Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17,Reference Pries, Erzin, van Os, Ten Have, de Graaf and van Dorsselaer18]. However, it should be noted that ES-SCZ was limited by the availability of exposure assessment, and therefore did not include all known environmental exposures relevant for risk or course, such as obstetric and pregnancy complications, as well as exposures such as ethnic minority and migration that were deliberately excluded to increase the utility of ES-SCZ in applications combined with genetic data [Reference Pries, Lage-Castellanos, Delespaul, Kenis, Luykx and Lin17]. Finally, our findings were based on cross-sectional analysis; therefore, future longitudinal studies are required to assess the prognostic performance of ES-SCZ.
Conclusion
In conclusion, our findings showing an association between ES-SCZ and functioning outcomes suggest that ES-SCZ shows promise for enhancing risk prediction and stratification in research practice. From a clinical perspective, ES-SCZ, a cumulative exposure score, may aid in efforts of clinical characterization, operationalizing future transdiagnostic clinical staging models, and personalizing the clinical management plan. Furthermore, as the effects of ES-SCZ on functioning outcomes were not reducible to the individual-level genomic risk score for schizophrenia, for future studies, supplementing PRS-SCZ with ES-SCZ is likely to yield better predictive performance than a pure genetic prediction.
Acknowledgments
The authors are grateful to the patients and their families for participating in the project. They also thank all research personnel involved in the GROUP project, in particular J. van Baaren, E. Veermans, G. Driessen, T. Driesen, E. van’t Hag, and J. de Nijs. All the DNA samples from Turkey were provided by the Ankara University Brain Research Center Biobank, which was supported by Ankara University Scientific Research Projects Coordination Unit (project no. 10A6055003, 2010).
Ethical statement
The projects were approved by the Medical Ethics Committees of all participating sites and conducted in accordance with the Declaration of Helsinki. All respondents provided written informed consent, and, in the case of minors, such consent was also obtained from parents or legal guardian.
Financial support
The EUGEI project was supported by the European Community’s Seventh Framework Program under grant agreement No. HEALTH-F2-2009-241909 (Project EU-GEI). Dr Erzin is supported by the Scientific and Technological Research Council of Turkey, 2219 International Postdoctoral Research Fellowship Program. Dr Pries is supported by the Kootstra Talent Fellowship of Maastricht University. Dr O’Donovan is supported by MRC Program grant (G08005009) and an MRC Centre grant (MR/L010305/1). Dr Rutten was funded by a VIDI award number 91718336 from the Netherlands Scientific Organization. Drs Guloksuz and van Os are supported by the Ophelia research project, ZonMw grant number: 636340001. Dr Arango was supported by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (SAM16PE07CP1, PI16/02012, and PI19/024), CIBERSAM, Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), Fundación Familia Alonso, and Fundación Alicia Koplowitz.
Conflict of interest
Celso Arango has been a consultant to or has received honoraria or grants from Acadia, Angelini, Gedeon Richter, Janssen-Cilag, Lundbeck, Minerva, Otsuka, Roche, Sage, Servier, Shire, Schering-Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda. Michael O’Donovan is supported by a collaborative research grant from Takeda Pharmaceuticals. Maria Paz Garcia-Portilla has been a consultant to and/or has received honoraria/grants from Angelini, Alianza Otsuka-Lundbeck, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, and Sage Therapeutics.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request under the condition of the approval of the EUGEI and GROUP steering committees.
Appendix
GROUP Investigators in EUGEI Investigators are Behrooz Z. Alizadeh,Footnote 1 Therese van Amelsvoort,Footnote 2 Richard Bruggeman,1 Wiepke Cahn,Footnote 3, Footnote 4 Lieuwe de Haan,Footnote 5 Bart P. F. Rutten,2 Jurjen J. Luykx,3,Footnote 6, Footnote 7 Jim van Os,2,3,Footnote 8 and Ruud van Winkel.2,Footnote 9









Comments
No Comments have been published for this article.