Introduction
Background
Cognitive impairment has been considered a core feature of schizophrenia since the first descriptions of the disorder [Reference Bleuler1,Reference Kraepelin2]. Several meta-analyses and systematic reviews consistently demonstrated that subjects with schizophrenia, compared to healthy controls, present a mild to severe impairment in different domains of cognition [Reference Fatouros-Bergman, Cervenka, Flyckt, Edman and Farde3–Reference Zhang, Wang, Hu, Zhu, Zhang and Wang9]. The involved domains encompass a wide range of functions, including neurocognitive domains, such as attention, speed of processing, memory, working memory, reasoning and problem solving, as well as social cognition domains, such as emotion processing, and theory of mind (ToM). Cognitive impairment is present since the first manifestations of the disease and in subjects at clinical high risk (CHR) for psychosis [Reference Fatouros-Bergman, Cervenka, Flyckt, Edman and Farde3,Reference Lee, Hong, Shin and Kwon6,Reference Bora, Lin, Wood, Yung, McGorry and Pantelis10,Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung and Howes11], as well as, in an attenuated form, in non-affected relatives of subjects with schizophrenia [Reference Sitskoorn, Aleman, Ebisch, Appels and Kahn12,Reference Mucci, Galderisi, Green, Nuechterlein, Rucci and Gibertoni13]. The overall magnitude and pattern of cognitive impairment remain substantially stable over the course of schizophrenia, after the first episode of the illness, with the exception of working memory and social cognition, which are less impaired in the early stages of the illness than in the chronic phases [Reference McCleery, Ventura, Kern, Subotnik, Gretchen-Doorly and Green14].
The deficits in multiple neurocognitive domains seem to interfere with real-life functioning more than negative and positive symptoms [Reference Green, Horan and Lee15–Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci18]. The impact of neurocognitive deficits on real-life functioning is mediated at least in part by social cognition; however, neurocognitive and social cognitive impairments are associated with different functional outcomes [Reference Green, Horan and Lee15,Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17–Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca and Bertolino21]. While currently available pharmacological treatments appear to exert only limited improvements in cognitive performance, psychosocial interventions such as cognitive remediation appear to provide consistent benefits, especially when integrated with a structured psychiatric rehabilitation program, as attested by several meta-analytic studies [Reference Kambeitz-Ilankovic, Betz, Dominke, Haas, Subramaniam and Fisher22–Reference Wykes, Huddy, Cellard, McGurk and Czobor26]. Physical exercise also appears to have positive effects [Reference Firth, Cotter, Carney and Yung27]. However, despite their important role in determining worse real-world outcomes and the existence of targeted and effective evidence-based treatment, cognitive deficits often remain an overlooked aspect in day-to-day clinical practice in mental health services.
In this perspective, the Schizophrenia Section of the European Psychiatric Association (EPA) proposed the development of a guidance paper aimed to provide recommendations for the assessment of cognitive impairment in people living with schizophrenia.
Aims
The aim of this work is to present a comprehensive and detailed overview of cognitive impairment in people living with schizophrenia and provide evidence-based recommendations for its assessment both in research settings and in everyday clinical practice.
The guidance will be structured into four sections:
-
• Conceptualization of cognitive impairment: detailing the identification of distinct domains and the factor structure of cognitive deficits.
-
• Impact of cognitive impairment in schizophrenia: describing the negative role of cognitive deficits on psychosocial functioning, real-world outcomes, and quality of life (QoL).
-
• Recognition and assessment of cognitive impairment: providing a review of available validated assessment instruments as well as recommendations regarding the feasibility and applicability of cognitive assessment tools in real-world psychiatric settings.
-
• Assessment of cognitive impairment in early intervention settings: focusing on recognition and assessment of cognitive impairment in high-risk and early psychosis subjects.
Methodology
Systematic literature search
The development of EPA guidance on the assessment of cognitive impairment in schizophrenia followed the standardized methods defined by the European Guidance Project of the EPA, as described in previous publications [Reference Gaebel, Großimlinghaus, Mucic, Maercker, Zielasek and Kerst28–Reference Galderisi, Kaiser, Bitter, Nordentoft, Mucci and Sabé33], and is based on a systematic literature search performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) indications [Reference Moher, Liberati, Tetzlaff, Altman and Group34,Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann and Mulrow35].
The literature search was conducted on three electronic databases, Medline/PubMed, Scopus, and PsychINFO, using the following research string: (Schizophrenia and (“cognitive impairment”, “cognitive function”, “cognitive symptoms”, “memory”, “attention”, “executive functions”, “processing speed”, “learning”, “reasoning”, “problem solving”, “social cognition”, “emotion processing”, “theory of mind”, “attributional style”, “social perception”, “metacognition”, “metacognitive”, “cognitive assessment”, or “neuropsychological assessment”)), considering results from January 01, 2010 to December 31, 2020 to avoid excessively outdated findings.
Studies were selected for inclusion in the EPA guidance according to pre-defined criteria.
Selection procedure
To be considered for inclusion, studies had to be meta-analyses, randomized controlled trials (RCTs), reviews, cohort studies, open studies, descriptive studies, or expert opinions regarding the assessment of cognitive deficits in people living with schizophrenia, with CHR or early psychosis. Reports were considered for inclusion if they were published in English language.
Reports were excluded if they were duplicates, comments, editorials, case reports, case series, theses, proceedings, letters, short surveys, and notes, or if they were studies irrelevant to the topic.
Retracted and outdated studies, studies whose results were included or pooled in subsequent works, and studies with relevant methodological issues were also excluded.
All documents were independently inspected by at least two screeners and discrepancies in the selection process were discussed and resolved with the support of a third researcher.
Results of the selection procedure are shown in Figure 1.

Figure 1. PRISMA flow diagram.
Grading of evidence
Included studies were graded regarding the level of evidence provided, according to previous literature [Reference Gaebel, Großimlinghaus, Heun, Janssen, Johnson and Kurimay29]. Grades were assigned according to the indications detailed by Gaebel et al. [Reference Gaebel, Großimlinghaus, Mucic, Maercker, Zielasek and Kerst28] and modified by Galderisi et al. [Reference Galderisi, Mucci, Dollfus, Nordentoft, Falkai and Kaiser32]. The grading criteria of included evidence are reported in Table 1. Discrepancies in the ratings were resolved by discussion among all coauthors.
Table 1. Grading of evidence.

Grading of recommendations
Based on the evidence level of the included documents, recommendations were developed and reviewed by all coauthors. Grades were then assigned to recommendations according to the indications detailed by Gaebel et al. [Reference Gaebel, Großimlinghaus, Mucic, Maercker, Zielasek and Kerst28] and modified by Galderisi et al. [Reference Galderisi, Mucci, Dollfus, Nordentoft, Falkai and Kaiser32]. The grading criteria of recommendations are reported in Table 2.
Table 2. Grading of recommendations.

Conceptualization of Cognitive Impairment
The definitions of the neurocognitive and social cognition domains, selected by expert consensus [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock36,Reference Pinkham, Penn, Green, Buck, Healey and Harvey37] and widely recognized as impaired in schizophrenia [Reference Green, Horan and Lee15], are provided in Box 1.
Box 1. Cognitive domains identified by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative and Social Cognition Psychometric Evaluation (SCOPE) consensus initiatives.
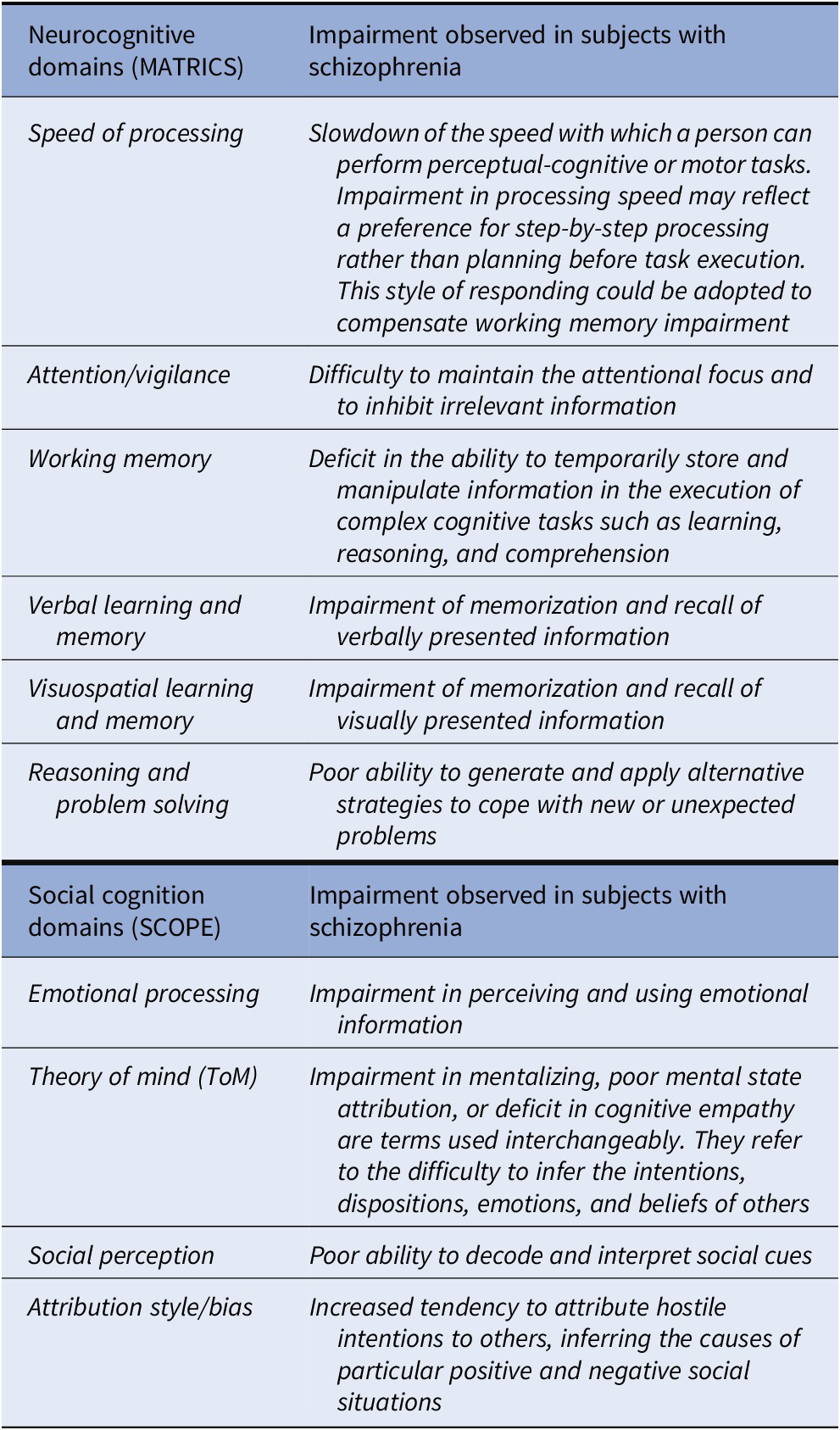
Cognitive Impairment in schizophrenia: identification of the distinct domains of impairment
The NIMH-Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative adopted a structured consensus-building process to identify the domains of cognitive impairment in schizophrenia and developed a consensus cognitive battery for use in clinical trials in schizophrenia. To this aim, factor analytic studies of cognitive performance in schizophrenia published until 2004 were examined and factors that were replicated across several studies were selected. Thirteen-factor analyses were scrutinized, and six separable factors were selected through consensus by the expert panel (see Box 1). Based on the feedback received, the Neurocognition Committee added social cognition as one of the domains, yielding a total of seven domains [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock36]. Relevant papers published after 2004, including several meta-analyses, consistently demonstrate that the six neurocognitive domains identified by the MATRICS initiative as well as the four social cognition domains are impaired in schizophrenia (see Supplementary Table 1). Apart from these domains, one meta-analysis indicated an impairment of autobiographical memory and another one of semantic memory [Reference Berna, Potheegadoo, Aouadi, Ricarte, Allé and Coutelle38,Reference Doughty and Done39]. Several meta-analyses focused on aspects of executive functions different from those selected by the MATRICS initiative [Reference Knapp, Viechtbauer, Leonhart, Nitschke and Kaller40–Reference Westerhausen, Kompus and Hugdahl44]. The relevant literature identified a wide range of subdomains: categorization, strategy forming, complex forward planning, cognitive flexibility, problem solving, working memory, attention, and set shifting, which overlap in part with each other and with the domains of “working memory” and “reasoning and problem solving” identified by the MATRICS consensus initiative.
However, factor analytic studies published in the period following the MATRICS consensus initiative did not report robust and consistent results (Supplementary Table 2). This heterogeneity seems to derive from differences in methodological and patient-related factors. Further studies, using large multicenter samples and a large array of standardized tests for the assessment of multiple domains of cognition, are needed to evaluate the factor structure with respect to what has been established by the MATRICS initiative.
Regarding social cognition, despite the agreement on its definition and the consistent demonstration of impairment in subjects with schizophrenia, there has been a lack of consensus on the identification of the independent domains which should be assessed [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37]. Therefore, the NIMH “Social Cognition Psychometric Evaluation” (SCOPE) study was initiated in 2013 in order to identify independent social cognition domains and identify the best existing measures of those domains [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37]. A panel of experts reviewed all available scientific information, following the “Research and Development (RAND) Corporation/University of California Los Angeles (UCLA) Appropriateness Method” (RAM), and four social cognition domains were identified (see Box 1).
Several meta-analyses [Reference Lee, Hong, Shin and Kwon6,Reference Bora, Yucel and Pantelis45–Reference Thibaudeau, Achim, Parent, Turcotte and Cellard52] reported that people with schizophrenia present deficits in social cognition, especially in emotion processing, ToM, social perception and, to a lesser extent, in social knowledge and attributional bias.
Literature focusing on ToM demonstrated severe and stable impairment in first-episode outpatients, and a mild impairment in both CHR individuals and unaffected relatives [Reference Bora, Yucel and Pantelis45,Reference Bora and Pantelis46]. ToM has also been found to be associated with overall neurocognition and each of its subdomains, and to be a moderator of neurocognitive task performance in subjects with schizophrenia [Reference Thibaudeau, Achim, Parent, Turcotte and Cellard52]. ToM might also play a role in poor insight [Reference Bora53] and could have a stronger correlation with functional outcome than emotion recognition [Reference Hajdúk, Krajčovičová, Zimányiová, Kořínková, Heretik and Pečeňák54].
Factor structures of cognitive impairment in schizophrenia
Since there is consistent evidence for the existence of distinct social cognition and neurocognition constructs, social cognition has been analyzed as a separate domain [Reference Mehta, Thirthalli, Subbakrishna, Gangadhar, Eack and Keshavan55].
Several factor-analytic studies examined the factor structure of neurocognitive deficits in subjects with schizophrenia. However, findings across studies were discrepant and, until now, there is no broad consensus on the number of independent domains and on the model of their factor structure (single, multiple, or hierarchical) (Supplementary Table 2).
The confirmatory factor analysis (CFA) conducted by Keefe et al. supported a single, second-order factor model, according to which the cognitive impairment could be sufficiently represented by a single cognitive factor, which hierarchically comprised five domains: speed of processing, vigilance, working memory, verbal memory, and reasoning [Reference Keefe, Bilder, Harvey, Davis, Palmer and Gold56]. In the multifactorial models, a certain number of cognitive domains were found to cluster as separable but inter-correlated factors. Some authors found that the seven-factor model had the best goodness-of-fit [Reference Genderson, Dickinson, Diaz-Asper, Egan, Weinberger and Goldberg57,Reference McCleery, Green, Hellemann, Baade, Gold and Keefe58], while others found that the six [Reference Noh, Kim, Hong, Kim, Nam and Lee59] or three [Reference Burton, Vella, Harvey, Patterson, Heaton and Twamley60–Reference Lo, Szuhany, Kredlow, Wolfe, Mueser and McGurk62] had the best fit. Three of these studies [Reference Burton, Vella, Harvey, Patterson, Heaton and Twamley60–Reference Lo, Szuhany, Kredlow, Wolfe, Mueser and McGurk62] used CFA on the MATRICS Consensus Cognitive Battery (MCCB) domains, demonstrating that a three-factor model, including processing speed, attention/working memory, and learning, provided a better fit than the unifactorial structure. Two CFA studies described a hierarchical model, consistent with models of intelligence in healthy samples, in which individual cognitive tests loaded on six cognitive domain factors, which in turn loaded on the general cognitive ability factor [Reference Dickinson, Goldberg, Gold, Elvevåg and Weinberger63,Reference Dickinson, Ragland, Calkins, Gold and Gur64].
The presence of discrepant findings between studies may be primarily due to heterogeneity in assessment instruments, or in sample sizes and characteristics. As the six domains of impairment were identified through expert consensus, future studies should provide external validation of the same domains and should investigate whether these domains demonstrate distinct associations with functional outcome measures or specific sensitivity to different pharmacological and psychosocial treatments.
Very few heterogeneous studies regarding the factor structure of social cognition have been conducted subsequently to the SCOPE initiative [Reference Mehta, Thirthalli, Subbakrishna, Gangadhar, Eack and Keshavan55]. Some exploratory factor analytic studies found multifactorial social cognition models [Reference Buck, Healey, Gagen, Roberts and Penn65–Reference Mike, Guimond, Kelly, Thermenos, Mesholam-Gately and Eack68]. Each study identified different factors, which seem to represent diverse latent constructs: the observed heterogeneity does not allow conclusions. The CFA study conducted by Browne et al. in subjects with schizophrenia and healthy controls, based on SCOPE structure, supports a one-factor model for both groups [Reference Browne, Penn, Raykov, Pinkham, Kelsven and Buck69]. Differences in the assessment of social cognition across studies and the inclusion of patients at different stages of the illness could explain discrepancies in results. Therefore, future studies need to focus on the use of psychometrically sound instruments to measure social cognition in large homogeneous populations.
Recommendations
Considering the available literature, the working group elaborated the following recommendations:
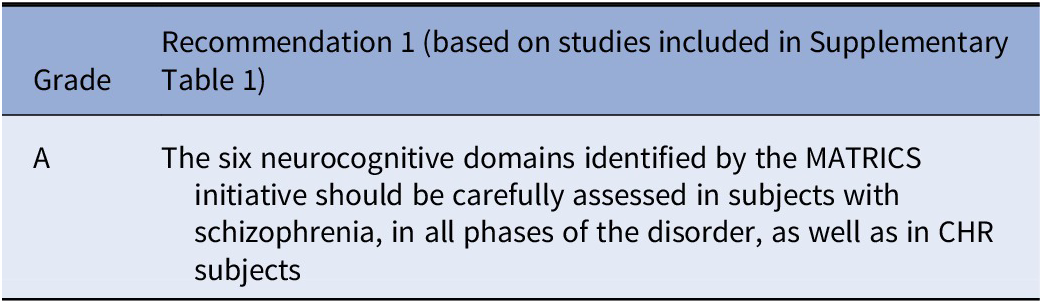

No recommendation is deemed appropriate by the EPA Guidance Group on Cognitive Impairment on the factor model of neurocognition and social cognition to be used in clinical trials. Further studies are deemed essential to validate the six neurocognitive domains identified by the MATRICS initiative and the four domains identified by the SCOPE initiative. Validation should be based on patterns of differential associations with functional outcome measures or pathophysiological mechanisms or differential sensitivity to pharmacological and non-pharmacological treatments.
Impact of Cognitive Impairment in Schizophrenia
This chapter identifies scientific results on neurocognitive and social cognitive impairments and their impact on real-life functioning and QoL.
To facilitate the analysis, results are shown in the following way:
-
• Effects of neurocognition and social cognition on functional outcome
-
• Effects of neurocognition on QoL
-
• Effects of social cognition on QoL
Effects of neurocognition and social cognition on functional outcome
Cognitive impairment is a central feature of schizophrenia [Reference Green, Horan and Lee15] and has been identified as the strongest predictor of functional outcome [Reference Bowie, Leung, Reichenberg, McClure, Patterson and Heaton70,Reference Green, Kern, Braff and Mintz71]. A large body of literature shows that cognitive dysfunction in patients with schizophrenia accounts for 20–60% of the variance in measures of functional outcome [Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17,Reference Galderisi, Rucci, Kirkpatrick, Mucci, Gibertoni and Rocca72]. These impairments are present across the course of the illness, from prodromal to early onset psychosis and to more chronic patients. Cognitive deficits are evident from an early stage [Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung and Howes11] and are often strongly associated with functional impairment [Reference Harvey and Strassnig16,Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17,Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam19]. This association is very robust as it was replicated in numerous studies, using different types of assessments and different patient groups across all phases of the illness, including high-risk subjects and subjects in prodromal states [Reference Green, Bearden, Cannon, Fiske, Hellemann and Horan73], first-episode patients (FEPs) [Reference Nuechterlein, Subotnik, Green, Ventura, Asarnow and Gitlin74], and older patients with chronic schizophrenia [Reference Holshausen, Bowie, Mausbach, Patterson and Harvey75,Reference McClure, Bowie, Patterson, Heaton, Weaver and Anderson76]. Cognitive deficits constitute one of the main limiting factors for recovery in the context of psychiatric treatment and rehabilitation. Despite treatment of symptoms with antipsychotics, impairments in daily functioning still represent a major treatment issue. Many studies demonstrated that functional outcome is more closely related to cognition than to positive or negative symptoms [Reference Green, Kern, Braff and Mintz71,Reference Green, Kern and Heaton77,Reference Strassnig, Bowie, Pinkham, Penn, Twamley and Patterson78]. The question has moved from whether cognitive dysfunction is related to functional outcome to how cognition is related to functional outcome. Composite measures of cognitive performance seem to account for 25–50% of the variance in real-world functioning, and the relationship between neuropsychological functioning and functional outcome seems to be mediated by functional capacity [Reference Bowie, Leung, Reichenberg, McClure, Patterson and Heaton70,Reference Bowie and Harvey79,Reference Moore, Harmell, Harvey, Bowie, Depp and Pulver80]. Other factors have a significant impact on real-life functioning, such as empathy, negative symptoms, and depression [Reference Green, Bearden, Cannon, Fiske, Hellemann and Horan73,Reference Robertson, Prestia, Twamley, Patterson, Bowie and Harvey81–Reference Bechi, Bosia, Spangaro, Buonocore, Cavedoni and Agostoni83]. In a sample of outpatients with chronic schizophrenia [Reference Bechi, Bosia, Spangaro, Buonocore, Cavedoni and Agostoni83], factors influencing both functional capacity and real-life behavior were investigated. Real-life behavior was significantly predicted by interpersonal reactivity. Functional capacity seems mainly related to neurocognition. Studies that have linked neurocognition to social cognition and/or social cognition to functional status have shown social cognition in schizophrenia to be a mediator of relations between neurocognition and functional status [Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17, Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca and Bertolino21,Reference Horan, Green, DeGroot, Fiske, Hellemann and Kee84,Reference Sergi, Rassovsky, Nuechterlein and Green85].
In the meta-analysis by Fett et al. [Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam19], social cognition explained relatively more variance in community outcome than neurocognition. This difference was largely due to the ability to infer other’s mental states, for instance mentalizing or ToM.
The estimated average correlations between community functioning and each of the neurocognitive and social cognitive domains were strongest for mentalizing, verbal fluency, social perception, and knowledge. A network analysis that investigated 740 patients with schizophrenia [Reference Galderisi, Rucci, Kirkpatrick, Mucci, Gibertoni and Rocca72] found that functional capacity and everyday life skills were the most central and highly interconnected nodes in the network. Functional capacity bridged cognition with everyday life skills, and the everyday life skills node was linked to disorganization and expressive deficits. Interpersonal relationships and work skills were connected to avolition; the interpersonal relationships node was also linked to social competence. The high centrality of functional capacity and everyday life skills in the network suggests that improving the ability to perform tasks is relevant for any therapeutic intervention in schizophrenia.
The meta-analysis by Halverson et al. [Reference Halverson, Orleans-Pobee, Merritt, Sheeran, Fett and Penn20] explored relationships between functional outcome in schizophrenia spectrum disorders and different domains of neurocognition and social cognition. Overall, associations between social cognition, neurocognition, and functional outcome showed significant small-to-medium effect sizes. Social cognition explained more variance in functioning than neurocognition [Reference Addington, Girard, Christensen and Addington86,Reference Schmidt, Mueller and Roder87]. In the mediation analysis associations between neurocognition, social cognition and different domains of functional outcome were found. Verbal learning and memory were shown to correlate with community functioning, working memory with social behavior in the milieu. For social cognition, the strongest associations were present for social knowledge and perception and community functioning. ToM was shown to correlate with social behavior in the milieu. Reasoning and problem solving demonstrated the strongest relationships with social problem solving, working memory with social skills, and ToM was shown to correlate with social skills.
Similar associations between neurocognition and social cognition with functional outcome are already present in the early stages of illness. The relationship between cognition and outcome was observed also in FEPs highlighting the importance of early intervention [Reference Salagre, Grande, Solé, Mezquida, Cuesta and Díaz-Caneja88,Reference Rojnic Kuzman, Makaric, Bosnjak Kuharic, Kekin, Madzarac and Koricancic Makar89]. The influence of cognitive reserve as a mediator between cognitive domains and function in FEP was shown by Amoretti et al. [Reference Amoretti, Rosa, Mezquida, Cabrera, Ribeiro and Molina90] and by Gonzalez-Ortega et al. [Reference González-Ortega, González-Pinto, Alberich, Echeburúa, Bernardo and Cabrera91].
In a study by Modinos et al. [Reference Modinos, Kempton, Tognin, Calem, Porffy and Antoniades92], individuals at CHR of psychosis were assessed. It was found that abnormalities in social cognition at baseline were associated with poor functional outcome after 12 months. Poor functional outcome was associated with baseline abnormalities in the recognition of angry emotion. These findings have potential implications for the stratification of individuals at CHR of psychosis according to subsequent outcome and suggest that functional outcome might be improved by interventions that target emotional processing.
Distinctions between self-report and observer reported measures of real-world functional outcome have become an area of focus in schizophrenia research. Self-report has been shown to be minimally correlated with observer reported real-world functional outcome.
Ho et al. [Reference Ho, Moore, Davine, Cardenas, Bowie and Patterson93] demonstrated that functional capacity partially mediates the relationship between overall cognitive ability and observer reported real-world functioning in work skills and community participation. It appears that cognitive impairment might be a precursor to poor acquisition of functional capacity, for instance what a person can do, which subsequently affects real-world functioning, for instance what a person actually does. Self-report of ability seems to be a further determinant of real-world functional outcome. This newly defined component addresses just how well individuals evaluate their own abilities and performance, and this type of self-awareness was referred as “introspective accuracy” (IA). The results of a study by Silberstein and Harvey [Reference Silberstein and Harvey94] indicate that IA of neurocognition strongly predicted nonsocial functional outcomes (everyday activities). IA of social cognition showed a significant correlation of medium strength to interpersonal relationships while showing a small relationship to everyday activities. These findings support the idea to combine observer ratings and patients’ self-reports because the discrepancy score adds some understanding of everyday social deficits.
The role of cognitive ability in observer versus self-reported real-world functioning may be explained by different mechanisms. Deviation between observed and expected cognitive ability is a core cognitive feature of schizophrenia related to neurophysiological, clinical, and psychosocial functioning [Reference Hochberger, Thomas, Joshi, Swerdlow, Braff and Gur95]. Hochberger et al. [Reference Hochberger, Thomas, Joshi, Swerdlow, Braff and Gur95] found that 24% of the total patient population exhibited significant deviation between observed and expected cognitive ability. The magnitude of this deviation was associated with worse psychosocial functioning. Since the relationship between psychopathology, neurocognitive deficits, and functional outcome is very complex, new statistical methods have been applied including computational model tools such as artificial neural networks [Reference Bosia, Bechi, Bosinelli, Politi, Buonocore and Spangaro96]. In the study by Bosia et al. [Reference Bosia, Bechi, Bosinelli, Politi, Buonocore and Spangaro96], processing speed turned out to be the first rank-predictor of functional outcome. Attention and verbal memory were also shown to have an impact on functioning. This finding stands in line with previous and recent findings.
By using a structural equation approach, Ojeda et al. [Reference Ojeda, Sánchez, Gómez-Gastiasoro, Peña, Elizagárate and Ezcurra97] found that processing speed, verbal memory, and premorbid functioning predicted outcome. Social cognition and processing speed explained 47% of the variance in community functioning in a study by Lewandowski et al. [Reference Lewandowski, Cohen and Ongur98].
Vita et al. [Reference Vita, Barlati, Deste, Rocca, Rossi and Bertolino99] showed that autistic symptoms may identify a subgroup of people with schizophrenia with worse outcome. Subjects with schizophrenia with more severe autistic symptoms showed poorer processing speed, attention, verbal memory, social cognition, poorer functional capacity, real-world interpersonal relationships, and participation in community-living activities [Reference Vita, Barlati, Deste, Rocca, Rossi and Bertolino99].
Supplementary Table 3 shows all systematic reviews and meta-analyses (level of evidence I) available on the effects of neurocognition and social cognition on functional outcome.
Recommendations
Considering the available literature, the working group elaborated the following recommendations:


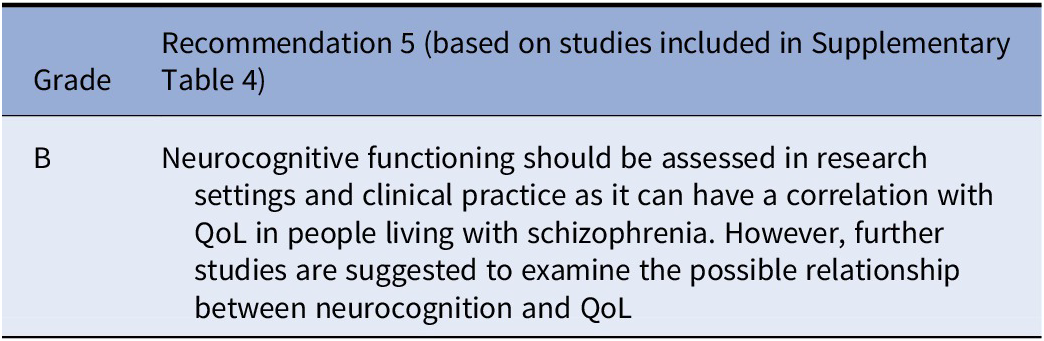
Effects of neurocognition on QoL
Studies on the effects of neurocognition on QoL are shown in Supplementary Table 4. Some small studies with evidence level III showed an association of neurocognition with QoL, and a large meta-analytic study revealed a moderate correlation between verbal ability and processing speed with subjective QoL, while a large study with 1032 subjects with schizophrenia showed no association of neurocognitive functioning with QoL [Reference Maat, Fett and Derks100] or a negative correlation [Reference Ehrminger, Roux, Urbach, André, Aouizerate and Berna101]. In a population of older adults with schizophrenia, neurocognitive impairment was associated with reduced overall functioning and low education with diminished QoL [Reference Hoertel, Rotenberg, Blanco, Camus, Dubertret and Charlot102].
Recommendations
Considering the available literature, the working group elaborated the following recommendations:
Effects of social cognition on QoL
Results of the few studies on the effects of social cognition on QoL (QOL) are shown in Supplementary Table 5. Only one study has investigated the relationship at evidence level I, showing an association of ToM but not emotion perception or neurocognition with QoL in subjects with schizophrenia.
Recommendations
Considering the available literature, the working group elaborated the following recommendations:

Recognition and Assessment of Cognitive Impairment
Assessment instruments
The systematic assessment of cognitive impairment still represents an unmet need for people with schizophrenia. Until 2004, this type of assessment was confined to specialized centers and limited to a few domains [Reference Green, Kern, Braff and Mintz71,Reference Green103,Reference Green, Llerena and Kern104]. Many different instruments were employed in the past decades: most of them were adapted from clinical neuropsychology and were too long and complex, as they assessed the entire neuropsychological profile of an individual. A systematic review, conducted by Bakkour in 2014 [Reference Bakkour, Samp, Akhras, El Hammi, Soussi and Zahra105], found that the batteries most often used in trials assessing cognition in schizophrenia were the Cambridge Neuropsychological Test Automated Battery (CANTAB), the CogState, and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); sometimes single subtests were adopted [Reference Bakkour, Samp, Akhras, El Hammi, Soussi and Zahra105]. However, these instruments were not specifically designed for schizophrenia and were developed to assess cognitive deterioration in elderly subjects with dementia, with possible ceiling effects in subjects with schizophrenia. Furthermore, the batteries were too complex and time-consuming for their use in clinical trials and routine clinical assessments.
A renewal of interest in the cognitive assessment of people with schizophrenia was related to the increasing acknowledgment of the strong relationships of cognitive deficits with functional outcome [Reference Green, Kern, Braff and Mintz71,Reference Green103,Reference Green, Llerena and Kern104]. In the early 2000s, research focused on those aspects of cognition that demonstrated a strong correlation with a variety of functional outcome measures (community functioning, functional capacity, social skills acquisition). Later on, social cognition, which was not included in neuropsychological batteries, became also a focus as it represents a mediator of the impact of neurocognition on functioning [Reference Harvey and Strassnig16,Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca and Bertolino21,Reference Green, Kern, Braff and Mintz71,Reference Galderisi, Rucci, Kirkpatrick, Mucci, Gibertoni and Rocca72,Reference Galderisi, Rucci, Mucci, Rossi, Rocca and Bertolino106]. The renewed interest and the association with functional outcome stimulated the development of batteries specifically devoted to the cognitive assessment of subjects with schizophrenia [Reference Keefe, Fox, Harvey, Cucchiaro, Siu and Loebel107–Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109].
Instruments Developed for Assessing Neurocognitive Impairment in Subjects with Schizophrenia
Performance-based instruments
Until 2004, no standardized battery for assessing neurocognitive impairment in schizophrenia was developed, contrary to what happened for other diseases, such as dementia. The first instrument specifically designed for the disorder was the Brief Assessment of Cognition in Schizophrenia (BACS), developed by Keefe and his group [Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour108]. The BACS had a rather short administration time (35 min) and a high completion rate. Its results correlated with those obtained using a standard battery [Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour108]. The included subtests explore six domains of neurocognition (Supplementary Table 6) and were chosen on the basis of test–retest reliability, practice effects, and sensitivity to impairment in schizophrenia (Supplementary Table 7). The BACS also showed a correlation with measures of functional outcome, the University of California Performance-based Skills Assessment (UPSA, a measure of functional capacity), and the Independent Living Skills Inventory (ILSI, a measure of real-life functioning) [Reference Keefe, Poe, Walker and Harvey110] and was found to have better psychometric properties, compared to RBANS, in subjects with schizophrenia [Reference Chianetta, Lefebvre, LeBlanc and Grignon111]. It has been translated and validated into nine languages [Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour108,Reference Bralet, Falissard, Neveu, Lucas-Ross, Eskenazi and Keefe112–Reference Anselmetti, Poletti, Ermoli, Bechi, Cappa and Venneri119] (Supplementary Table 6). Normative data in different cultural contexts (USA, Italy, Taiwan-Mandarin-speaking Chinese population; Singapore-English-speaking Chinese population) are available [Reference Anselmetti, Poletti, Ermoli, Bechi, Cappa and Venneri119–Reference Wang, Huang, Hung, Chen, Chen and Lee122]; they show a good degree of consistency in western countries [Reference Anselmetti, Poletti, Ermoli, Bechi, Cappa and Venneri119,Reference Keefe, Harvey, Goldberg, Gold, Walker and Kennel121], while those collected Taiwan and Singapore appear significantly different from US data, demonstrating the need for adaptation. Western samples performed better on language-related tasks, while Chinese samples performed better on non-language-related tasks, indicating that theapplication of BACS western norms to determine the performance of Chinese populations might result in data misinterpretation [Reference Eng, Lam, Bong, Subramaniam, Bautista and Rapisarda120,Reference Wang, Huang, Hung, Chen, Chen and Lee122]. Even if BACS proved to be a valid tool, it was developed by a single group, without a large consensus on the domains to be assessed and on criteria for test selection [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock36].
To analyze the efficacy of new compounds on cognition, the Food and Drug Administration required the development of an instrument through the acquisition of a consensus among experts. To this aim, the MATRICS initiative developed a consensus cognitive battery for subjects with schizophrenia, designed for use in clinical trials [Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109]. The process of test selection was conducted following the RAND/UCLA appropriateness method (an internationally recognized method to define the appropriateness of tests or clinical procedures using consensus among expert panels): 74 experts were questioned about the criteria for including subtests in the battery [Reference Fitch, Bernstein, Aguilar, Burnand and LaCalle123]. Five criteria were selected as most important: 1) test–retest reliability; 2) high utility as a repeated measure; 3) relationship with the functional outcome; 4) potential response to pharmacologic agents; and 5) tolerability and practicality [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock36]. Of note, these criteria only partially overlap with those applied for BACS. Through the evaluation of adherence to the five criteria cited above, 10 tests were selected. The resulting MCCB explored the seven domains of cognition that were identified by the consensus conference. Supplementary Tables 6 and 7 provide details on the cognitive domains assessed by the MCCB and the psychometric properties of this instrument. At the present time, the MCCB is the only performance-based assessment battery that includes a subtest exploring a social cognition domain. Furthermore, despite a rather long administration time (60–90 min), 95% of participants returned for re-administration: this was underlined as high tolerability index (Supplementary Table 7). Several studies demonstrated that the MCCB is a sensitive instrument, with a good correlation to functional outcome [Reference Keefe, Fox, Harvey, Cucchiaro, Siu and Loebel107,Reference Kiwanuka, McMahon and Gold124]; in addition to that, its psychometric properties were found adherent to key criteria individuated by the MATRICS initiative [Reference Georgiades, Davis, Atkins, Khan, Walker and Loebel125,Reference Roseberry and Kristian Hill126]. Based on its psychometric properties and the strong relationship with functional outcome, the MCCB has been proposed as the gold standard in assessing cognitive impairment in subjects with schizophrenia. It has been translated into 24 languages (Supplementary Table 6) and validated in different countries, USA, Brazil, Italy, Czech Republic, Poland, Japan, Norway, China, Singapore, and Spain [Reference Mucci, Galderisi, Green, Nuechterlein, Rucci and Gibertoni13,Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109,Reference Bezdicek, Michalec, Kališová, Kufa, Děchtěrenko and Chlebovcová127–Reference Shi, Kang, Yao, Ma, Li and Liang134]. Normative data in different cultural contexts (USA; Brazil; Italy, Czech Republic; Norway; China; Singapore; Spain) are available [Reference Mucci, Galderisi, Green, Nuechterlein, Rucci and Gibertoni13,Reference Bezdicek, Michalec, Kališová, Kufa, Děchtěrenko and Chlebovcová127,Reference Fonseca, Berberian, de Meneses-Gaya, Gadelha, Vicente and Nuechterlein128,Reference Mohn, Sundet and Rund131–Reference Kern, Nuechterlein, Green, Baade, Fenton and Gold135]. Consistent normative data for MCCB are available for western countries, while normative data from Singapore significantly differ from US data [Reference Rapisarda, Lim, Lim, Collinson, Kraus and Keefe132]. The overall performance of Singaporeans, compared to the US population, was poorer in all subtests except the Spatial Span subtest in which no mean difference between samples was detected [Reference Rapisarda, Lim, Lim, Collinson, Kraus and Keefe132]. The most important obstacle to the use of MCCB in routine clinical assessment of patients with schizophrenia is the long administration time (60–90 min). Therefore, other short-administered tools were developed, aimed at use in everyday clinical routine (the Screen for Cognitive Impairment in Psychiatry [SCIP], the Brief Neurocognitive Assessment (BNA), and the Brief Cognitive Assessment Tool for Schizophrenia [B-CATS]).
The SCIP is a tool for the screening of cognitive impairment in subjects with affective disorders and psychoses. In particular, this test was specifically developed in order to provide a short instrument assessing neurocognitive impairment, thus facilitating the implementation into everyday clinical practice. It is a brief, pencil‐and‐paper test that requires about 15 min for its administration [Reference Purdon136]. Three equivalent forms are available and include the assessment of processing speed, attention, verbal fluency, and verbal memory domains (Supplementary Table 6). SCIP showed adequate psychometric properties in terms of test–retest reliability, temporal stability and internal consistency, specificity and sensitivity, as well as criterion and discriminant validity [Reference Belvederi Murri, Folesani, Costa, Biancosino, Colla and Zerbinati137–Reference Sachs, Lasser, Purdon and Erfurth141] (Supplementary Table 7).
The BNA has a 10-min administration time and was developed with the aim of capturing the maximum possible variance, using only two subtests that explored two domains (Supplementary Table 6). The BNA was able to capture 76% of the total variance found using a standard neurocognitive battery (The Clinical Antipsychotic Trials of Intervention Effectiveness-CATIE-cognitive battery) (Supplementary Table 7) [Reference Fervaha, Agid, Foussias and Remington142].
Another instrument, the B-CATS, was similarly developed, choosing subtests from batteries employed in previous trials and selecting those tests that account for a large proportion of variance in the global score per minute of administration time. It has an administration time of about 10 min and offers a global score of cognition, rather than exploring single domains [Reference Hurford, Marder, RSE, Reise and Bilder143] (Supplementary Table 6–7). Both BNA and B-CATS were subsequently compared to the MCCB and the measures were found to be highly correlated. The BNA showed a test–retest reliability that was similar to MCCB, low practice effects, and sensitivity to longitudinal changes [Reference Fervaha, Hill, Agid, Takeuchi, Foussias and Siddiqui144]. The B-CATS was found to have good internal consistency and test–retest reliability; it was highly correlated with the composite score of MCCB and total score of UPSA [Reference Hurford, Ventura, Marder, Reise and Bilder145]. However, the subtests included in both the above short batteries are included in the MCCB: this is to be considered when correlations between these instruments are evaluated. Therefore, further validation of these tools is advised. A study that compared the ability of two of these short tests (SCIP and B‐CATS) found that the SCIP was better than the B‐CATS in predicting global cognitive impairment in subjects with psychosis [Reference Cuesta, Pino, Guilera, Rojo, Gómez-Benito and Purdon146]. According to a systematic review conducted in 2014 [Reference Bakkour, Samp, Akhras, El Hammi, Soussi and Zahra105], the MCCB was the instrument adopted in most trials (N = 69), followed by the BACS (N = 24). In addition, the BACS has proven to be a feasible tool in a clinical rehabilitation setting [Reference John, Yeak, Ayres, Sevastos and Moore147]; however, more research is advisable. BNA and B-CATS were not used in any clinical trial. At the present time, given the recent introduction of these short batteries (BNA and B-CATS) and the small amount of evidence, more studies should be conducted in order to assess the validity of these instruments in routine clinical assessment of cognition in schizophrenia.
Interview-based instruments
Alongside performance-based tests, the MATRICS initiative adopted interview-based cognitive assessment as a co-primary measure that has face validity for patients and clinicians [Reference Green, Nuechterlein, Kern, Baade, Fenton and Gold148]. There is no universal agreement on how much change in performance-based instruments is clinically meaningful, while interview-based measures are designed to evaluate the impact on the functioning of the cognitive deficits and might capture better the clinical meaning of changes over time or following pharmacological or psychosocial treatments [Reference Buchanan, Keefe, Umbricht, Green, Laughren and Marder149–Reference Keefe, Davis, Spagnola, Hilt, Dgetluck and Ruse151]. Subjects with schizophrenia might have poor insight about their impairment; thus, interview-based measures usually require informants and an expert rater, as it is the case for the assessment of cognitive deterioration in elderly subjects with dementia. Two interview-based instruments, the Clinical Global Impression of Cognition in Schizophrenia (CGI-CogS) and the Schizophrenia Cognition Rating Scale (SCoRS), were evaluated within the MATRICS Psychometric and Standardization Study [Reference Green, Nuechterlein, Kern, Baade, Fenton and Gold148] to verify if they fitted the five MATRICS criteria stated above. These scales were both found to be acceptable and comparable across the various criteria [Reference Green, Nuechterlein, Kern, Baade, Fenton and Gold148].
CGI-CogS was modeled after the development of the Clinical Interview-Based Impression of Severity, a tool widely used for assessing dementia. The interview consists of 38 items, divided into two major categories: (a) activities of daily living and (b) neurocognitive state (Supplementary Table 6). Each item can be evaluated on a scale from 0 to 7 and the administration time is of approximately 30 min. CGI-Cogs shows high internal consistency, good inter-rater reliability, and high test–retest reliability; in addition, the caregiver and rater global scores correlate with neurocognition in the moderate range and with functioning in the moderate-high range [Reference Ventura, Cienfuegos, Boxer and Bilder152]. No other study using CGI-CogS was conducted; this is probably due to the development of shorter interview-based instruments. Therefore, further studies are needed.
The other interview-based instrument developed within the MATRICS initiative, the SCoRS, is composed of 18 items, based on the Brief Cognitive Scale, and modified by an experts’ panel. The SCoRS items can be rated on a scale of four levels of severity and assess six cognitive domains (Supplementary Table 6). The administration time is of 20–30 min. The composite score ranges from 1 to 10. The SCoRS provides a measure strongly correlated with real-world functioning [Reference Keefe, Poe, Walker, Kang and Harvey153] and is regarded as a valid co-primary measure as intended by the MATRICS initiative; this is also supported by a narrative review of all research conducted using SCoRS [Reference Harvey, Khan, Atkins, Walker and Keefe154]. However, a work by Vita and his group on the validity of the SCoRS demonstrated that the pattern of correlations between SCoRS and functioning varied in different samples; in particular, the relationship with functioning was found only in clinically stable patients, but not in recently hospitalized ones, suggesting a limited value of the SCoRS in acute phases [Reference Vita, Deste, Barlati, De Peri, Giambra and Poli155]. Further studies are needed to confirm this finding, thus allowing a better characterization of potential factors that influence the correlation between SCoRS and functioning. Furthermore, SCoRS seems to be a valid instrument for assessing the patient and caregiver’s insight into her/his cognitive functioning. A study [Reference Poletti, Anselmetti, Riccaboni, Bosia, Buonocore and Smeraldi156] showed that the patient’s rating scores of SCoRS did not correlate with neurocognitive performance assessed with BACS, while the caregiver’s rating scores correlated only with performance on executive functions. This result suggests a poor awareness of cognitive impairment from both the patient and caregiver perspectives. SCoRS has been translated into 22 languages (Supplementary Table 6) and validated in the US, Italy, Iran, Japan, Korea, and Singapore [Reference Mazhari, Parvaresh, Eslami Shahrbabaki, Sadeghi, Nakhaee and Keefe114,Reference Vita, Deste, Barlati, De Peri, Giambra and Poli155,Reference Chia, Chan, Chua, Lee, Lee and Lee157–Reference Kang, Kim, Seo, Jung, Seo and Ryu159]. Validation studies of SCoRS translated versions found patterns of correlations between cognitive performance and interviewer and informant scores similar to those observed in the US, although some differences were found, likely due to differences in patient populations [Reference Mazhari, Parvaresh, Eslami Shahrbabaki, Sadeghi, Nakhaee and Keefe114,Reference Vita, Deste, Barlati, De Peri, Giambra and Poli155,Reference Chia, Chan, Chua, Lee, Lee and Lee157,Reference Higuchi, Sumiyoshi, Seo, Suga, Takahashi and Nishiyama158].
To shorten the administration time, the Cognitive Assessment Interview (CAI), a semi-structured interview, was developed by experts within the MATRICS initiative, using both CGI-CogS and SCoRS as “parent instruments.” The development of this interview was based on the item-response theory analysis of the original scales, which showed that only 10–12 items were necessary to achieve an accurate estimate of the neuropsychological deficits [Reference Reise, Ventura, Keefe, Baade, Gold and Green160]. CAI explored six out of the seven domains of cognition identified by the MATRICS initiative (Supplementary Table 6). The severity of the deficit was assessed on a 7-level scale [Reference Ventura, Reise, Keefe, Baade, Gold and Green161]. The measure was subsequently validated by administering it to 150 stable patients diagnosed with schizophrenia and found to be correlated with neurocognition, functional capacity, and everyday functioning. Its short administration time (15 min) and the absence of practice effects made it a reliable instrument in detecting changes across time [Reference Ventura, Reise, Keefe, Hurford, Wood and Bilder162]. CAI has been translated and validated in the US, Italy, Turkey, and Spain [Reference Ventura, Reise, Keefe, Baade, Gold and Green161,Reference Bosgelmez, Yildiz, Yazici, Inan, Turgut and Karabulut163–Reference Sánchez-Torres, Elosúa, Lorente-Omeñaca, Moreno-Izco, Peralta and Ventura165] (Supplementary Table 6). A qualitative research study, which investigated the cross-cultural adaptability of four intermediate measures of functioning (Independent Living Scales, UCSD Performance-Based Skills Assessment, Test of Adaptive Behavior in Schizophrenia, and CAI) reported that, while the majority of subscales required major adaptations, CAI required considerably less cultural adaptation [Reference Gonzalez, Rubin, Fredrick and Velligan166].
The systematic review [Reference Bakkour, Samp, Akhras, El Hammi, Soussi and Zahra105] on all tools used for assessing cognition in schizophrenia in clinical trials found that SCoRS was used in few trials (N = 9) and CGI-CogS was used once. The exclusion of the CAI is probably due to the initial stage of development of the interview-based measures in 2014 [Reference Bakkour, Samp, Akhras, El Hammi, Soussi and Zahra105].
A summary of the characteristics of neurocognition assessment tools is provided in Supplementary Table 6.
Recommendations
The working group elaborated the following recommendations on the instruments to be used for the assessment of cognitive impairment in schizophrenia, taking into account the level of evidence concerning their psychometric properties, the coverage of the seven cognitive domains identified by the MATRICS Consensus Initiative as the most frequently impaired in subjects with schizophrenia, the administration time, and the availability of translations and validation in the largest number of languages.






Instruments developed for assessing social cognition impairment in subjects with schizophrenia
As emerged in the previous paragraphs, some instruments developed for the assessment of cognition also included a test or an item to evaluate social cognition impairment in subjects with schizophrenia (MCCB, CGI-CogS, and CAI). Therefore, over the years, some research groups have developed different customized instruments to assess exclusively social cognition domains. However, most of them tend to be poorly validated and standardized and show substandard psychometric properties [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Bora, Yucel and Pantelis45,Reference Hoekert, Kahn, Pijnenborg and Aleman48,Reference Green, Penn, Bentall, Carpenter, Gaebel and Gur167–Reference Yager and Ehmann171]. Therefore, as mentioned in the paragraph on conceptualization, in 2013 the NIMH “Social Cognition Psychometric Evaluation” (SCOPE) study was designed to identify not only core domains of social cognition but also instruments that best assess these domains [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37]. Based on the literature data, during the first and second phases of the SCOPE study, over 100 measures were nominated, and, after a survey of experts, 21 were forwarded to the RAND panel for consideration. The Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) [Reference Mayer, Salovey, Caruso and Sitarenios172], a test included within the MCCB to explore social cognition, was not considered in this project since the basic psychometrics properties of this instrument have been already established before the SCOPE study [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109,Reference Eack, Mermon, Montrose, Miewald, Gur and Gur173]. Eight instruments were positively evaluated and selected for psychometric evaluation via RAND panel ratings, based on previous data concerning the following factors: (a) reliability, test–retest and interrater reliability as applicable, as well as internal consistency; (b) distributions, floor and/or ceiling effects, and normality of distributions; (c) utility as a repeated measure, stability over time in the absence of intervention, or sensitivity to intervention associated change; (d) convergent and discriminant validity, relationship to social cognitive measures relative to other abilities and constructs; (e) relationship to functional outcomes; (f) practicality for administration; and (g) tolerability for patients. The eight included instruments were: Bell Lysaker Emotion Recognition Task (BLERT) and Penn Emotion Recognition Task (ER-40) assessing the emotional processing domain; Reading the Mind in the Eyes Test and The Hinting task assessing ToM; The Awareness of Social Inference Test (TASIT), assessing the emotional processing and ToM; Relationships Across Domains (RAD) assessing social perception; Ambiguous Intentions Hostility Questionnaire (AIHQ) and Trustworthiness Task assessing the attributional bias/style domain.
During the third phase of the SCOPE study, psychometric properties of all the above-mentioned eight instruments have been evaluated in a sample of 179 stable outpatients with schizophrenia and 104 healthy controls. Finally, during the fourth and fifth phases of the SCOPE study, psychometric properties of the eight instruments were evaluated in a sample of 218 stable outpatients with schizophrenia and 154 healthy controls. These instruments included those that were classified during the third phase as adequate for the use in clinical trials (BLERT and Hinting task), or acceptable with modifications (ER-40, Eyes task, and TASIT), and three new measures, the Mini Profile of Nonverbal Sensitivity (MiniPONS) and the Social Attribution Task-Multiple Choice (SAT-MC), assessing social perception; the Intentional Bias Task (IBT), assessing attributional bias/style.
In the following paragraphs, we will illustrate all the above-mentioned instruments, dividing them on the basis of the social cognition domain explored.
Emotional processing
The BLERT [Reference Bryson, Bell and Lysaker174] (Supplementary Table 8) measures the ability of a person to identify affect cues. It is an audio-visual task designed to elicit a person’s ability to properly discriminate seven emotional states (happiness, sadness, fear, disgust, surprise, anger, or no emotion) expressed by facial, voice-tonal, and upper-body movement of a male actor during 21 10-s video clips. BLERT has a short administration time of about 6–8 min and good psychometric properties in line with the key criteria individuated by the SCOPE initiative (Supplementary Table 9). In particular, BLERT showed good test–retest reliability and internal consistency, limited potential for floor/ceiling effects, good utility as a repeated measure, good convergent and discriminant validity, good practicality for administration, tolerability for patients, and good sensitivity to differentiate between patients and healthy controls [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170,Reference Lim, Lee, Pinkham, Lam and Lee175]. BLERT also showed significant correlations with measures of functioning, functional capacity, and social competence [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170] and significantly predicted real-world functioning [Reference Pinkham, Penn, Green and Harvey170]. The modified version of the BLERT, which included response time and confidence ratings, tested by Pinkham et al. [Reference Pinkham, Harvey and Penn169] had psychometric properties similar to the original version, although it showed greater practice effects and reduced criterion validity as compared to SCOPE phase 3. Of note, one of the weaknesses of BLERT is that the same Caucasian male actor is used for all stimuli, which might lead to a non-appropriate use of this instrument in non-Caucasian populations [Reference Pinkham, Kelsven, Kouros, Harvey and Penn176].
The Penn Emotion Recognition Task (ER-40) [Reference Kohler, Turner, Gur and Gur177] (Supplementary Table 8) assesses facial emotion recognition ability. It includes 40 color photographs of faces depicting a given emotion (i.e., happiness, sadness, anger, or fear) or a neutral expression. Stimuli are balanced for poser’s gender, age, and ethnicity, and for each emotion category. Participants are instructed to examine a series of faces and identify the expressed emotion from five possible choices. ER-40 has a short administration time of about 3–4 min and good psychometric properties in line with key criteria defined by the SCOPE initiative (Supplementary Table 9). In particular, studies have reported good test–retest reliability and internal consistency, limited potential for floor/ceiling effects, a good utility as a repeated measure, practicality for administration and tolerability for patients, good sensitivity to differentiate between patients and healthy controls, and good convergent validity with modest discriminant validity [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170,Reference Lim, Lee, Pinkham, Lam and Lee175,Reference Sachs, Steger-Wuchse, Kryspin-Exner, Gur and Katschnig178]. ER-40 also showed significant correlations with measures of functional capacity [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170] and social competence [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170,Reference Beck179], but not of real-life functioning [Reference Pinkham, Penn, Green and Harvey170], at odds with what observed for BLERT [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170]. The modified version of the ER-40, which included response time and confidence ratings, tested by Pinkham et al. had the advantage that confidence ratings correlated with measures of real-life functioning [Reference Pinkham, Harvey and Penn169]. In addition, ER-40 accuracy and response time emerged as unique significant predictors of social competence, even when controlling for all other cognitive and social cognitive variables [Reference Pinkham, Harvey and Penn169]. However, further studies are needed to confirm the associations between the ER-40-modified version and real-life functioning.
The MSCEIT [Reference Yager and Ehmann171] (Supplementary Table 8) is a 141-item scale, made up of eight tasks, measuring four branches of emotional intelligence (EI): perceiving emotions, using emotions to facilitate thoughts, understanding emotions, and managing emotions (ME). MSCEIT-ME represents to date the only measure that assesses emotional regulation, a subdomain of the emotional processing domain. Each subscale consists of two tasks. The MSCEIT can be scored at three levels: (a) an overall score reflecting a general level of EI; (b) two area scores, experiencing EI and strategic EI; and (c) four branch scores (each measured by two subtests). Each of these scores is obtained through two scoring criteria: expert scoring criterion and consensus scoring criterion. The expert scoring criterion is based on responses to the test items from 21 members of the International Society for Research on Emotion. The consensus scoring criterion is based on the responses to the test items from a large and heterogeneous standardization sample of over 5,000 subjects. Actually, MSCEIT scores are computed using the general consensus approach based on a large community sample rather than the expert rating approach. However, the interpretation of the consensus-based scoring method is controversial as it is unclear whether the scores reflect true variations in EI [Reference Roberts, Schulze, O’Brien, MacCann, Reid and Maul180]. MSCEIT has been shown to have a long administration time of about 35 min and modest to good psychometric properties [Reference Eack, Mermon, Montrose, Miewald, Gur and Gur173–Reference Lim, Lee, Pinkham, Lam and Lee175]. In particular, good internal consistency has been reported for MSCEIT branch scores, as well as for MSCEIT total score [Reference Eack, Mermon, Montrose, Miewald, Gur and Gur173,Reference Lim, Lee, Pinkham, Lam and Lee175,Reference Extremera, Fernández-Berrocal and Salovey181], good test–retest reliability for MSCEIT branches 1 and 4 [Reference Horan, Green, DeGroot, Fiske, Hellemann and Kee84,Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109], good sensitivity to differentiate between patients and healthy controls [Reference Eack, Mermon, Montrose, Miewald, Gur and Gur173,Reference Lim, Lee, Pinkham, Lam and Lee175], and good tolerability and good discriminant validity [Reference Eack, Mermon, Montrose, Miewald, Gur and Gur173]. A low degree of convergent validity, as well as practicality and utility as a repeated measure, have also been reported [Reference Eack, Mermon, Montrose, Miewald, Gur and Gur173], [Reference Lim, Lee, Pinkham, Lam and Lee175]. Furthermore, MSCEIT total score and branch scores demonstrated modes correlations with measures of functioning [Reference Green, Bearden, Cannon, Fiske, Hellemann and Horan73,Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109,Reference Eack, Mermon, Montrose, Miewald, Gur and Gur173,Reference DeTore, Mueser and McGurk182,Reference McCleery, Lee, Fiske, Ghermezi, Hayata and Hellemann183].
Theory of mind
The Hinting task [Reference Corcoran, Mercer and Frith184] (Supplementary Table 8) was devised to test the ability of subjects to infer the true intent of indirect speech utterances. The task comprises 10 short passages read aloud presenting an interaction between two characters and involving one of the characters dropping a hint. The participants are then asked to say what the character dropping the hint intended. The Hinting task has a short administration time of about 5–7 min and overall good psychometric properties (Supplementary Table 9). In particular, it has good internal consistency and test–retest reliability, small practice effects, limited potential for floor/ceiling effects [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170], good practicality for administration and tolerability for patients [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170], good sensitivity to differentiate between patients and healthy controls [Reference Pinkham, Penn, Green and Harvey170], and good convergent but weak discriminant validity [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37]. The Hinting task also demonstrated a correlation with measures of functioning, functional capacity, and social competence [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170]; furthermore, it was the only significant predictor or showed significant incremental validity in the prediction of functional capacity and social competence [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170]. However, a recent study [Reference Lim, Lee, Pinkham, Lam and Lee175], investigating psychometric properties of different social cognitive measures in an Asian sample, found the Hinting task less favorable psychometric properties in terms of internal consistency in comparison with previous studies. Probably, this was due to the cultural sensitivity of the task that examines inferential ability through the presentation of short vignettes, which might require cultural adaptation.
The Reading the Mind in the Eyes Test (Eyes Test) [Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb185] (Supplementary Table 8) measures the capacity to discriminate the mental state of others from expressions in the eye region of the face. It includes 36 photographs of male and female eyes, rather than the whole face, depicting emotional states. For each photograph, participants are asked to choose the emotional state that best describes the eyes, choosing among four possible emotions. Each stimulus is presented with four response options. Participants are awarded one point for each correct item. It has a short administration time and adequate psychometric properties (Supplementary Table 9): good internal consistency and test–retest reliability; good utility as a repeated measure; good convergent and good to modest discriminant validity; practicality for administration, tolerability for patients, and good sensitivity to differentiate between patients and healthy controls [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170]. The Eyes Task also was significantly correlated with measures of functional capacity [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170] and social competence [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170,Reference Bora, Eryavuz, Kayahan, Sungu and Veznedaroglu186], but not of real-life functioning [Reference Pinkham, Penn, Green and Harvey170]. The modified version of the Eyes Task, which included definitions of terms used, tested by Pinkham et al. [Reference Pinkham, Harvey and Penn169], in order to reduce the dependence of performance on vocabulary, did not ameliorate the lack of relationship with measures of real-life functioning [Reference Pinkham, Harvey and Penn169]. Overall, the Eyes Task represents a promising instrument to assess the ToM domain. However, due to some limitations in its psychometric properties, even in the modified versions of this instrument, further validation studies are needed.
Emotional processing and ToM
TASIT [Reference McDonald, Flanagan, Rollins and Kinch187] (Supplementary Table 8) consists of videotaped vignettes of everyday social interactions and includes three parts, each with alternative forms (form A and B): Part I: The Emotion Evaluation Test, which evaluates the ability to recognize the basic emotions expressed by others in 28 video sequences; the subject is asked to identify the emotion expressed by a character, choosing from 7 options (surprise, happiness, anger, sadness, anxiety, disgust, neutral); Part 2: Social Inference—Minimal (SI-M), which includes 15 vignettes that represent dialogues between two actors and assess comprehension of sincere versus sarcastic exchanges; Part 3: Social Inference—Enriched (SI-E), which includes 16 vignettes that provide additional information before or after the dialogue of interest to “set the scene” and assess lies versus sarcasm. The administration time of the entire test is about 30–45 min; Part III, which is used to detect lies and sarcasm, lasts about 17–19 min. The total score and the score for part III have good internal consistency and test–retest reliability [Reference Browne, Penn, Raykov, Pinkham, Kelsven and Buck69,Reference Pinkham, Harvey and Penn169,Reference McDonald, Flanagan, Rollins and Kinch187]. All three parts showed good convergent and weak discriminant validity, good tolerability for patients but weak practicality due to the administration time, and significant practice effect [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170,Reference Lim, Lee, Pinkham, Lam and Lee175] (Supplementary Table 9). Part III has shown correlations with measures of functional capacity [Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17,Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca and Bertolino21,Reference Galderisi, Rucci, Kirkpatrick, Mucci, Gibertoni and Rocca72,Reference Galderisi, Rucci, Mucci, Rossi, Rocca and Bertolino106,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170,Reference Rocca, Galderisi, Rossi, Bertolino, Rucci and Gibertoni188], social competence [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170], and real-life functioning [Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17,Reference Mancuso, Horan, Kern and Green67,Reference Galderisi, Rucci, Kirkpatrick, Mucci, Gibertoni and Rocca72,Reference Galderisi, Rucci, Mucci, Rossi, Rocca and Bertolino106,Reference Pinkham, Penn, Green and Harvey170,Reference Rocca, Galderisi, Rossi, Bertolino, Rucci and Gibertoni188,Reference Sparks, McDonald, Lino, O’Donnell and Green189]. The modified version of the TASIT-III, which includes response time and counterbalanced administration of test forms across visits, tested by Pinkham et al., has psychometric properties similar to the original version, although response time scores do not correlate with measures of real-life functioning [Reference Pinkham, Harvey and Penn169] (Supplementary Table 9). Counterbalancing form administration reduced the discrepancy between forms noted in phase 3 of the SCOPE study [Reference Pinkham, Harvey and Penn169]. In a large sample of subjects with schizophrenia, recruited within a large multicenter study of the Italian Network for Research on Psychoses (NIRP), it has been demonstrated that social cognition, whose assessment included the TASIT, as well as the MSCEIT and FEIT, was correlated with real-life functioning and functional capacity at baseline [Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17,Reference Rocca, Galderisi, Rossi, Bertolino, Rucci and Gibertoni188] and at 4-year follow-up [Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca and Bertolino21]. In the same sample, through a network analysis, the authors found that TASIT-1 was connected to all the other social cognition nodes and bridged the social cognition domain with the functional capacity node and, through the latter one, with the real-life functioning nodes [Reference Galderisi, Rucci, Kirkpatrick, Mucci, Gibertoni and Rocca72]. These results were also confirmed at 4-year follow-up [Reference Galderisi, Rucci, Mucci, Rossi, Rocca and Bertolino106]. Different factors might play a role in discrepancies of results concerning the presence or the absence of association between TASIT and real-life functioning. In particular, the smaller sample size, a milder impairment of social cognition, or a reduced variance in real-life functioning observed in Pinkham et al.’s studies [Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170], as compared to other studies [Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17,Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci18,Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca and Bertolino21,Reference Galderisi, Rucci, Kirkpatrick, Mucci, Gibertoni and Rocca72,Reference Galderisi, Rucci, Mucci, Rossi, Rocca and Bertolino106], might account for the lack of associations between TASIT and real-life functioning [Reference Browne, Penn, Raykov, Pinkham, Kelsven and Buck69,Reference Pinkham, Harvey and Penn169]. Therefore, further studies including large populations of subjects with schizophrenia are needed.
Social perception
RAD [Reference Sergi, Fiske, Horan, Kern, Kee and Subotnik190] (Supplementary Table 8) is a 75-item paper-and-pencil measure of competence in relationship perception. The content and format of RAD are based on relational model theory, which proposes that individuals use their implicit knowledge of four relational models to understand social relationships and predict the behavior of others: (a) communal sharing, (b) authority ranking, (c) equality matching, and (d) market pricing. The administration time is estimated at 35 min (16 min for the abbreviated version). The test has weak psychometric properties [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Lim, Lee, Pinkham, Lam and Lee175] (Supplementary Table 9), in particular, considerable floor effects, low practicality, and poor tolerability by patients [Reference Pinkham, Penn, Green and Harvey170].
The SAT-MC [Reference Bell, Fiszdon, Greig and Wexler191] (eTable 8). Participants viewed a short animation of geometric shapes enacting a social drama. The animation was shown twice, and participants then answered 19 multiple-choice questions about what happened. This instrument showed weak psychometric properties, in particular, poor test–retest reliability, a great floor effect, a modest relationship with measures of functional capacity and social competence, and no relationship with measures of functioning [Reference Pinkham, Harvey and Penn169] (Supplementary Table 9).
The MiniPONS [Reference Bänziger, Scherer, Hall and Rosenthal192] (Supplementary Table 8) tests accuracy in decoding interpersonal cues (face, body, and voice tone). Participants were presented 64 two-s auditory or visual segments of a Caucasian female exhibiting facial expressions, voice intonations, and/or gestures and had to choose which of two behavioral labels best described the situation. This instrument showed weak psychometric properties, in particular a great floor effect, a poor tolerability, a modest relationship with measures of functional capacity and social competence, and no relationship with measures of real-life functioning [Reference Pinkham, Harvey and Penn169,Reference Lim, Lee, Pinkham, Lam and Lee175] (Supplementary Table 9).
At the present time, given the low psychometric properties of tasks assessing social perception in schizophrenia, and the limited amount of evidence, further studies are needed in order to validate a measure to assess this social cognition domain.
Attributional bias/style
The AIHQ [Reference Combs, Penn, Wicher and Waldheter193] (Supplementary Table 8) evaluates hostile social cognitive biases. Participants read five hypothetical negative social situations with ambiguous causes and record a reason why the scenario occurred. For each situation, participants rate the intentionality of the other’s action, how angry it would make the participant feel, and how much s/he would blame the other. These three items are summed up for an overall blame score with higher scores indicating greater blame, perceived intention, and anger. Additionally, participants provide two open-ended responses: an explanation of why the event occurred, and what they would do in response to the event. These open-ended questions are evaluated by two independent raters according to the extent to which the participant interpreted the situation in a hostile manner (hostility bias, rating a hostility index) and the extent to which the individual reports aggression in her or his behavioral response (aggression bias, rating an aggression index). AIHQ has a short administration time of about 5–7 min. It shows good internal consistency [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37] but a low test–retest reliability (with the exception of the blame score) [Reference Pinkham, Penn, Green and Harvey170]. The instrument has low sensitivity in differentiating between patients and healthy controls [Reference Pinkham, Penn, Green and Harvey170], low to moderate degree of utility as a repeated measure [Reference Pinkham, Penn, Green and Harvey170,Reference Horan, Kern, Shokat-Fadai, Sergi, Wynn and Green194–Reference Roberts and Penn197], and moderate to not significant convergent validity scores [Reference Mancuso, Horan, Kern and Green67,Reference Combs, Penn, Michael, Basso, Wiedeman and Siebenmorgan198]. However, the study of Lim et al. [Reference Lim, Lee, Pinkham, Lam and Lee175] found acceptable test–retest reliability for the AIHQ Hostility Bias subscale (AIHQ-HB) in the controls and slightly poorer test–retest for the AIHQ Aggression Bias subscale (AIHQ-AB) in patients. A possible reason for different results between Pinkham et al. [Reference Pinkham, Penn, Green and Harvey170] and Lim et al. [Reference Lim, Lee, Pinkham, Lam and Lee175] is that the SCOPE [Reference Pinkham, Penn, Green and Harvey170] only administered items on ambiguous scenarios (Pinkham et al. [170]), while Lim et al. [Reference Lim, Lee, Pinkham, Lam and Lee175] administered the full AIHQ, which consisted of vignettes on ambiguous, accidental, and intentional scenarios. Further evaluation of this aspect is needed. Furthermore, AIHQ did not correlate with measures of real-life functioning, functional capacity, and social competence [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Pinkham, Penn, Green and Harvey170] (Supplementary Table 9).
The Trustworthiness task [Reference Adolphs, Tranel and Damasio199]. Participants are shown 100 (or 42 in the abbreviated version of the task) faces of unfamiliar people, showing ethnically diverse males and females in natural poses, and are asked to judge how much they would trust the person by providing a rating on a 7-point scale (Supplementary Table 8). This scale has often been used in patients with brain injury and with autism spectrum disorder [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37], and rarely in subjects with schizophrenia, in which it showed weak psychometric properties (Supplementary Table 9), in particular, low convergent validity, low sensitivity in discriminating patients from healthy controls, and no relationship with measures of real-life functioning, functional capacity, and social competence [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Hajdúk, Krajčovičová, Zimányiová, Kořínková, Heretik and Pečeňák54,Reference Pinkham, Penn, Green and Harvey170]. On the other hand, the instrument showed good reliability, utility as a repeated measure, practicality of administration, and tolerability for patients [Reference Pinkham, Penn, Green and Harvey170,Reference Pinkham, Hopfinger, Pelphrey, Piven and Penn200] (Supplementary Table 9).
The IBT [Reference Rosset201] (Supplementary Table 8) assesses the tendency to attribute intentionality to the actions of others. Participants read 24 brief descriptions of ambiguous actions and quickly indicate whether that action occurred “on purpose” or “by accident.”. IBT showed acceptable psychometric properties (good internal consistency, utility as a repeated measure, relationship with functional capacity and real-life functioning, tolerability and practicality, sensitivity in differentiating patients from controls) [Reference Pinkham, Harvey and Penn169,Reference Buck, Hester, Pinkham, Harvey, Jarskog and Penn202], but some limitations, in particular, low test–retest reliability scores, and high rate of missing data due to limited response times [Reference Pinkham, Harvey and Penn169] (Supplementary Table 9). Even if IBT represents a promising instrument to evaluate attributional style/bias, the relevant literature is scant at the present time. Therefore, this instrument needs more validation studies to be recommended for assessing impairment in attributional style/bias in subjects with schizophrenia.
At the present time, given the low psychometric properties of tasks assessing attributional bias/style in schizophrenia, and the small amount of evidence, further studies are needed in order to validate a measure to assess this social cognition domain.
In conclusion, to date, different instruments are available to assess emotional processing and ToM in subjects with schizophrenia, while no validated instrument might be indicated in assessing social perception and attributional bias/style. In addition, unfortunately, a unified battery assessing all social cognition domains impaired in subjects with schizophrenia is not available. Therefore, much more effort involving experts in this field is needed to identify and develop broader measures of social cognition relevant to schizophrenia. The cross-cultural validity of the different instruments also requires scrutiny, in light of the considerable influence of cultural factors on social interactions and difficulties arising from transferring subtle nuances of social communication from one language to the other. In the Italian version of the TASIT, adopted in the studies of the Italian Network for Research on Psychoses [Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17,Reference Bora, Eryavuz, Kayahan, Sungu and Veznedaroglu186], the videotaped vignettes of the test had to be dubbed in Italian by a prestigious society in the field of film industry, as the job requires the professional expertise of actors and of course, entails not negligible costs.
A summary of the characteristics of social cognition assessment tools is provided in Supplementary Table 8.
Recommendations
Considering the available literature, the working group elaborated the following recommendations:




Assessment of Cognitive Impairment in Early Intervention Settings
Patients with FEP and UHR subjects show widespread and persistent cognitive impairments [Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung and Howes11,Reference Aas, Dazzan, Mondelli, Melle, Murray and Pariante203,Reference Bora and Murray204] that can impede functional recovery. Indeed, cognitive deficits have consistently been reported to be associated significantly with the functional prognosis of UHR and FEP patients in both cross-sectional and longitudinal studies [Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam19,Reference Ali, Jahshan and Cadenhead205–Reference Stanford, Messinger, Malaspina and Corcoran216].
Cognitive assessment forms an important part of the assessment battery used in early intervention facilities as the cognitive deficits in themselves may be the focus of targeted interventions, but furthermore, as the cognitive assessment allows for taking the cognitive deficits into account when offering therapy in early intervention settings; that is by tailoring the therapy, so it fosters areas of growth and does not exceed the individual’s cognitive capacity. Additionally, cognitive assessment is of relevance when facilitating and supporting the patient in his/her vocational pursuits. A comprehensive cognitive assessment should comprise both neurocognitive and social cognitive deficits.
Assessment of neurocognitive deficits
The assessment of neurocognitive impairments has a longer tradition than social cognitive assessments, and thus the selection of neurocognitive batteries and the literature on the topic are more elaborated and have already been described in previous sections [Reference Mesholam-Gately, Giuliano, Goff, Faraone and Seidman7,Reference Zhang, Wang, Hu, Zhu, Zhang and Wang9–Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung and Howes11,Reference McCleery, Ventura, Kern, Subotnik, Gretchen-Doorly and Green14].
Reviewing the literature on cognitive assessment batteries used in cognitive remediation trials in early phases of psychoses revealed that many of the studies used an assessment battery that adhered to the cognitive domains defined in the MATRICS initiative [Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109] with 3 out of 13 studies specifically using the MCCB [Reference Østergaard Christensen, Vesterager, Krarup, Olsen, Melau and Gluud217–Reference Mendella, Burton, Tasca, Roy, St. Louis and Twamley219]. The additional studies used a variety of tests assessing selected or multiple aspects of cognitive function with the domains of memory functions and executive functions being the most commonly assessed [Reference Drake, Day, Picucci, Warburton, Larkin and Husain220–Reference Vidarsdottir, Roberts, Twamley, Gudmundsdottir, Sigurdsson and Magnusdottir227]. Regarding cognitive remediation trials in the UHR subjects, fewer studies have been conducted [Reference Glenthøj, Hjorthøj, Kristensen, Davidson and Nordentoft228]. To date, evidence from only three RCTs and three cohort studies are available. Two of the three RCTs and one cohort study used the MCCB [Reference Loewy, Fisher, Schlosser, Biagianti, Stuart and Mathalon229–Reference Hooker, Carol, Eisenstein, Yin, Lincoln and Tully231], while the remaining studies used tests predominantly indexing processing speed and verbal learning [Reference Choi, Corcoran, Fiszdon, Stevens, Javitt and Deasy232–Reference Rauchensteiner, Kawohl, Ozgurdal, Littmann, Gudlowski and Witthaus234].
To achieve a comprehensive understanding of the potential areas of deficits, and strengths, cognitive assessments in early intervention facilities should comprise a battery that covers the six core neurocognitive domains and not merely providing a neurocognitive global score. The findings from cognition studies on FEPs and UHR individuals indicate the utility of a standardized cognitive assessment battery such as the MCCB to be used in early intervention facilities. An alternative shorter battery that can be used in these facilities is the BACS, which covers four of the six MATRICS-defined neurocognitive domains. Domain scores and a neurocognitive composite score can be derived from the BACS battery. The BACS battery and BACS subtests have also shown efficacy in detecting neurocognitive deficits in patients with a first-episode psychosis [Reference Bliksted, Fagerlund, Weed, Frith and Videbech235,Reference Albert, Melau, Jensen, Emborg, Jepsen and Fagerlund236] and UHR individuals [Reference Friedman-Yakoobian, Parrish, Thomas, Lesser, Gnong-Granato and Eack233,Reference Bolt, Amminger, Farhall, McGorry, Nelson and Markulev237,Reference Glenthøj, Jepsen, Hjorthøj, Bak, Kristensen and Wenneberg238]. Both the MCCB and the BACS have exhibited robust correlations with functional measures [Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109,Reference Keefe, Harvey, Goldberg, Gold, Walker and Kennel121]. The CANTAB [Reference Sahakian and Owen239] offers a selection of cognitive tests, representing the core neurocognitive domains, and has proven sensitive to cognitive deficits in patients with a FEP [Reference Lee, Redoblado-Hodge, Naismith, Hermens, Porter and Hickie223] and UHR individuals [Reference Glenthøj, Fagerlund, Bak, Hjorthøj, Gregersen and Kristensen240,Reference Kristensen, Mandl, Raghava, Jessen, Jepsen and Fagerlund241]. Hence, the CANTAB tests may show utility to form a neurocognitive assessment battery, with the limitation already discussed, that is, the included tests were developed for elderly patients with dementia and might present a ceiling effect especially in young FEP or UHR individuals.
Finally, the Measure of Insight into Cognition-Self Report (MIC-SR) represents a measure of self-perceived cognitive ability that has been recently found to be related with overall functioning and QoL in UHR individuals [Reference Glenthøj, Mariegaard, Kristensen, Wenneberg, Medalia and Nordentoft242].
In conclusion, assessing neurocognitive deficits in early intervention facilities is recommended to be in keeping with the MATRICS-designated separate cognitive domains. The standardized neurocognitive batteries the MCCB and the BACS constitute psychometrically sound instruments shown to be sensitive to the neurocognitive deficits in the UHR population and in FEP and may therefore be the preferred choices. However, normative data for the adolescent population should be collected to improve the accuracy of the batteries in detecting neurocognitive impairment in UHR and the FEP subjects under 18 and to help diagnostic decision-making in clinical settings. The CANTAB may offer supplementary tests to index the range of neurocognitive deficits in the putative prodromal and early stages of psychosis, but with the limitation reported above.
Recommendations
Considering the available literature, the working group elaborated the following recommendations:



Assessment of social cognitive deficits
Social cognition is a relatively young research field that has suffered a significant problem of lacking social cognitive tests with good psychometric properties. The BLERT [Reference Vohs, Lysaker, Francis, Hamm, Buck and Olesek243], Hinting task [Reference Bliksted, Fagerlund, Weed, Frith and Videbech235,Reference Vohs, Lysaker, Francis, Hamm, Buck and Olesek243–Reference Sullivan, Lewis, Mohr, Herzig, Corcoran and Drake246], ER-40 [Reference Kuharic, Makaric, Kekin, Lovrencic, Savic and Ostojic247], Reading the Mind in the Eyes task [Reference Vohs, Lysaker, Francis, Hamm, Buck and Olesek243,Reference Couture, Penn, Addington, Woods and Perkins248–Reference Mazza, Catalucci, Pino, Giusti, Nigri and Pollice250], and TASIT [Reference Horan, Green, DeGroot, Fiske, Hellemann and Kee84,Reference Bliksted, Fagerlund, Weed, Frith and Videbech235] have been used in studies with FEP patients and found to be effective in detecting social cognitive deficits in this population. Likewise, the TASIT [Reference Green, Bearden, Cannon, Fiske, Hellemann and Horan73,Reference Glenthøj, Jepsen, Hjorthøj, Bak, Kristensen and Wenneberg238,Reference Piskulic, Liu, Cadenhead, Cannon, Cornblatt and McGlashan251] and the ER-40 [Reference Piskulic, Liu, Cadenhead, Cannon, Cornblatt and McGlashan251] have been found to be efficient in detecting deficits in mental attributions and emotion recognition in UHR patients. Additionally, the Reading the Minds in the Eyes (Eyes) task has been used in UHR studies [Reference Barbato, Liu, Penn, Keefe, Perkins and Woods206,Reference Couture, Penn, Addington, Woods and Perkins248], but the test’s ability to discriminate between UHR individuals and healthy controls still need to be established [Reference Couture, Penn, Addington, Woods and Perkins248]. Consequently, the UHR research is in vital need of further validation of social cognitive tests.
Considering the subjective experience of social cognition, only preliminary findings are currently available in FEP and UHR subjects [Reference Pelizza, Poletti, Azzali, Garlassi, Scazza and Paterlini252].
In conclusion, there is currently no standardized social cognitive test battery available to be recommended for use in early intervention settings. Based on the findings from the comprehensive SCOPE test evaluation, the best available social cognitive measures to be used in psychosis research are the BLERT, the Hinting task, and the ER-40 which have proven strong psychometric properties. The Eyes, TASIT, and IBT have acceptable psychometric properties and may show utility as measures of ToM and attributional style. These six tests are therefore suitable for use in early intervention settings, but the psychometric limitations to the latter three measures must be kept in mind. Furthermore, it must be recognized that the SCOPE initiative validated the tests for use in patients with established psychosis, and while they have shown efficacy in detecting social cognitive deficits in FEP and UHR populations, the psychometric properties of the tests may not apply as consistently for the FEP and UHR populations (e.g., they may show ceiling effects). This underscores a need for the development and validation of social cognitive tests specifically for use in the putative prodromal state of psychosis and FEP to capture the potential subtleties of social cognitive deficits occurring in these phases.
Recommendations
Considering the available literature, the working group elaborated the following recommendations:



Discussion
The aim of the present work is to provide a comprehensive and detailed overview of cognitive impairment in schizophrenia and to give evidence-based recommendations for its assessment both in research settings and in real-world clinical practice. In fact, assessment generally represents the first step for improvement, both for implementing currently available evidence-based treatment options and to appropriately develop new and more focused pharmaco- and psychotherapeutic remedies, including rehabilitation, aiming for precision psychiatry [Reference Maj, van Os, De Hert, Gaebel, Galderisi and Green253].
Research supports that cognitive impairment is a core feature of schizophrenia and is not just the result of other symptomatological dimensions or the consequence of current treatments [Reference Heinrichs and Zakzanis5,Reference Keefe254]. Several meta-analyses and systematic reviews consistently demonstrated that at least 80% of subjects with schizophrenia present a mild to severe impairment in different cognitive domains, involving both neurocognitive and social cognitive domains [Reference Fatouros-Bergman, Cervenka, Flyckt, Edman and Farde3–Reference Zhang, Wang, Hu, Zhu, Zhang and Wang9,Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17]. In line with the NIMH consensus conference and major systematic reviews, six neurocognitive domains as well as four social cognition domains are impaired in schizophrenia [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock36,Reference Green, Kern and Heaton77,Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109,Reference Kern, Nuechterlein, Green, Baade, Fenton and Gold135,Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton255]. The key neurocognitive domains include speed of processing, attention/vigilance, working memory, verbal learning and memory, visuospatial learning and memory, reasoning, and problem solving, while the key social cognitive domains include emotional processing, social perception, ToM, and attributional bias [Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam19,Reference Pinkham, Harvey and Penn169,Reference Pinkham, Penn, Green and Harvey170,Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton255]. Cognitive impairment is present since the first episode of psychosis and also in subjects at risk for psychosis [Reference Fatouros-Bergman, Cervenka, Flyckt, Edman and Farde3,Reference Lee, Hong, Shin and Kwon6,Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung and Howes11,Reference Bora and Murray204], as well as in non-affected first-degree relatives of people with schizophrenia [Reference Sitskoorn, Aleman, Ebisch, Appels and Kahn12,Reference Mucci, Galderisi, Green, Nuechterlein, Rucci and Gibertoni13]. The overall magnitude and pattern of cognitive impairment remain substantially stable over the course of schizophrenia [Reference McCleery, Ventura, Kern, Subotnik, Gretchen-Doorly and Green14]. The deficits in multiple neurocognitive domains seem to affect real-life functioning more than negative and positive symptoms [Reference Green, Horan and Lee15–Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci18]. The impact of neurocognitive deficits on real-life functioning seems to be mediated at least in part by social cognition [Reference Green, Horan and Lee15,Reference Galderisi, Rossi, Rocca, Bertolino, Mucci and Bucci17–Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca and Bertolino21,Reference Irani, Seligman, Kamath, Kohler and Gur256,Reference Ventura, Hellemann, Thames, Koellner and Nuechterlein257]. However, despite their crucial role in determining a worse real-world functioning, cognitive deficits are often not assessed in clinical practice.
The fifth edition of DSM includes cognition as a domain that needs to be evaluated by clinicians in the course of a diagnostic assessment [258]. In the last decades, the identification of cognitive impairment in schizophrenia is improved and studies reviewed in this paper provide evidence that cognition can be reliably assessed using appropriate instruments. The present guidance for the optimal assessment of cognitive symptoms in schizophrenia is based on expert consensus and systematic reviews [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock36,Reference Pinkham, Penn, Green, Buck, Healey and Harvey37,Reference Green, Kern and Heaton77,Reference Bakkour, Samp, Akhras, El Hammi, Soussi and Zahra105,Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen109,Reference Kern, Nuechterlein, Green, Baade, Fenton and Gold135,Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton255]. Based on the reviewed evidence, we recommend a systematic assessment of the neurocognitive domains identified by the MATRICS initiative in subjects with schizophrenia, in all phases of the disorder, as well as in subjects at risk to develop psychosis.
This assessment should be conducted as early as possible, also in the perspective of developing a personalized treatment program [Reference Maj, van Os, De Hert, Gaebel, Galderisi and Green253], and repeated to observe potential changes, particularly following interventions that specifically target cognitive performance in order to assess their effectiveness and document potential improvements.
However, it must also be considered that a thorough assessment of the different domains of cognition requires human and technical resources, which are globally—and even in many European countries—either not available or neglected; this issue has been particularly problematic also in wealthier countries given the recent difficulties faced by mental health services linked to the COVID-19 pandemic [Reference Barlati, Nibbio and Vita259–Reference Unützer, Kimmel and Snowden262]. Therefore, the profile of cognitive impairment of many patients currently goes unrecognized [Reference Vita and Barlati263]. Moreover, the still restricted treatment options available for cognitive impairment, including pharmacotherapy effectiveness, may not balance favorably with an extensive and elaborate assessment.
How does that translate into current clinical practice? The DSM-5 provides specifiers for clinician-rated symptom severity profiling in psychotic disorders, including “impaired cognition”—in the chapter Assessment Measures of Section III Emerging Measures and Models [258] hence not in Section II on Diagnostic Criteria and Codes of Mental Disorders. The ICD-11 [Reference Gaebel264] in its chapter on schizophrenia or other primary psychotic disorders with pre-coordinated course specifiers provides somewhat similar symptom severity specifiers, including cognitive impairment with operationalizations in the “Clinical Descriptions and Diagnostic Guidelines” (now “Requirements” [265]), which can be directly post-coordinated with the respective psychotic disorders. The expected global mandatory assessment of cognitive impairment represents a step forward to at least recognize and judge the severity of cognitive impairment compared to its full neglect, which still represents an often-observed issue [Reference Vita and Barlati263]. In fact, this development in both DSM-5 and ICD-11 points in the right direction of the current global clinical situation in which many countries or institutions do not have even the option and the resources to perform detailed assessments, let alone have specific and effective treatments available.
In this perspective, providing some kind of evaluation of cognitive performance for people living with schizophrenia in everyday, real-world clinical practice, even with simpler assessment tools that have lower levels of recommendation, or with interview-based instruments, or with clinical observation,-following symptoms severity specifiers, represents a substantially better alternative compared to leaving cognition completely unassessed, particularly if available resources are limited.
Likewise, providing an assessment of cognitive performance at least once in the lifetime of the patient is considered preferable than never performing any kind of evaluation.
As to the factor structure of neurocognition and social cognition to be used in clinical trials, no recommendation is deemed appropriate by the EPA Guidance Group on Cognitive Impairment on the basis of the available evidence. Further studies are needed to validate the six neurocognitive domains identified by the MATRICS initiative [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock36], and the four social cognitive subdomains identified by the SCOPE initiative [Reference Pinkham, Penn, Green, Buck, Healey and Harvey37]. Validation should be based on patterns of differential associations with functional outcome measures or pathophysiological mechanisms or differential sensitivity to pharmacological and non-pharmacological interventions. In recent years, the assessment of cognitive functions progressed with the development of validated, reliable, and comprehensive clinician-rated neuropsychological batteries and self-rated instruments with better assessment of experiential cognitive symptoms. This guidance paper provides evidence-based recommendations for using both observer reports and self-reports of cognitive performance, as they give additive and complementary information [Reference Hochberger, Thomas, Joshi, Swerdlow, Braff and Gur95]. In particular, self-report of neurocognition predicts everyday activities and self-report of social cognition predicts interpersonal relationships [Reference Silberstein and Harvey94]. In this context, MCCB should be used because it covers the seven cognitive domains identified by the MATRICS Consensus Initiative, BACS could be used for its short time of administration, and SCIP should be used as a screening tool. We also advise a carefully social cognitive assessment, taking into account the following subdomains: emotion processing, social perception, and ToM. Due to their good psychometric properties, BLERT and Hinting tasks should be used for emotional processing and ToM assessment, respectively, while TASIT could be used for emotional processing and ToM assessment. Furthermore, it is essential to highlight that the MATRICS initiative also adopted interview-based cognitive assessment tools as a co-primary measure [Reference Green, Nuechterlein, Kern, Baade, Fenton and Gold148]. Interview-based measures are designed to evaluate the impact of cognitive impairment on functioning and might capture better the clinical meaning of changes over time or following pharmacological or psychosocial interventions [Reference Green, Schooler, Kern, Frese, Granberry and Harvey150,Reference Keefe, Davis, Spagnola, Hilt, Dgetluck and Ruse151,Reference Ventura, Reise, Keefe, Baade, Gold and Green161]. Due to their good psychometric properties and face validity, we recommend SCoRS or CAI as co-primary measures in the assessment of cognitive impairment of subjects with schizophrenia in routine clinical contexts and clinical trials.
It should also be noted that, when performing a thorough assessment of cognitive performance, interviews should also be conducted with caregivers and family members. They could provide further valuable insight regarding the patient’s cognitive functioning, as well as relevant details regarding the cognitive strategies and mechanisms adopted in real-world situations in daily life. Obtaining a clear overview of the patient’s cognitive strengths and weaknesses, in fact, represents a valuable asset when devising personalized treatment programs [Reference Maj, van Os, De Hert, Gaebel, Galderisi and Green253, Reference Vita, Barlati, Ceraso, Deste, Nibbio and Wykes266].
The guidance also provides a systematic review of the state of the art of assessment in FEP and in individuals at risk for psychosis, highlighting the need to extending to the early stages of schizophrenia the use of a comprehensive cognitive assessment battery and further development of these assessment tools in the prodromal phases, and in subjects at risk.
As regards the limitations of the present guidance paper, no manual and non-systematic literature search was conducted on additional databases. However, a systematic search comprising three different electronic databases can be considered an accurate literature search [Reference Higgins and Green267].
Only the results of works published in English language were reported in the guidance; however, this represents a source of bias in systematic literature that is often considered of small effect [Reference Jüni, Holenstein, Sterne, Bartlett and Egger268, Reference Morrison, Polisena, Husereau, Moulton, Clark and Fiander269].
Future research perspectives include different areas of interest, but a number of topics of relevance emerged as requiring further studies. In particular, future research should focus on a better understanding of the factorial structure of cognitive deficits in schizophrenia, better exploring the relationship between deficits in neurocognitive and social cognition performance and both objective and subjective QoL, and developing valid and reliable tools to assess attributional style bias and social perception.
A summary of the reported findings and of the key points discussed in the guidance, providing several take-home messages, is provided in Box 2.
Box 2. Summary and take-home messages.
-
• Neurocognitive and social cognition performance should always be carefully assessed in people living with schizophrenia and with clinically high-risk states, considering the important negative effects of cognitive deficits on functional outcomes and QoL.
-
• This assessment should be performed not only in the perspective of better characterizing the patient but also to help in the development of personalized treatment and rehabilitation programs.
-
• Cognitive performance should be assessed at least once in the lifetime of the patient, and optimally several times, in different phases of the illness and at the start and at the completion of dedicated treatment programs.
-
• The MCCB represents the most appropriate and complete validated tool that is currently available to assess neurocognitive performance in people living with schizophrenia and with clinically high-risk states.
-
• The BACS could be used as an alternative for its short time of administration.
-
• The SCIP could be used as a screening instrument.
-
• Interview-based instruments (SCoRS, CAI) can be used to obtain useful supplemental information.
-
• Social cognition performance should be assessed with the BLERT and the Hinting task for the emotional processing and ToM domains, respectively.
-
• The TASIT can be used to obtain useful additional information on both domains.
-
• For other domains of social cognition, no available test has sufficiently reliable psychometric proprieties to be recommended.
-
• In contexts with limited or very limited available resources, providing any type of cognitive assessment is better than providing no cognitive assessment at all.
The construct of schizophrenia, even after about 110 years since its origin [Reference Bleuler1] and despite many modifications, has still made it into the contemporary classification systems, on the basis of which most of our evidence-based treatments have been clinically tested. However, although no paradigm shift has as yet happened in the classification of schizophrenia, research on transdiagnostic domain-specific deconstruction (Research Domains Criteria or RDoC) or reconstruction of hierarchical higher-order constructs (Hierarchical Taxonomy Of Psychopathology or HiTOP), including schizophrenia and other psychotic disorders, is fully underway [Reference Gaebel and Salveridou-Hof270-Reference Cuthbert and Morris273].
Depending on whether and which new and practically useful entities will inherit which kind of domains of cognitive impairment in which new configuration, the kind and direction of development of new treatment concepts may also depend on using new methodological approaches for “precision psychiatry” [Reference Maj, van Os, De Hert, Gaebel, Galderisi and Green253].
Overall, the comprehensive review of the evidence and the elaboration of recommendations might contribute to advance the field, allowing a better cognitive assessment, avoiding overlaps with other psychopathological dimensions, such as negative symptoms, and antipsychotics side effects, such as extrapyramidal and secondary negative symptoms. Despite the clinical relevance of cognitive deficits identification in order to improve outcomes and to achieve recovery in people living with schizophrenia, the low grade of some recommendations reflects the still limited literature available in this field.
Conclusions
Neurocognitive and social cognitive impairments in schizophrenia should be assessed in research settings and in clinical practice as they have an important impact on functional outcomes. This guidance paper aimed to promote the adoption of shared assessment protocols both in clinical trials and in real-world clinical setting, leading the way to further progress in the field of cognitive functions assessment and recognition in schizophrenia patients. Studies specifically aimed to assess neurocognitive and social cognitive functions at all the stages of schizophrenia should be carried out, in order to optimize the recognition and management of these symptoms, with the ultimate goal to improve outcomes and achieve recovery. Furthermore, sound methodological longitudinal studies aimed to assess the natural course and the trajectory of neurocognitive and social cognitive functions from the earliest phases of schizophrenia are extremely needed. Despite many steps forward are achieved in the last 15 years, much remains to be done, in order to reach a standardization of the neurocognitive and social cognitive construct and to identify effective strategies for cognitive deficits recognition in clinical practice. The dissemination of this guidance paper may promote the development of shared guidelines concerning the assessment of cognitive functions in schizophrenia, with the purpose to improve the quality of care and to obtain recovery.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1192/j.eurpsy.2022.2316.
Data Availability Statement
All the data that support the findings of this study are available within the article and its supplementary material.
Author Contributions
Conceptualization: A.V., W.G., G.S., S.G.; Data curation: A.M., G.S., A.E., F.Z., G.M.G., L.B.G., M.N., S.G.; Formal analysis: A.M., G.S., A.E., G.M.G., L.B.G., M.N.; Methodology: A.V., W.G., A.M., G.S., F.Z., G.M.G., L.B.G.; Project administration: A.V., S.G.; Supervision: A.V., W.G., A.M., G.S., A.E., M.N., S.G.; Validation: A.V., W.G., G.S.; Writing—original draft: A.M., G.S., A.E., S.B., G.M.G., M.N., S.G.; Writing—review and editing: A.V., W.G., S.B., L.B.G., S.G.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
S. Galderisi has received consulting fees or payments for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Angelini, Gedeon-Richter, Recordati, Innova Pharma, Janssen Pharmaceutica, Janssen-Cilag, Lundbeck a/S, Lundbeck Italia, and Sunovion. A. Erfurth declares Lecture fees from and/or Advisory Boards for Angelini, Germania, Janssen, Lundbeck, Mylan, Neuraxpharm, Recordati, and Sandoz. A. Mucci received honoraria, advisory board, or consulting fees, outside the present work, from the following companies: Angelini-Acraf, Astra Zeneca, Bristol-Myers Squibb, Gedeon Richter Bulgaria, Innova-Pharma, Janssen Pharmaceuticals, Lundbeck, Otsuka, Pfizer, and Pierre Fabre. G. Sachs declares Lecture fees from and/or Advisory Boards for Janssen, Mylan, Recordati, and Schwabe. A. Vita received advisory board, lecture, or consulting fees, outside the present work, from Angelini, Innova Pharma-Recordati, Janssen Pharmaceuticals, Lundbeck, Otsuka, Pfizer. All other authors declare no conflict of interest.






Comments
No Comments have been published for this article.